Abstract
Context
Drug abuse (DA) strongly runs in families. Does this result solely from genetic factors or does the family environment contribute?
Objective
To determine the familial environmental contribution to the risk for DA.
Design
Follow-up in 9 public databases (1961–2009) in siblings and spouses.
Setting
Sweden.
Participants
A total of 137 199 sibling pairs and 7561 spousal pairs containing a proband with DA and matched control probands.
Main Outcome Measures
Drug abuse recorded in medical, legal, or pharmacy registry records.
Results
In the best-fit model, which contained significant linear, quadratic, and cubic effects, among full sibling pairs containing a proband with DA, the relative risk for DA in the sibling declined from more than 6.0 for siblings born within 2 years of each other to less than 4.5 when born 10 years apart. Controlling for age differences in full sibling pairs, the hazard rate for DA in a sibling when the affected proband was older vs younger was 1.42 (95% CI, 1.31–1.54). In the best-fit model, which contained significant linear, quadratic, and cubic effects, among spousal pairs containing a proband with DA, the relative risk for DA in the spouse declined from more than 25.0 within 1 year of proband DA registration to 6.0 after 5 years.
Conclusions
Controlling for genetic effects by examining only full siblings, sibling resemblance for the risk for DA was significantly greater in pairs closer vs more distant in age. Older siblings more strongly transmitted the risk for DA to their younger siblings than vice versa. After one spouse is registered for DA, the other spouse has a large short-lived increase in DA risk. These results support strong familial environmental influences on DA at various life stages. A complete understanding of the familial transmission of DA will require knowledge of how genetic and familial environmental risk factors act and interact over development.
Drug abuse (DA) runs strongly in families.1,2 Twin and adoption studies have demonstrated that genetic factors contribute substantially to this familial aggregation.3–9 However, most studies also suggest that family environment contributes to the risk for DA.3,5,6,8,9 In a national Swedish adoption study of DA, we found that genetic risk—indexed by DA and other externalizing disorders in biological parents—and familial environmental risk—indexed by disruptions in the adoptive home and DA in adoptive siblings—contributed nearly equally to the risk for DA in the adopted child.10
We herein attempt to further quantify and characterize the familial environmental contribution to the risk for DA in Sweden. Traditionally, assessments of the family environment compare the degree of resemblance in special kinds of relationships with inherent power to distinguish nature from nurture: monozygotic and dizygotic twins and adopted children and their biological and adoptive relatives. In this article, we detect and characterize the impact of familial environmental factors on the risk for DA by examining 2 of the most common relationships—full siblings and spouses—using as our laboratory the entire population of Sweden.
With siblings, we evaluated 2 hypotheses. First, age differences between siblings vary widely. Given that peer environment influences the risk for DA and other externalizing behaviors,11–15 we reasoned that the closer siblings were in age, the more likely they would share their social environment. Therefore, we predicted that within sibships containing 1 individual with DA (the proband), the risk for DA would be higher in siblings close vs far apart in age from the proband.
Second, the relationship between older and younger siblings is generally not symmetric. Older siblings typically influence their younger siblings more than vice versa.16–20 In a review on sibling relationships, Brody stated that “[n]aturalistic observations of sibling interactions indicate that siblings enact asymmetrical, complementary roles…. Older siblings act as teachers [and] managers … when playing with their younger brothers and sisters, and the younger siblings assume the corresponding learner [and] managee … roles.”16(p16)
Furthermore, Newman18 found that second-born individuals in large families reported having more influence on their younger siblings than on their older siblings. We predicted that in sibships with a drug-abusing proband, the risk for DA would be higher in the proband’s younger siblings than in the proband’s older siblings.
Spouses are correlated in the risk for substance use disorders.21–27 The degree to which this results from assortative mating or environmental effects remains unclear.21,22,26–28 We tested for shared environmental influences on DA between spouses by examining whether, after 1 was registered for DA, their spouse had a temporal spike in DA registration.
We hope that these analyses will contribute to a better understanding of the social transmission of DA, thereby facilitating effective prevention strategies.
METHODS
As outlined elsewhere,10 we linked comprehensive register and health care data from multiple nationwide Swedish sources to form a DA database using the unique individual Swedish 10-digit personal identification number assigned at birth for all residents. This identification number was replaced by a serial number to preserve confidentiality. Our database contained 9 sources:
The Swedish Hospital Discharge Register included all hospitalizations for people in Sweden from 1964 through 2009.
The Swedish Prescribed Drug Register included all prescriptions in Sweden picked up by patients from July 1, 2005, through December 31, 2009.
The Swedish Mortality Register contained all causes of death and time of death from 1961 through 2009.
The National Censuses provided information on education and marital status in 1960, 1970, 1980, and 1990.
The Total Population Registry included annual data on education and marital status from 1990 through 2009.
The Multi-Generation Register included information on family relationships for all individuals born in Sweden in 1932 and later.
The Outpatient Care Register included information from outpatient clinics covering all geographic regions in Sweden from 2001 through 2009, with information on an increasing number of clinics for each year during this period.
The Primary Health Care Register included outpatient primary care data on diagnoses and time of diagnoses from 2001 through 2007 for 1 million patients from Stockholm and middle Sweden.
The Swedish Suspicion Register included nationwide data on all individuals strongly suspected of crime, including DA, from 1998 through 2007.
