Abstract
OBJECTIVE
Diabetes mellitus (DM) increases cardiovascular risk, at least in part, through shortage of vascular regenerative cells derived from the bone marrow (BM). In experimental models, DM causes morphological and functional BM alterations, but information on BM function in human DM is missing. Herein, we sought to assay mobilization of stem and proangiogenic cells in subjects with and without DM.
RESEARCH DESIGN AND METHODS
In a prospective trial (NCT01102699), we tested BM responsiveness to 5 μg/kg human recombinant granulocyte colony–stimulating factor (hrG-CSF) in 24 individuals with DM (10 type 1 and 14 type 2) and 14 individuals without DM. Before and 24 h after hrG-CSF, we quantified circulating stem/progenitor cells and total and differential white blood cell counts. We also evaluated in vivo the proangiogenic capacity of peripheral blood mononuclear cells using the Matrigel plug assay.
RESULTS
In response to hrG-CSF, levels of CD34+ cells and other progenitor cell phenotypes increased in subjects without DM. Patients with DM had significantly impaired mobilization of CD34+, CD133+, and CD34+CD133+ hematopoietic stem cells and CD133+KDR+ endothelial progenitors, independently of potential confounders. The in vivo angiogenic capacity of peripheral blood mononuclear cells significantly increased after hrG-CSF in control subjects without DM, but not in patients with DM. DM was also associated with the inability to upregulate CD26/DPP-4 on CD34+ cells, which is required for the mobilizing effect of granulocyte colony–stimulating factor.
CONCLUSIONS
Stem and proangiogenic cell mobilization in response to hrG-CSF is impaired in DM, possibly because of maladaptive CD26/DPP-4 regulation. These alterations may hamper tissue repair and favor the development of cardiovascular complications.
Diabetes mellitus (DM) increases cardiovascular disease, and this is attributed, at least in part, to shortage of vascular regenerative cells derived from the bone marrow (BM) (1). DM is associated with reduced levels of several circulating progenitor cell phenotypes (2). We have previously shown that DM prevents postischemic progenitor cell mobilization in rats, which translates into impaired vascular recovery after ischemia (3). Recent data from experimental models of type 1 DM and type 2 DM highlight BM pathologies that include microangiopathy (4), neuropathy (5), altered gene expression (6), and niche dysfunction (7). These changes may account for an impaired mobilizing capacity in DM compared with control animals (8). Data on BM function in human DM are scant, whereas there is no information on BM structure. In a retrospective case series of patients undergoing BM autotransplantation, DM was statistically associated with poor mobilization in response to chemotherapy plus human recombinant granulocyte colony–stimulating factor (hrG-CSF) (7). Moreover, in support of the existence of a BM defect in human DM, we have shown a reduction in BM CD34+ cells, compared with nondiabetic subjects (9).
The mechanism of action of the mobilizing factor granulocyte colony–stimulating factor (G-CSF) is complex and involves cleavage of stromal-derived factor (SDF)-1α through release of proteases, elastases, and matrix metalloprotease-9, suppression of osteoblastic function, and modulation of integrins (10). The mechanism whereby DM impairs stem cell mobilization may depend on altered local concentrations of the chemokine SDF-1α. It is noteworthy that SDF-1α is a natural substrate of the protease CD26/DPP-4, the activity of which is dysregulated in DM (11). The impaired stem cell mobilization in DM has important implications for the care of patients in the hematology clinic. Furthermore, because the BM harbors a variety of regenerative nonhematopoietic progenitors, including endothelial progenitor cells (EPCs), BM dysfunction may contribute to the onset of chronic DM complications (12). Unfortunately, exploration of BM structure and function in humans is limited by the intrinsic low availability of BM samples from nonhematologic patients. Therefore, to confirm the diabetic stem cell “mobilopathy” in humans, we devised a pharmacologic test of BM reserve in a prospective trial of BM stimulation with a single subcutaneous injection of hrG-CSF in individuals with DM and without DM.