Drug abuse was identified in the Swedish medical registries by the eighth, ninth, and tenth revisions of the International Classification of Diseases (ICD) codes (ICD-8: drug dependence [304]; ICD-9: drug psychoses [292] and drug dependence [304]; ICD-10: mental and behavioral disorders due to psychoactive substance use [F10-F19], except those due to alcohol [F10] or tobacco [F17]) as well as in the Suspicion Register by codes 5011 and 5012, which reflect crimes related to DA. Crimes related only to alcohol abuse or to trafficking in or possession of drugs of abuse were excluded. Drug abuse was identified in individuals (excluding those suffering from cancer) in the Prescribed Drug Register who had retrieved (on average) more than 4 defined daily doses for 12 months of either hypnotics and sedatives (Anatomical Therapeutic Chemical Classification System codes N05C and N05BA) or opioids (Anatomical Therapeutic Chemical Classification System code N02A). We restricted the diagnosis of DA to individuals older than the age of 10 years, except from the Prescribed Drug Register, where the age limit was set at 18 years. Registration for DA in the birth cohort from 1950 through 1993 was highly correlated across our sources with all odds ratios (ORs), except for one (crime and prescription) exceeding 20.10 This study was approved by the ethics committee of Lund University in Malmö, Sweden.
SAMPLES
These analyses were based on 2 databases. The first began by double entering all sibling pairs in the Swedish population and required that (1) the proband was born between 1950 and 1993, (2) the proband’s sibling was born between 1940 and 1993, and (3) both the proband and the sibling were alive after 1972. In total, the database consisted of 7 210 949 sibling pairs, from which we selected pairs where at least 1 sibling who we defined as the proband was registered with DA during the period from 1973 through 2009.
For the second database, we double entered all married couples in Sweden during the period from 1990 through 2007. From this total database of 4 778 556 couples, we selected pairs where at least 1 was registered with DA during the period from 1990 through 2009.
International Classification of Diseases codes from the Hospital Discharge and Outpatient Care registers included the abused substance. No such information was available from the Suspicion Register. For the 31 661 and 3648 unique cases from these registers used in the sibling and spousal analyses, respectively, the 4 most common drug classes were sedatives/hypnotics (75%), stimulants (29%), opiates (25%), and cannabis (22%) among the sibling pairs and sedatives/hypnotics (49%), opiates (29%), stimulants (11%), and cannabis (7%) among the spousal pairs (the percentages of cases sum to greater than 100% because subjects had multiple diagnoses).
STATISTICAL METHODS
We conducted 3 different analyses. First, we looked at aggregation of DA among siblings and examined resemblance as a function of their age differences. Probands were defined as individuals registered for DA from 1973 through 2009, who had at least 1 nontwin full sibling living in Sweden from 1973 through 2009. For each DA proband, we specified all possible case-sibling pairs consisting of the proband and each of his or her full siblings (who were or were not registered with DA). For each pair, we randomly selected 5 control sibling pairs matched to case pairs by sex, birth year, country of birth, and education. Individuals were eligible as control subjects if they lived in Sweden at the time of the case proband’s DA registration and were not registered with DA prior to the time of the case proband’s registration. However, they could be registered later with DA.
Analyses were conducted using conditional logistic regression. In the first set of analyses, DA in a sibling (yes/no) was used as the independent variable. In the second set of analyses, the age difference between the sibling and the proband was used as the main predictor variable. We modeled this as a continuous variable where it was most sensible to define the absence of DA in the sibling as zero. Therefore, for cases where there was DA in the sibling, we transformed the age differences between the proband and sibling as follows: DA in the sibling and 0 to less than 2 years age difference between siblings was recoded to 15, DA in the sibling and 2 to less than 3 years age difference was recoded to 14, and so on, ending with DA in the sibling and 15+ years age difference recoded to 1. Note that given this transformation, a positive linear effect predicted the highest rate of DA in the sibling when the age difference between the proband and sibling was 0 to less than 2 years. We examined linear, quadratic, and cubic effects for this age difference. All analyses were stratified based on the 4 types of sibling pairs: male-male, male-female, female-male, and female-female. Because a proband could be included several times, we adjusted for the nonindependence by using a robust sandwich estimator. A total of 25 416 case probands could not be matched to 5 control probands and were excluded. The most common reason for failure was that the proband was born outside Sweden.
Our second set of analyses examined the difference in risk to a younger vs older sibling of a DA proband. These analyses began with all sibling pairs (excluding twins) born from 1950 through 1993 who were double entered. From this database, we selected all pairs where at least 1 individual—the proband—had DA. For sibling pairs where both had DA, the first individual found in the DA register was selected as the proband and the second as the sibling. Because of the possibility of differential right censoring (the older sibling will always have more years of exposure), we used a Cox proportional hazard regression with DA in the sibling as the outcome. The key predictor was a dummy variable that defined whether the sibling was younger or older than the proband. Control variables included linear and quadratic effects of age difference between the sibling and the proband, the sex of the proband and sibling, birth cohort, and relevant interactions. Because a proband could be included several times in the analyses, we adjusted for the nonindependence with a robust sandwich estimator.
Our third set of analyses examined aggregation of DA among spouses. We began by selecting, as case probands, all subjects who were in the state of being married the year prior to their first registration for DA. Five control probands were chosen randomly among individuals who lived in Sweden at the time of the case proband’s DA and not registered for DA prior to the time of the case proband’s DA. Control probands were matched to case probands by age, sex, country of birth, and education (measured the year before the date of diagnosis). The control proband also had to be married the same year as the case proband. When spouses were registered for DA in the Suspicion Register in the same year, we eliminated them to avoid biasing upward the spousal association solely from both potentially being arrested for the same criminal event.