RESEARCH DESIGN AND METHODS
Patients and treatment
The study was approved by the local ethics committee and is registered in ClinicalTrials.gov (NCT01102699). This was a prospective, parallel group study of direct BM stimulation with hrG-CSF in subjects with and without DM. The primary end point was change in circulating CD34+ cells from baseline. Secondary end points were changes in other progenitor cell phenotypes, proangiogenic capacity of peripheral blood mononuclear cells (PBMCs), white blood cells, and safety. The study was not designed and powered to detect baseline differences in progenitor cell levels. DM patients were recruited at the outpatient clinic of the University Hospital of Padova, and healthy control subjects were volunteers from the local community. Both type 1 DM and type 2 DM patients were eligible because preclinical studies have shown similar BM alterations and progenitor cell reductions in both types of DM (4,5). Exclusion criteria were as follows: age <25 or >65 years; any acute disease or infection; recent trauma, surgery, or cardiovascular event; chronic immune or infectious diseases; current or past hematological disorders or malignancy; leukocytosis, leukopenia, or thrombocytopenia; organ transplantation or immune suppression; advanced diabetic retinopathy; altered liver function; severe renal failure (estimated glomerular filtration rate <30 mL/min/m2); anomalies in lymphocytes subpopulations; allergy to Filgrastim; bronchial asthma or other chronic lung disorders; and impossibility to provide informed consent. For each patient, we collected anthropometric measures, data on concomitant risk factors, HbA1c, eventual DM complications, and therapy.
After providing informed consent, patients were subjected to baseline examination and blood samples, including determination of the complete leukocyte counts and lymphocyte subpopulations, liver enzymes, renal function, plasma protein electrophoresis, erythrocyte sedimentation rate, C-reactive protein, prothrombin time, uric acid, and standard urine examination. After verification of inclusion and exclusion criteria, at 8:30 a.m. after an overnight fast, eligible patients were subjected to a baseline peripheral blood sampling for circulating progenitor cell quantification and collection of PBMCs. Immediately after, they were injected subcutaneously with 5 μg/kg Filgrastim (Granulokine; Amgen). Twenty-four hours later, another peripheral blood sample was obtained to evaluate the effects of Filgrastim. Study subjects were invited to register and report any eventual side effect occurred after Filgrastim injection. Dosage of the drug was chosen as the minimum effective dose based on available pharmacodynamic data on Filgrastim, showing that 5 μg/kg is sufficient to increase absolute count of circulating CD34+ cells in healthy control subjects (13).
Fluorescence-activated cell sorter analysis
Circulating progenitor cells were quantified using flow cytometry as previously described in detail (14). In brief, after erythrocyte lysis, 150 μL peripheral blood were stained with 10 μL fluorescein isothiocyanate-conjugated anti-human CD34 mAb (Becton Dickinson), 10 μL phycoethrin-conjugated anti-human KDR mAb (R&D Systems), and 10 μL allophycocyanin-conjugated anti-CD133 mAb (Miltenyi Biotech). The frequency of peripheral blood cells positive for these reagents was determined by a two-dimensional side-scatter fluorescence dot plot analysis after appropriate gating. We gated CD34+ or CD133+ peripheral blood cells in the mononuclear cell fraction and then examined the resulting population for the dual expression of KDR. At the intersection of the CD34 and CD133 gates, we identified CD34+CD133+ cells, which were examined for KDR expression. In all patients, we also quantified the expression of CD26/DPP-4 on CD34+ cells using a phycoethrin-labeled anti-CD26 mAb (Becton Dickinson). In separate analyses, CD45 costaining was performed and showed that >90% of CD34+ cells are CD45dim. For fluorescence-activated cell sorter analysis, 5 × 105 cells were acquired and scored using a FACSCalibur (BD). Data were processed using the Macintosh CELLQuest software program (BD). The same trained operators, blind to the clinical status of the patients, performed the tests throughout the study. Absolute progenitor cell counts per unit of blood were derived by multiplying fractional data per white blood cell count. We previously have shown that reproducibility of CD34+ cell quantification with this method is high (intraclass correlation coefficient 0.94; 95% CI 0.88–0.96; coefficient of variation [CV] 6.3%) (14).