Using conditional logistic regression, we first investigated whether the risk for DA in the spouse of the case proband was elevated compared with the risk for DA in the spouse of the control proband. In the second analysis, the differences (in years) in registration for DA between spouses were used as the main predictor variable. As with the sibling analyses, we treated this as a continuous variable, where we defined the absence of DA in the spouse as zero. Therefore, for cases where the spouse did have DA, we transformed the years between registration for DA in the proband and the spouse as follows: DA in the spouse and 0 to less than 1 year difference between spouses in the registration of DA was recoded to 20, DA in the spouse and 1 to less than 2 years difference among spouses in registration of DA was recoded to 19, and so on, ending with DA in spouse and 20+ years difference recoded to 1. We examined the linear, quadratic, and cubic effects, as well as the sex of the spouse. Given the transformation of this variable, a positive linear effect would predict the highest rate of DA when the spouse was registered for DA within 1 year of proband registration. A total of 452 case probands could not be matched to 5 control probands and were excluded from the analysis.
RESULTS
DA RISK IN SIBLINGS AS A FUNCTION OF AGE DIFFERENCES
We identified 137 199 proband sibling pairs with DA (mean [SD] age, 29 [11] years; 65% male) who could be matched with 685 995 control proband sibling pairs. The sources of registration for the siblings with DA are seen in Table 1. The mean (SD) number of siblings for cases and control subjects were 1.84 (1.17) (range, 1–16) and 1.80 (1.11) (range, 1–17), respectively. We first examined whether the risk for DA in the sibling of the case proband was elevated compared with the risk for DA in the sibling of the control proband. The results from the conditional logistic regression showed that the risk for DA was substantially higher in siblings of case vs control probands (OR, 5.29; 95% CI, 5.19–5.40). The association for a diagnosis of DA was considerably higher in same-sex pairs (brother-brother: OR, 6.37; 95% CI, 6.19–6.56, and sister-sister: OR, 5.37; 95% CI, 5.08–5.67) than in opposite-sex pairs (brother-sister: OR, 4.27; 95% CI, 4.09–4.46, and sister-brother: OR, 4.33; 95% CI, 4.15–4.52). If the proband sibling with DA was male, 9.1% (95% CI, 8.9–9.3) of his male siblings and 3.1% (95% CI, 3.0–3.2) of his female siblings had DA. The corresponding figures when the proband was female were 6.6% (95% CI, 6.4–6.8) and 4.0% (95% CI, 3.8–4.2), respectively.
Table 1.
Sources of Registration for Drug Abuse in the Sibling and Spouse Samples
| Register | No. (%)
|
|
|---|---|---|
| Siblings | Spouses | |
| Suspicion | 40 774 (51.4) | 1979 (26.2) |
| Hospital Discharge | 39 210 (49.4) | 3927 (51.9) |
| Outpatient Care | 24 014 (30.3) | 2190 (29.0) |
| Primary Health Care | 489 (0.6) | 68 (0.9) |
| Mortality | 737 (0.9) | 21 (0.3) |
| Prescription Drug | 6407 (8.1) | 1710 (22.6) |
We then fitted a conditional logistic regression model with the between-sibling age difference as the independent variable. As outlined in the “Methods” section, this variable was transformed so that ORs of greater than 1.0 indicated an inverse association between the age difference of the proband and sibling and the risk for DA in the sibling. We found significant linear (OR per year, 1.62; 95% CI, 1.56–1.69), quadratic (OR, 0.95; 95% CI, 0.95–0.96), and cubic (OR, 1.002; 95% CI, 1.001–1.002) effects. We also fitted similar models for the 4 types of sibling pairs: male-male, male-female, female-male, and female-female.
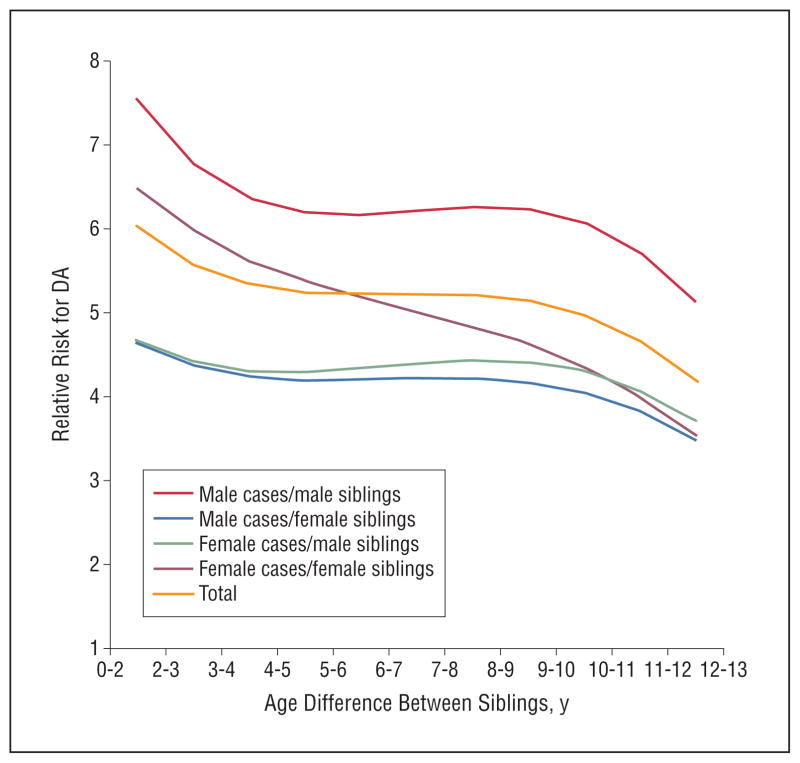
The results of these analyses are depicted in Figure 1. Examining first all pairs, the relative risk for DA was more than 6 for a sibling born within 1 year of the proband and declined to around 5 when the age span was 3 to 4 years. The relative risk then declined more slowly to approximately 4 for siblings born 10 years from the proband. The curve is very similar for male-male pairs. For female-female pro-band sibling pairs, the decline in risk was steeper. The 2 opposite-sex proband sibling combinations were similar. The relative risk exceeded 4.5 for those born 0 to 2 years apart and gradually declined to slightly less than 4 for those born 10 years apart. The decline was significant for all types of sibling pairs.