In vivo proangiogenic cell function
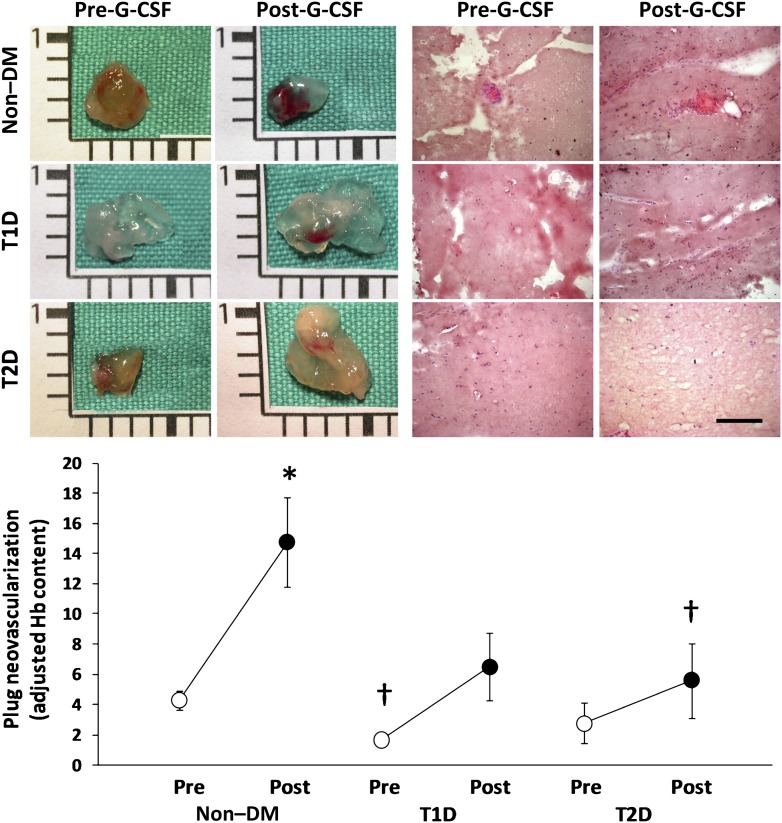
To gather information on the presence of functional circulating proangiogenic cells and how they are modulated by hrG-CSF in subjects with and without DM, we used the in vivo Matrigel plug angiogenesis assay with patients’ PBMCs. Data suggest that diverse monocyte subsets, including monocytic EPCs and Tie2-expressing monocytes, have proangiogenic capacity (15,16). In brief, PBMCs were isolated with Histopaque (Sigma-Aldrich). Cell count and viability were assayed with an automated BioRad TC20 cell counter, and then, 3 × 106 PBMCs were resuspended in 500 μL phenol-free Matrigel (catalog number 356237; BD) and implanted subcutaneously into the dorsum of immunodeficient RAG-2/γ(c) double knockout mice (in-house colony). The experiment was performed with pre-hrG-CSF and post-hrG-CSF PBMCs of five non–DM control subjects, five type 1 DM patients, and five type 2 DM patients. To minimize variability, the same mouse received pre-GCSF and post-GCSF PBMCs of the same subject. Plugs were explanted 10 days later for macroscopic inspection, histology (hematoxylin and eosin staining), and determination of the hemoglobin-to-protein content ratio (Drabkin solution and Bradford reagent, respectively; Sigma-Aldrich), which is a surrogate of perfusion. Hemoglobin-to-protein ratio was adjusted for the change in monocyte count after hrG-CSF to gather information on the proangiogenic capacity of circulating PBMCs at each time point.
Statistical analysis
Data are expressed as mean ± SE. Normal distribution of the variables of interest was verified with the Kolmogorov-Smirnov test. Comparisons between the diabetic group and nondiabetic group were performed using Student t test for normal variables, Mann-Whitney U test for nonnormal variables, and the χ2 test for categorical variables. Because CD34+ cell count is a normally distributed variable, the change in CD34+ cell count from baseline to 24 h after Filgrastim was assessed using paired Student t test. We then calculated the mean ± SE change of CD34+ cells in the diabetic and nondiabetic groups, which were compared using unpaired Student t test. Linear associations were assayed using the Pearson r correlation coefficient. To control for variables that were different between the two groups at P ≤ 0.10 and that may bias results, a multiple linear regression analysis was performed with change in progenitor cell levels as the dependent variable. Nonnormal dependent variables in secondary end point analyses were log transformed. Statistical significance was accepted at P < 0.05; SPSS version 17.0 was used.
RESULTS
Characteristics of the study population
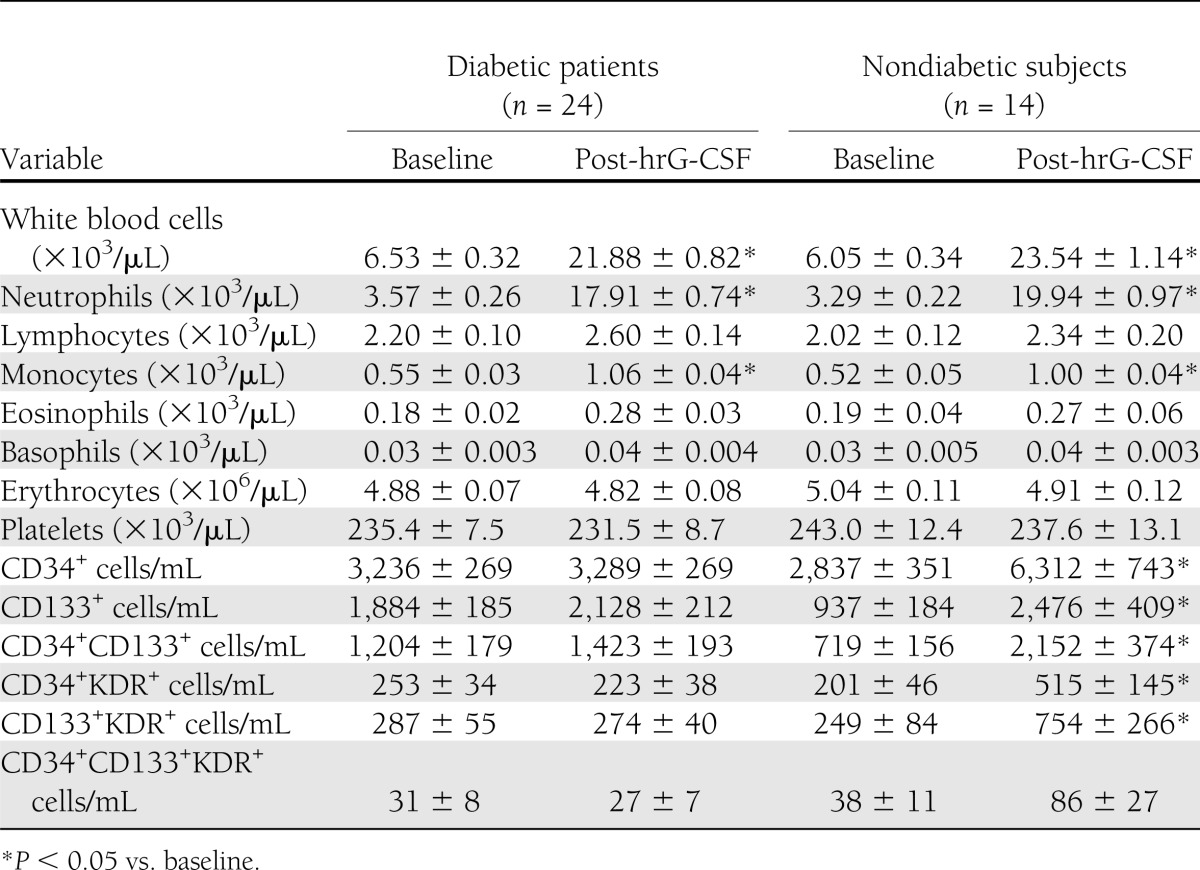
A total of 24 DM patients (10 type 1 DM and 14 type 2 DM patients) and 14 control subjects without DM have been enrolled and treated. DM patients had a higher prevalence of hypertension and tended to be older than control subjects. Type 1 DM patients had significantly longer disease duration and lower prevalence of cardiovascular disease compared with type 2 DM patients (Table 1).
Table 1.
Characteristics of the study subjects
Stem and progenitor cell mobilization
In subjects without DM, absolute CD34+ cell level significantly increased 2.2-fold after hrG-CSF, whereas CD34+ cells completely failed to mobilize in DM patients (mean ± SE change vs. baseline; non–DM: 3,475 ± 800 cells/mL; DM: 52 ± 332 cells/mL; P = 5 × 10−5) (Fig. 1A). Results were similar when type 1 or type 2 DM patients, considered separately, were compared with age-matched non–DM control subjects (Fig. 1B and C). Clinical characteristics of the subgroups are shown in Table 1.
Figure 1.