Figure 1.
The relationship between age difference and the risk for drug abuse (DA) among siblings of case probands with DA vs siblings of matched control subjects. We show results, predicted by the best-fit model up to 11 years difference between siblings, for all sibling pairs and the 4 sex combinations: male case/male sibling, male case/female sibling, female case/male sibling, and female case/female sibling.
DA RISK IN OLDER VS YOUNGER SIBLINGS OF A PROBAND SIBLING WITH DA
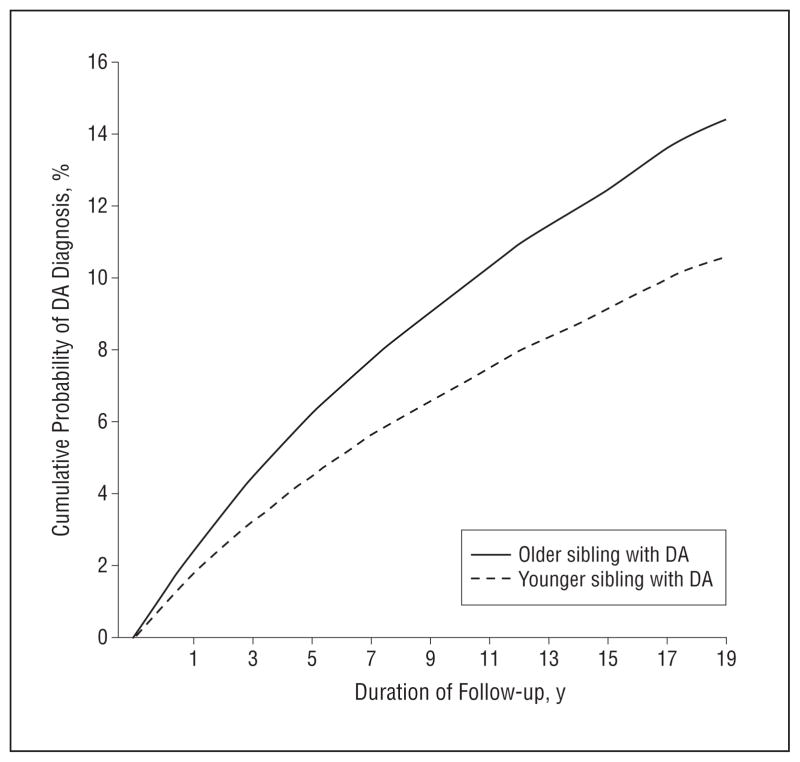
We examined 142 159 sibling pairs containing at least 1 proband sibling with DA. Our Cox model predicted the hazard ratio for DA in siblings of an affected proband. The proportional hazards assumption was fulfilled for the main variable of interest, DA in siblings. However, it was violated for the control variable of age differences. Therefore, we included an interaction term between follow-up time and age differences in the model (not shown in Table 2). Controlling for linear and quadratic effects of the age difference between the siblings, sex combination of the sibling pair, birth cohort, and interaction terms, the hazard ratio for DA in siblings was significantly greater when the proband was older than when the proband was younger (hazard ratio, 1.42, 95% CI, 1.31–1.54) (Table 2). This overall effect is illustrated in Figure 2.
Table 2.
Results From Full Cox Proportional Hazards Model for the Risk for Drug Abuse as a Function of Whether the Drug Abuse–Affected Sibling Was Older vs Younger
| Variable | Hazard Ratio (95% CI) |
|
P Value | |
|---|---|---|---|---|
| Older vs younger sibling | 1.42 (1.31–1.54) | 70.2 | <.001 | |
| Age differences | 0.88 (0.87–0.90) | 128.9 | <.001 | |
| Age differences2 | 1.01 (1.00–1.01) | 38.8 | <.001 | |
| Sibling pair by sex | ||||
| Male-male | 1.0 [Reference] | |||
| Male-female | 0.35 (0.33–0.37) | 1341.5 | <.001 | |
| Female-female | 0.52 (0.47–0.58) | 135.1 | <.001 | |
| Female-male | 0.73 (0.66–0.80) | 42.4 | <.001 | |
| Birth cohort | ||||
| 1950–1959 | 1.0 [Reference] | |||
| 1960–1969 | 1.65 (1.54–1.77) | 195.4 | <.001 | |
| 1970–1979 | 2.95 (2.75–3.17) | 885.2 | <.001 | |
| 1980–1993 | 3.90 (3.63–4.20) | 1390.1 | <.001 | |
| Interaction terms | ||||
| Older sibling, age differences | 1.03 (1.02–1.04) | 15.6 | <.001 | |
| Older sibling, female-female | 0.85 (0.74–0.98) | 5.0 | .03 | |
| Older sibling, female-male | 0.87 (0.77–0.98) | 5.1 | .02 | |
Figure 2.
The cumulative probability of receiving a drug abuse (DA) diagnosis over a 20-year follow-up period for siblings where the older vs younger sibling had DA as predicted from the full Cox model, the results of which are given in Table 2.