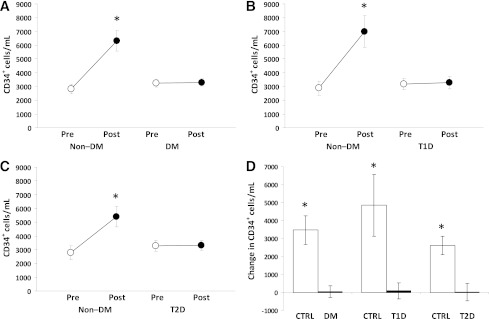
CD34+ cell mobilization after hrG-CSF. A: Absolute pre-G-CSF and post-G-CSF CD34+ cell count in all DM and all non–DM control (Ctrl) subjects (study primary end point). *P < 0.05 vs. baseline. Absolute pre-G-CSF and post-G-CSF CD34+ cell count in type 1 DM (T1D) compared with young control subjects (B) and in type 2 DM (T2D) compared with age-matched control subjects (C). *P < 0.05 vs. baseline. D: Changes in the absolute levels of circulating CD34+ cell counts. *P < 0.05 vs. DM.
After adjusting for potential confounders that were different between the two groups at P ≤ 0.10 (age, hypertension, and cardiovascular disease), DM remained significantly associated with reduced CD34+ cell increase after hrG-CSF (P = 0.002).
In response to hrG-CSF, DM was associated with impaired mobilization of all the other progenitor cell phenotypes, such as CD133+, CD34+CD133+, CD34+KDR+, CD133+KDR+, and CD34+CD133+KDR+ cells (Fig. 2). On correction for potential confounders, DM remained significantly associated with defective mobilization of CD133+ (P = 0.015), CD34+CD133+ (P = 0.011), and CD133+KDR+ (P = 0.013) cells, whereas the association between defective CD34+KDR+ mobilization and DM was marginally significant (P = 0.056) and blunted by age (P = 0.024).
Figure 2.
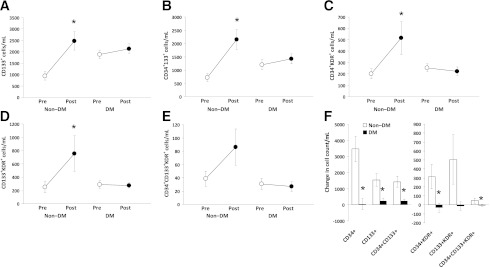
Mobilization of other progenitor cell phenotypes after hrG-CSF. A–E: Absolute pre-G-CSF and post-G-CSF cell counts of circulating hematopoietic stem cells (CD133+, CD34+CD133+) and endothelial progenitor cells (CD34+KDR+, CD133+KDR+, and CD34+CD133+KDR+) in DM and non–DM patients. *P < 0.05 vs. baseline. F: Changes in the absolute levels of stem/progenitor cell phenotypes in DM and non–DM patients. *P < 0.05 vs. non–DM control subjects.
Percentage expression of CD26/DPP-4 on CD34+ cells significantly increased after hrG-CSF in non–DM control subjects (δ = +14.1 ± 3.9%), consistent with previous findings in vitro (17). In DM, CD26/DPP-4 expression was elevated at baseline and tended to decline after hrG-CSF treatment (δ = −8.8 ± 5.5%; P = 0.013 vs. non–DM) (Fig. 3).
Figure 3.
Effects of hrG-CSF on CD26/DPP-4 expression. Percentage CD26/DPP-4 expression on CD34+ cells was significantly (*P < 0.05) increased in non–DM control subjects, whereas it was reduced in DM patients.
In the DM groups, progenitor cell mobilization was not significantly correlated to HbA1c, disease duration, pattern of complications, or treatment regimen.
After treatment with hrG-CSF, white blood cell counts, neutrophil counts, and monocyte counts significantly increased in both groups, and there were no differences between patients with DM and control subjects without DMs (Table 2 and Supplementary Fig. 1). This suggests that DM affects immature, but not mature, cell mobilization.
Table 2.