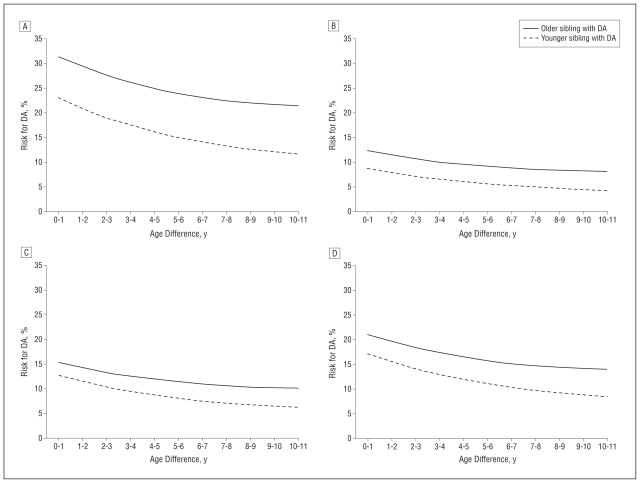
Interaction analyses indicated that the impact on the risk for DA of having an older vs a younger affected sibling significantly differed across the 4 sibling pair sex combinations. As seen in Figure 3 (A–D), which depicts the predicted risk for DA at a 20-year follow-up, this effect was greatest in male-male pairs, least in female-female pairs, and intermediate in the 2 opposite-sex pair combinations. Our analysis also showed that the increase in risk for DA to a sibling who is younger vs older than an affected proband increased 3% for every year of age difference between the siblings.
Figure 3.
The risk for drug abuse (DA) as predicted from a Cox model, the details of which are presented in Table 2, at 20-year follow-up from the diagnosis of DA in the proband. A, The results depict the risk when the male proband with DA is older vs younger than the male sibling, as a function of the age difference between them. B, The results depict the risk when the male proband with DA is older vs younger than the female sibling, as a function of the age difference between them. C, The results depict the risk when the female proband with DA is older vs younger than the female sibling, as a function of the age difference between them. D, The results depict the risk when the female proband with DA is older vs younger than the male sibling, as a function of the age difference between them.
DA RISK IN SPOUSES AS A FUNCTION OF TIME AFTER PROBAND’S DA REGISTRATION
We identified 7561 married case probands with DA (mean [SD] age, 40 [8] years; 52% male) and 37 805 matched married control probands. The sources of registration for the spouses with DA are seen in Table 1. The results from the conditional logistic regression showed that the risk for DA was substantially higher in spouses of case vs control probands (OR, 8.51; 95% CI, 7.58–9.56). Little difference was seen in the risk to wives of male case vs control probands (OR, 8.31; 95% CI, 7.05–9.81) and husbands of female case vs control probands (OR, 8.71; 95% CI, 7.41–10.23). Among the proband spouses who were male, 8.9% (95% CI, 8.0–9.8) of their spouses had DA. The corresponding figure when the proband spouse was female was 10.4% (95% CI, 9.4–11.4).
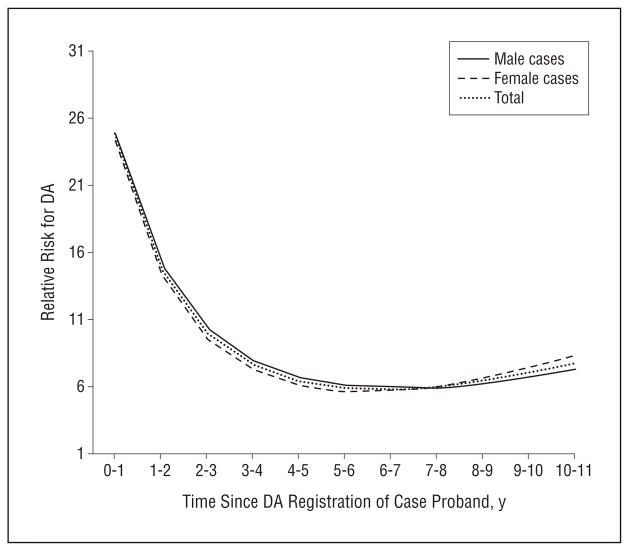
We then fitted a conditional logistic regression model with years since DA registration in the proband as an independent variable. As outlined in the “Methods” section, this variable was transformed so that ORs of greater than 1.0 indicated an inverse association between the years from DA registration in the proband and the risk for DA in the spouse. We found a strong positive linear effect (OR per year, 2.15; 95% CI, 1.88–2.46), a substantial negative quadratic effect (OR, 0.92; 95% CI, 0.91–0.94), and a slightly positive cubic effect (OR, 1.003; 95% CI, 1.002–1.003). The results were very similar when examined in the wives of affected men and husbands of affected women.
The risk curves predicted from our model are seen in Figure 4. The relative risk for DA registration in spouses of case vs control probands was dramatically elevated with a relative risk of approximately 25 within 1 year of the proband spouse’s DA registration. This declined steadily during the ensuing 4 years to a stable relative risk of approximately 6. A nearly identical pattern was seen in spouses of male vs female DA probands.
Figure 4.
The relative risk of registration for drug abuse (DA) in the spouse of a case proband with DA vs a control proband.
COMMENT
We previously found strong evidence for the impact of familial environmental factors on the risk for DA in Swedish adopted children.10 In this article, we examined whether these results could be confirmed and generalized in the more typical familial relationships of siblings and spouses in the entire population of Sweden. From this rich set of findings, 4 are particularly worthy of emphasis.
First, consistent with much prior data,1,2,21–23,25–27 we found that the risk for DA in Sweden was strongly correlated in siblings and spouses. Our estimate of sibling resemblance for DA (OR, 5.3) was similar to a 4.5-fold increased risk for DA obtained by Merikangas et al2 in first-degree relatives of drug-abusing probands and a 6.7-fold increased risk for DA seen in first-degree relatives of opiate-abusing probands by Rounsaville et al.29 In our sibling pairs, opposite-sex pairs resembled one another less strongly for the risk for DA than same-sex pairs. From such data alone, it was not possible to determine whether this pattern arose because of genetic or environmental factors.