Hematological parameters and absolute progenitor cell counts in DM and non–DM patients before and after administration of hrG-CSF
Proangiogenic cell function in vivo
Mononuclear cells collected from patients before and after hr-GSCF administration were embedded into Matrigel plugs and implanted in immunodeficient mice to assess the presence of proangiogenic cells. Baseline PBMC from non–DM subjects showed higher neovascularization capacity compared with DM patients, which was statistically significant versus type 1 DM. Plugs with non–DM PBMCs before hrG-CSF (baseline) showed vascular invasion at gross inspection and the presence of vascular structures containing erythrocytes at histology. Plugs implanted with type 1 DM PBMCs at baseline showed almost no vascularization, lower hemoglobin content, and no evidence of perfused vascular structures. Plugs containing baseline type 2 DM PBMCs showed nonsignificantly lower vascularization capacity compared with non–DM at baseline. After hrG-CSF, the proangiogenic capacity of patients’ cells significantly increased in non–DM, but not in DM groups, and post-hrG-CSF neovascularization capacity was lower in type 1 DM (P = 0.058) and type 2 DM (P = 0.045) versus non–DM (Fig. 4). Matrigel plug neovascularization capacity was significantly correlated with circulating CD34+ (r = 0.47; P = 0.003), CD34+KDR+ (r = 0.38; P = 0.024), CD133+KDR+ (r = 0.46; P = 0.005), and CD34+CD133+KDR+ (r = 0.41; P = 0.014) cell levels (Supplementary Fig. 2). These data suggest that hrG-CSF mobilizes functional proangiogenic cells in non–DM subjects but not in DM patients.
Figure 4.
In vivo proangiogenic cell function. Patients’ PBMCs before collected pre-G-CSF and post-G-CSF were embedded into Matrigel plugs and implanted in immunodeficient mice. Representative gross appearance of the plugs (scale bar = 1 cm) and histological sections (scale bar = 100 μm) are shown. In the bottom, quantification of hemoglobin content, a quantitative surrogate of perfusion, adjusted for mobilized monocytes is shown. *P < 0.05 vs. pre-G-CSF. †P < 0.05 vs. non–DM.
Safety
Treatment with hrG-CSF was safe and uneventful. Five subjects in the non–DM group (35.7%) and five patients in the DM group (20.8%; P = 0.53 DM vs. non–DM) reported mild back pain 12 to 18 h after Filgrastim injection, which resolved at 24 h and required analgesic therapy with acetaminophen in three non–DM cases.
CONCLUSIONS
We show that DM is associated with impaired stem and progenitor cell mobilization after direct BM stimulation, independently of potential confounders. Remarkably, this was true for both hematopoietic stem cells and EPCs despite baseline cell levels not being reduced in this DM cohort. Therefore, the present prospective trial substantiates the existence of a BM defect in human DM and suggests that the BM mobilization failure precedes reduction of circulating progenitors. Both type 1 DM and type 2 DM patients showed almost complete unresponsiveness to stem/progenitor cell mobilization, suggesting that this complication is independent of DM etiology; however, the role of autoimmunity in determining BM response may be worthy of investigation.
In mice, long-term DM causes BM microangiopathy and altered oxygen gradients (4) that, in addition to reduced expression of prosurvival genes (6), lead to a pauperization of the stem cell pool. Microvascular BM alterations in experimental DM include capillary rarefaction, increased permeability, endothelial cell apoptosis, and dysfunction (4), features that resemble diabetic microangiopathy of other organs, such as the kidney and retina. These histopathological aspects suggest that the BM is a hitherto unrecognized site of DM complication and is likely responsible for the mobilization failure. Data on the amount of BM stem cells in DM are discordant, with some studies showing normal (3,18) or even increased (7) primitive Sca-1+c-kit+Lin− hematopoietic progenitors. Therefore, the low CD34+ cell count in BM aspirates from type 2 DM patients that we have shown previously (9) might reflect true stem cell deficiency or reduced accessibility of the niches to aspiration, attributable to the sticky property of the diabetic niche, which is more prone to stem cell retention than mobilization (7). It is noteworthy that DM did not impair mobilization of mature leukocytes, which are more loosely retained by the BM stroma than stem cells.
Normally, G-CSF stimulates expression and activity of CD26/DPP-4 and other proteases, with subsequent degradation of the chemokine and retention signal SDF-1α (17,19). Thus, stem/progenitor cells migrate to the peripheral circulation following SDF-1α gradients. Herein, we suggest a possible mechanism of stem cell unresponsiveness to G-CSF in DM by showing a maladaptive CD26/DPP-4 response. Systemic CD26/DPP-4 activity is increased in DM (11), and our new data indicate that G-CSF fails to upregulate CD26/DPP-4 on BM-derived cells in DM, likely preventing modification of the SDF-1α gradient. Studies show that DM mice mobilize stem cells after treatment with the SDF-1α receptor CXCR4 antagonists AMD3100 (7,18,20) and NIBR1816 (21). Therefore, the vascular niche containing stem cells that can be readily mobilized on disruption of the SDF-1α retention signal seems to be preserved, but responsiveness to CXCR4 antagonists should be confirmed in DM patients.
The existence of a BM mobilopathy in DM might be ascribed to both structural alterations affecting the stem cell niche (microangiopathy) and functional defects preventing the cells from being mobilized (e.g., the CD26/SDF-1α/CXCR4 axis). In addition, DM alters the activity of endothelial nitric oxide synthase (22), which is pivotal for EPC mobilization (23), and although G-CSF activity is mainly endothelial nitric oxide synthase–independent (24), this may be another mechanism accounting for depressed mobilization in DM. Among strategies to reverse BM dysfunction, our data showing no correlation between HbA1c and mobilization suggest that glucose control might not be effective, whereas experimental data indicate that boosting the antioxidative defense is a suitable strategy to prevent BM alterations (4). Although CD26/DPP-4 inhibition increases EPCs (25), whether this represents a therapeutic target to restore BM responsiveness in DM needs to be ascertained.
We also found that DM impairs neovascularization by mononuclear cells mobilized by hrG-CSF, as shown by the in vivo Matrigel plug assay. It is noteworthy that type 1 DM patients already had defective neovascularization capacity at baseline. This is possibly related to the longer disease duration in type 1 DM compared with type 2 DM patients (Table 1). Both type 1 DM and type 2 DM patients were unable to increase significantly their PBMCs proangiogenic capacity after hr-GCSF, again suggesting that mobilization failure precedes reduction of proangiogenic cells. Although we did not identify the subpopulation of PBMCs involved (15,16), change in neovascularization capacity was correlated with stem/progenitor mobilization. These important results indicate that the stem cell mobilization failure can be pathophysiologically linked to impaired tissue repair and development of cardiovascular DM complications, which are characterized by defective angiogenesis (26,27).
Our findings have clinical implications. Reduced progenitor cell levels are powerful predictors of future cardiovascular events (28), and replenishment of progenitor cells may lower cardiovascular risk. Although the pathogenesis of diabetic vascular complications is complex and possibly diversified in type 1 DM and type 2 DM, it is remarkable that alterations in BM-derived cells have been consistently reported for both type 1 DM (29) and type 2 DM patients (30). Understanding the causes of progenitor cell reduction and the role of BM can identify novel strategies to reverse this defect and prevent vascular disease. Moreover, the immunomodulatory activity of mobilized BM-derived progenitors may be important in type 1 DM (21). In addition, clinicians should be aware of the likelihood of mobilization failure in DM patients undergoing stem cell collection for BM autotransplantation or for angiogenic cell therapy (7,8).
The study has limitations. First, replication in other, possibly larger, cohorts is needed and comparison between recent-onset and long-term type 1 DM patients would allow a description of the natural history of BM dysfunction. Second, whereas the imbalances in clinical characteristics between groups were adjusted by multivariate analyses, residual confounding may be present. Finally, whereas a single-dose hrG-CSF was used in this study, DM patients might respond to a full 5-day course of hrG-CSF, but it would not be ethical to perform maximal BM stimulation for research purposes only. Several DM patients have been treated with high-dose hrG-CSF in cell therapy protocols (31), but a formal comparison of the mobilization effect between DM and non–DM patients never has been performed. Nonetheless, our data represent a proof-of-concept of diabetic BM dysfunction in humans. As the BM emerges as a novel target organ in DM, intensive investigation to reverse this complication becomes compelling.
Supplementary Material
Acknowledgments
This study was supported by grants from the University of Padova (60A07-8452) and the European Foundation for the Study of Diabetes (EFSD)/Lilly Fellowship and EFSD/Sanofi Clinical Investigator Fellowship to G.P.F.
No potential conflicts of interest relevant to this article were reported.
G.P.F. designed the study and wrote the manuscript. G.P.F., M.A., S.V.d.K., E.B., R.C., M.M., and N.P. researched data. C.A. designed the study and contributed to discussion. A.A. reviewed and edited the manuscript, contributed to discussion, and supervised the project. G.P.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01102699, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1084/-/DC1.