Second, in sibships containing a proband with DA, the risk for DA in other siblings was strongly correlated with the age differences between them. The risk for DA was nearly twice as great for a sibling born in the same year as a drug-abusing proband sibling as it was for a sibling born 10 years apart. Critically, these results isolated environmental effects because we only examined full siblings, all of whom had the same degree of genetic relationship. The decline in risk as a function of age difference was considerably steeper in sister-sister vs brother-brother pairs. This has no plausible genetic explanation. Rather, these results suggest that the environmental effects impacting resemblance for DA in brothers decay more slowly as a function of sibling differences in age than the environmental effects shared by sisters.
Third, controlling for multiple factors including the age difference between siblings, having an older sibling with DA conveys a 42% greater risk for DA than having a younger drug-abusing sibling. This effect also cannot result from genetic factors as we again examined only full siblings. However, these effects were much stronger in male-male vs female-female sibling pairs. That is, with respect to the risk for DA, an abusing older brother carried much more risk for his younger brother than vice versa. With sisters, this difference was more modest. Interestingly, the overall difference in risk from an older vs a younger sibling grew greater as the difference in years between the siblings increased. As shown in Figure 3, this was because the risk for DA declined more quickly with increasing age difference from younger than from older affected probands.
Fourth, after a proband spouse was registered with DA, his or her spouse had a 25-fold increased risk for DA registration the same year, which declined to a 6-fold increased risk after 4 to 5 years, thereafter remaining relatively stable. The main question in interpreting spousal resemblance for DA is the degree to which this resemblance is the result of environmental factors (including the effects of drug use in one spouse on the other spouse) or assortative mating where spouses pick one another because of traits correlated with the risk for DA. We sought to discriminate between these 2 causes of spousal resemblance by using the temporal pattern of DA registration. We reasoned that if social environmental factors impacted the risk for DA in spouses, after one spouse was registered for DA, we would see a temporal spike in the registrations for DA of the other spouse. We indeed observed this pattern. However, the relative risk for DA in the spouse did not return to unity but rather to a substantially elevated baseline. This stably elevated basal risk for DA likely reflected the results of assortative mating. Unlike the results with older siblings, we found no appreciable sex effects in our spousal analysis. Husbands with drug-abusing wives and wives with drug-abusing husbands had equally elevated risks.
To summarize, our results in siblings and spouses—where we could unconfound the impact of genetic vs environmental factors—robustly replicated our previous adoption findings and prior results from twin and adoption studies, suggesting that environmental factors contributed substantially to the familial transmission of DA.
We are not the first to try to understand the environmental transmission of DA in families by examining sibships. A number of prior studies suggested that siblings in general, or older siblings specifically, are frequently important role models and suppliers of drugs.30–36 However, we could locate no studies that explicitly compared the transmission of risk for DA to older vs younger siblings. We are aware of 1 study that used alcohol use as the outcome variable to examine the relationship between sibling similarity in age and substance use. Consistent with our results for DA, McGue et al37 found that the resemblance for alcohol use was significantly greater among sibling pairs who were near vs distant in age in adoptive families.
Brook et al38 usefully suggested that siblings can influence the risk for DA in 3 major ways: (1) influencing attitudes toward drug use, (2) providing a behavioral model for drug use and misuse, and (3) serving as a source of drugs of abuse. It is plausible that the potency of all 3 of these potential mechanisms would be greater in older vs younger siblings, as well as greater in siblings closer vs more distant in age. Social learning theory39 predicts that the potency of a role model depends on the strength of the identification of the subject with the model. Consistent with our data, this would predict stronger modeling for siblings of the same sex and siblings close in age to one another.
Prior studies have consistently documented that spouses are substantially correlated for drug use, DA, and related externalizing syndromes.21–27 For example, in 519 Canadian spouses, the OR for DA in spouses was 12.4.25 More interest has been paid to the nature and etiology of assortative mating than the shared environmental effects contributing to spousal resemblance, which have been variously termed marital influence,21 marital contamination,24 convergence,28 or contagion.22 Of those studies that directly examined the etiology of spousal resemblance for illicit drug use, abuse, or smoking and alcohol consumption, most21,26,27 but not all28 suggested that it arises largely through assortative mating. Our results are inconsistent with this conclusion. While our findings are insufficiently fine-grained to determine the precise mechanism (eg, does one spouse actively encourage the other to join in illicit substance use and misuse or do common friends or other social influences impact on both spouses), the strong temporal association between DA registration in members of spousal pairs strongly suggests an environmental influence at work. However, our finding that, after the temporal spike is over, spouses return to a quite elevated baseline risk for DA suggests that assortative mating also occurs for DA-related traits.
These results should be interpreted in the context of 3 potentially important method limitations. First, this study was confined to 1 Scandinavian country and only further research can determine whether the findings would generalize to other cultural and ethnic groups.
Second, we detected subjects with DA from medical, legal, and pharmacy records. This method has the advantage of not relying on accurate respondent recall and reporting. However, we cannot rule out false-negative or false-positive diagnoses, precisely estimate these biases, or know the distribution of the classes of abused psychoactive substances for the entire sample. However, a large-scale epidemiologic study of DA conducted in neighboring Norway, which has similar rates of drug use and abuse,40,41 found lifetime prevalence rates of DSM-III-R42 DA quite similar to those found using our registry-based methods.43 This makes large-scale underascertainment unlikely, at least for the more severe forms of DA.
Third, could our results artifactually stem from systematic police practices? If an individual was arrested for DA, would police provide closer surveillance to his siblings or spouse leading to an increased likelihood of arrest and conviction? Might police keep under closer surveillance the younger vs the older sibling of a convicted drug abuser? To address this possible bias, we repeated all major analyses presented here removing cases of DA identified only through the Suspicion Register. In no case did the pattern of findings substantially change.
In conclusion, using objective nationwide data from Sweden, we found evidence from both sibling and spousal relationships that, controlling for genetic factors, familial environmental factors were potent influences on the risk for DA. We robustly replicated and generalized our previous findings from our Swedish adoption sample in the present study population, which was considerably larger and more representative. Given prior strong evidence for the etiologic role of genetic factors in DA, these results clearly illustrate the etiologic complexity of DA. Furthermore, they illuminate the essential research task awaiting us, which is to understand how genetic and familial environmental risk factors act and interact over development to render individuals at low vs high risk for DA.
Acknowledgments
Role of the Sponsors: The National Institute of Drug Abuse and the Swedish Research Council were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Funding/Support: This study was funded by grant RO1 DA030005 from the National Institute of Drug Abuse; Swedish Research Council K2012-70X-15428-08-3, The Swedish Council for Information on Alcohol and Other Drugs (CAN); and an ALF project grant, Lund, Sweden.
Footnotes
Conflict of Interest Disclosures: None reported.
References
- 1.van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug Alcohol Depend. 1998;52(3):231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55(11):973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- 3.Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet. 1996;67(5):473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155(8):1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- 5.Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med. 2002;32(2):195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57(3):261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- 7.Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Adoption study demonstrating two genetic pathways to drug abuse. Arch Gen Psychiatry. 1995;52(1):42–52. doi: 10.1001/archpsyc.1995.03950130042005. [DOI] [PubMed] [Google Scholar]
- 8.Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. An adoption study of drug abuse/dependency in females. Compr Psychiatry. 1996;37(2):88–94. doi: 10.1016/s0010-440x(96)90567-2. [DOI] [PubMed] [Google Scholar]
- 9.Cadoret RJ, Troughton E, O’Gorman TW, Heywood E. An adoption study of genetic and environmental factors in drug abuse. Arch Gen Psychiatry. 1986;43(12):1131–1136. doi: 10.1001/archpsyc.1986.01800120017004. [DOI] [PubMed] [Google Scholar]
- 10.Kendler KS, Sundquist K, Ohlsson H, Palmer K, Maes H, Winkleby MA, Sundquist J. Genetic and familial environmental influences on the risk for drug abuse: a national Swedish adoption study. Arch Gen Psychiatry. 2012;69(7):690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris JR. The Nurture Assumption: Why Children Turn Out the Way They Do. New York, NY: Touchstone/Simon and Schuster; 2002. [Google Scholar]
- 12.Kandel DB. On processes of peer influences in adolescent drug use: a developmental perspective. Adv Alcohol Subst Abuse. 1985;4(3–4):139–163. doi: 10.1300/J251v04n03_07. [DOI] [PubMed] [Google Scholar]
- 13.Allen M, Donohue WA, Griffin A, Ryan D, Turner MM. Comparing the influence of parents and peers on the choice to use drugs: a meta-analytic summary of the literature. Crim Justice Behav. 2003;30(2):163–186. doi: 10.1177/0093854802251002. [DOI] [Google Scholar]
- 14.Petraitis J, Flay BR, Miller TQ, Torpy EJ, Greiner B. Illicit substance use among adolescents: a matrix of prospective predictors. Subst Use Misuse. 1998;33 (13):2561–2604. doi: 10.3109/10826089809059341. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins JD, Herrenkohl T, Farrington DP, Brewer D, Catalano RF, Harachi TW. A review of predictors of youth violence. In: Loeber R, Farrington DP, editors. Serious and Violent Juvenile Offenders: Risk Factors and Successful Interventions. London, England: Sage Publications Inc; 1998. pp. 106–146. [Google Scholar]
- 16.Brody GH. Sibling relationship quality: its causes and consequences. Annu Rev Psychol. 1998;49:1–24. doi: 10.1146/annurev.psych.49.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Slomkowski C, Rende R, Conger KJ, Simons RL, Conger RD. Sisters, brothers, and delinquency: evaluating social influence during early and middle adolescence. Child Dev. 2001;72(1):271–283. doi: 10.1111/1467-8624.00278. [DOI] [PubMed] [Google Scholar]
- 18.Newman J. College students relationships with siblings. J Youth Adolesc. 1991;20(6):629–644. doi: 10.1007/BF01537366. [DOI] [PubMed] [Google Scholar]
- 19.Lamb ME, Sutton-Smith B. Sibling Relationships: Their Nature and Significance Across the Lifespan. Oxford, England: Psychology Press; 1982. [Google Scholar]