References
- 1.Fadini GP, Avogaro A. It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications. Exp Diabetes Res 2012;2012:742976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fadini GP. An underlying principle for the study of circulating progenitor cells in diabetes and its complications. Diabetologia 2008;51:1091–1094 [DOI] [PubMed] [Google Scholar]
- 3.Fadini GP, Sartore S, Schiavon M, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia 2006;49:3075–3084 [DOI] [PubMed] [Google Scholar]
- 4.Oikawa A, Siragusa M, Quaini F, et al. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol 2010;30:498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busik JV, Tikhonenko M, Bhatwadekar A, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 2009;206:2897–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol 2010;105:703–712 [DOI] [PubMed] [Google Scholar]
- 7.Ferraro F, Lymperi S, Mendez-Ferrer S, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med 2011;3:104ra101 [DOI] [PMC free article] [PubMed]
- 8.DiPersio JF. Diabetic stem-cell “mobilopathy”. N Engl J Med 2011;365:2536–2538 [DOI] [PubMed] [Google Scholar]
- 9.Fadini GP, Boscaro E, de Kreutzenberg S, et al. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care 2010;33:1097–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts AWG-CSF. a key regulator of neutrophil production, but that’s not all! Growth Factors 2005;23:33–41 [DOI] [PubMed] [Google Scholar]
- 11.Fadini GP, Albiero M, Menegazzo L, de Kreutzenberg SV, Avogaro A. The increased dipeptidyl peptidase-4 activity is not counteracted by optimized glucose control in type 2 diabetes, but is lower in metformin-treated patients. Diabetes Obes Metab 2012;14:518–522 [DOI] [PubMed] [Google Scholar]
- 12.Fadini GP. Is bone marrow another target of diabetic complications? Eur J Clin Invest 2011;41:457–463 [DOI] [PubMed] [Google Scholar]
- 13.van Der Auwera P, Platzer E, Xu ZX, et al. Pharmacodynamics and pharmacokinetics of single doses of subcutaneous pegylated human G-CSF mutant (Ro 25-8315) in healthy volunteers: comparison with single and multiple daily doses of filgrastim. Am J Hematol 2001;66:245–251 [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, de Kreutzenberg SV, Coracina A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J 2006;27:2247–2255 [DOI] [PubMed] [Google Scholar]
- 15.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003;107:1164–1169 [DOI] [PubMed] [Google Scholar]
- 16.Pucci F, Venneri MA, Biziato D, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood 2009;114:901–914 [DOI] [PubMed] [Google Scholar]
- 17.Christopherson KW, 2nd, Uralil SE, Porecha NK, Zabriskie RC, Kidd SM, Ramin SM. G-CSF- and GM-CSF-induced upregulation of CD26 peptidase downregulates the functional chemotactic response of CD34+CD38- human cord blood hematopoietic cells. Exp Hematol 2006;34:1060–1068 [DOI] [PubMed] [Google Scholar]
- 18.Tepper OM, Carr J, Allen RJ, Jr, et al. Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes 2010;59:1974–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christopherson KW, 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood 2003;101:4680–4686 [DOI] [PubMed] [Google Scholar]
- 20.Nishimura Y, Ii M, Qin G, et al. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol 2012;132:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorina P, Jurewicz M, Vergani A, et al. Targeting the CXCR4-CXCL12 axis mobilizes autologous hematopoietic stem cells and prolongs islet allograft survival via programmed death ligand 1. J Immunol 2011;186:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 2001;108:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 2003;9:1370–1376 [DOI] [PubMed] [Google Scholar]
- 24.de Resende MM, Huw LY, Qian HS, Kauser K. Role of endothelial nitric oxide in bone marrow-derived progenitor cell mobilization. Handb Exp Pharmacol 2007;(108):37–44 [DOI] [PubMed]
- 25.Fadini GP, Boscaro E, Albiero M, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care 2010;33:1607–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boodhwani M, Sodha NR, Mieno S, et al. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation 2007;116(Suppl):I31–I37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caporali A, Meloni M, Völlenkle C, et al. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation 2011;123:282–291 [DOI] [PubMed] [Google Scholar]
- 28.Fadini GP, Maruyama S, Ozaki T, et al. Circulating progenitor cell count for cardiovascular risk stratification: a pooled analysis. PLoS ONE 2010;5:e11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004;53:195–199 [DOI] [PubMed] [Google Scholar]
- 30.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002;106:2781–2786 [DOI] [PubMed] [Google Scholar]
- 31.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 2010;209:10–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.