- 20.Brook JS, Whiteman M, Gordon AS, Brenden C. Older brother’s influence on younger sibling’s drug use. J Psychol. 1983;114(1st half):83–90. doi: 10.1080/00223980.1983.9915400. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi K, Kandel D. Marital homophily on illicit drug use among young adults: assortative mating or marital influence? Soc Forces. 1993;72(2):505–528. doi: 10.2307/2579859. [DOI] [Google Scholar]
- 22.Vanyukov MM, Neale MC, Moss HB, Tarter RE. Mating assortment and the liability to substance abuse. Drug Alcohol Depend. 1996;42(1):1–10. doi: 10.1016/0376-8716(96)01255-0. [DOI] [PubMed] [Google Scholar]
- 23.Maes HH, Neale MC, Kendler KS, Hewitt JK, Silberg JL, Foley DL, Meyer JM, Rutter M, Simonoff E, Pickles A, Eaves LJ. Assortative mating for major psychiatric diagnoses in two population-based samples. Psychol Med. 1998;28(6):1389–1401. doi: 10.1017/s0033291798007326. [DOI] [PubMed] [Google Scholar]
- 24.Galbaud du Fort G, Boothroyd LJ, Bland RC, Newman SC, Kakuma R, Galbaud du FG. Spouse similarity for antisocial behaviour in the general population. Psychol Med. 2002;32(8):1407–1416. doi: 10.1017/s0033291702006530. [DOI] [PubMed] [Google Scholar]
- 25.Galbaud du Fort G, Bland RC, Newman SC, Boothroyd LJ. Spouse similarity for lifetime psychiatric history in the general population. Psychol Med. 1998;28 (4):789–802. doi: 10.1017/s0033291798006795. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal A, Heath AC, Grant JD, Pergadia ML, Statham DJ, Bucholz KK, Martin NG, Madden PA. Assortative mating for cigarette smoking and for alcohol consumption in female Australian twins and their spouses. Behav Genet. 2006;36(4):553–566. doi: 10.1007/s10519-006-9081-8. [DOI] [PubMed] [Google Scholar]
- 27.Sakai JT, Stallings MC, Mikulich-Gilbertson SK, Corley RP, Young SE, Hopfer CJ, Crowley TJ. Mate similarity for substance dependence and antisocial personality disorder symptoms among parents of patients and controls. Drug Alcohol Depend. 2004;75(2):165–175. doi: 10.1016/j.drugalcdep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Price RA, Vandenberg SG. Spouse similarity in American and Swedish couples. Behav Genet. 1980;10(1):59–71. doi: 10.1007/BF01067319. [DOI] [PubMed] [Google Scholar]
- 29.Rounsaville BJ, Kosten TR, Weissman MM, Prusoff B, Pauls D, Anton SF, Merikangas K. Psychiatric disorders in relatives of probands with opiate addiction. Arch Gen Psychiatry. 1991;48(1):33–42. doi: 10.1001/archpsyc.1991.01810250035004. [DOI] [PubMed] [Google Scholar]
- 30.Clayton RR, Lacy WB. Interpersonal influences on male drug use and drug use intentions. Int J Addict. 1982;17(4):655–666. doi: 10.3109/10826088209053009. [DOI] [PubMed] [Google Scholar]
- 31.Needle R, McCubbin H, Wilson M, Reineck R, Lazar A, Mederer H. Interpersonal influences in adolescent drug use: the role of older siblings, parents, and peers. Int J Addict. 1986;21(7):739–766. doi: 10.3109/10826088609027390. [DOI] [PubMed] [Google Scholar]
- 32.Gfroerer J. Correlation between drug use by teenagers and drug use by older family members. Am J Drug Alcohol Abuse. 1987;13(1–2):95–108. doi: 10.3109/00952998709001502. [DOI] [PubMed] [Google Scholar]
- 33.Duncan TE, Duncan SC, Hops H. The role of parents and older siblings in predicting adolescent substance use: modeling development via structural equation latent growth methodology. J Fam Psychol. 1996;10(2):158–172. doi: 10.1037//0893-3200.10.2.158. [DOI] [Google Scholar]
- 34.Brook JS, Brook DW, Whiteman M. Older sibling correlates of younger sibling drug use in the context of parent-child relations. Genet Soc Gen Psychol Monogr. 1999;125(4):451–468. [PubMed] [Google Scholar]
- 35.Windle M. Parental, sibling, and peer influences on adolescent substance use and alcohol problems. Appl Dev Sci. 2000;4(2):98–110. doi: 10.1207/S1532480XADS0402_5. [DOI] [Google Scholar]
- 36.Ary DV, Tildesley E, Hops H, Andrews J. The influence of parent, sibling, and peer modeling and attitudes on adolescent use of alcohol. Int J Addict. 1993;28(9):853–880. doi: 10.3109/10826089309039661. [DOI] [PubMed] [Google Scholar]
- 37.McGue M, Sharma A, Benson P. Parent and sibling influences on adolescent alcohol use and misuse: evidence from a US adoption cohort. J Stud Alcohol. 1996;57(1):8–18. doi: 10.15288/jsa.1996.57.8. [DOI] [PubMed] [Google Scholar]
- 38.Brook JS, Whiteman M, Gordon AS, Brook DW. The role of older brothers in younger brothers’ drug use viewed in the context of parent and peer influences. J Genet Psychol. 1990;151(1):59–75. doi: 10.1080/00221325.1990.9914644. [DOI] [PubMed] [Google Scholar]
- 39.Bandura A, Huston AC. Identification as a process of incidental learning. J Abnorm Soc Psychol. 1961;63:311–318. doi: 10.1037/h0040351. [DOI] [PubMed] [Google Scholar]
- 40.Kraus L, Augustin R, Frischer M, Kümmler P, Uhl A, Wiessing L. Estimating prevalence of problem drug use at national level in countries of the European Union and Norway. Addiction. 2003;98(4):471–485. doi: 10.1046/j.1360-0443.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- 41.Hibell B, Guttormsson U, Ahlstrom S, Balakireva O, Bjarnason T, Kokkevi A, Kraus L. The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries. Stockholm, Sweden: The Swedish Council for Information on Alcohol and Other Drugs; [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1987. Revised. [Google Scholar]
- 43.Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. Am J Psychiatry. 2001;158(7):1091–1098. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]