Abstract
Hemophilia is an X-linked bleeding disorder, and patients with hemophilia are deficient in a biologically active coagulation factor. This study was designed to combine the efficiency of lentiviral vector transduction techniques with murine adipose tissue-derived stem/stromal cells (mADSCs) as a new method to produce secreted human coagulation factor IX (hFIX) and to treat hemophilia B. mADSCs were transduced with simian immunodeficiency virus (SIV)-hFIX lentiviral vector at multiplicities of infection (MOIs) from 1 to 60, and the most effective dose was at an MOI of 10, as determined by hFIX production. hFIX protein secretion persisted over the 28-day experimental period. Cell sheets composed of lentiviral vector-transduced mADSCs were engineered to further enhance the usefulness of these cells for future therapeutic applications in transplantation modalities. These experiments demonstrated that genetically transduced ADSCs may become a valuable cell source for establishing cell-based gene therapies for plasma protein deficiencies, such as hemophilia.
Watanabe and colleagues demonstrate that transduction of murine adipose tissue-derived stem/stromal cells (mADSCs) with a simian immunodeficiency virus-based lentivirus encoding human coagulation factor IX (hFIX) results in effective and persistent hFIX secretion. Transduced mADSC sheets were also engineered as a possible alternative treatment modality.
Introduction
Hemophilia is a congenital bleeding disorder that is attributed predominantly to a hereditary lack of biologically active coagulation factor VIII (FVIII) or factor IX (FIX). Worldwide, 105 to 160 per million of the male population suffer from this disease (Bolton-Maggs and Pasi, 2003). Current standard therapy is generally provided after the onset of bleeding episodes and relies on the infusion of FVIII or FIX concentrates. Unfortunately, these treatments are expensive, limiting access to this type of therapy for a majority of patients with hemophilia (Pipe et al., 2008). Thus, alternative molecular and cellular methods are needed for the treatment of hemophilia. Studies have shown that even a small increase in clotting activity (∼1–2%) over normal levels can improve the bleeding from severe to mild-to-moderate (Bolton-Maggs and Pasi, 2003), representing a dramatic improvement in quality of life by reducing the need for immediate clotting factor injections to prevent uncontrolled bleeding.
Cell-based therapies have received a great deal of attention as a next-generation therapeutic approach for hemophilia (Oh et al., 2006; Follenzi et al., 2008; Kasuda et al., 2008; Tatsumi et al., 2008a,b; Ohashi et al., 2010). There has been enormous interest in the transplantation of stem cells to produce clotting factors (Chuah et al., 2004; Oh et al., 2006; Coutu et al., 2011). Some types of stem cells can be readily isolated from human patients with minimal invasiveness (Lin et al., 2008), such as adipose tissue-derived stem/stromal cells (ADSCs, also known as adipose tissue-derived mesenchymal stem cells, AT-MSCs) (Zuk et al., 2001; Li et al., 2011b). ADSCs are actively proliferative in vitro and are multipotent, with the potential to differentiate into mesodermal, endodermal, and ectodermal lineages (Lee et al., 2004; Peister et al., 2004; Wang et al., 2004; Seo et al., 2005; Aurich et al., 2007; Banas et al., 2007; Liu et al., 2007). These cells would be an ideal autologous source of stem cells with the potential to reduce the need for immunosuppression after reimplantation back into patients.
In terms of hemophilia, native ADSCs need to be genetically modified to produce and secrete FVIII or FIX because ADSCs do not naturally express coagulation factors. A multitude of genetic approaches (Anjos-Afonso et al., 2004; Haleem-Smith et al., 2005; Oh et al., 2006; Talens-Visconti et al., 2006; Sugii et al., 2010; Coutu et al., 2011; Li et al., 2011a,b) have been applied to exogenously produce clotting factors.
In this study, we used simian immunodeficiency virus (SIV) lentiviral vectors derived from SIVagmTYO-1, a simian immunodeficiency virus strain isolated from African green monkeys (Nakajima et al., 2000). The SIV vector has been reported to have a different safety profile compared with other lentiviral vectors, in that this strain lacks the ability to become pathogenic in its natural host, African green monkeys, and in experimentally inoculated Asian macaques (Honjo et al., 1990; Nakajima et al., 2000; Kikuchi et al., 2004; Ogata et al., 2004). SIV also has low sequence homology to the HIV genome (Jin et al., 1994). Therefore, SIV vectors are assumed to hold low or almost no risk of causing homologous recombination that generates a replication-competent virus, even in circumstances in which the SIV vector coexists with HIV in the same cells inside a patient. The SIV vector is likely safer than other vectors and maintains the inherent ability to integrate into both proliferating and nonproliferating cells (Coffin et al., 1997; Walther and Stein, 2000; Li and Lu, 2009), making it an ideal vector to provide persistent expression of exogenous genes and possibly making it advantageous for application in future clinical studies.
Another approach to increasing the level of engraftment of transplanted cells at local sites is to engineer functional tissues. Our laboratory has established a cell sheet-engineering technology using temperature-responsive culture dishes that are grafted with a temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm) (Kikuchi and Okano, 2005; Yang et al., 2005). This technology allows us to recover monolithic cell sheets without any enzymatic digestion and has already been applied to regenerative medicine (Nishida et al., 2004; Obokata et al., 2011). To establish a tissue engineering-based treatment modality with murine ADSCs (mADSCs) for hemophilia, this method was applied to create a contiguous cell sheet of vector-transduced mADSCs.
Materials and Methods
Mice
C57BL/6J male mice (8 weeks old) were purchased from a commercial vendor (CLEA Japan, Tokyo, Japan). All animal procedures were conducted in accordance with the institutional guidelines of the Animal Care Committee of Tokyo Women's Medical University (Tokyo, Japan).
Preparation of mouse ADSCs
Adipose tissues were isolated from the inguinal region in the mice, minced with forceps, and enzymatically digested with 0.1% type I collagenase (17100-017; Invitrogen/Life Technologies, Carlsbad, CA) at 37°C for 1 hr. The stromal–vascular fraction (SVF) was collected by centrifugation at 700×g for 5 min and washed twice. The SVF was resuspended with Dulbecco's modified Eagle's medium (DMEM)–F12 (11320-033; Invitrogen/Life Technologies) supplemented with 10% fetal bovine serum (FBS, 04110101; Japan Bio-Serum, Hiroshima, Japan) and GlutaMAX-I supplement (35050-061; Invitrogen/Life Technologies). This medium is referred to in text as “basic medium.” The SVF was plated on PRIMARIA tissue culture-treated dishes (35-3803; BD Biosciences, Franklin Lakes, NJ) and cultured at 37°C in a 5% CO2 incubator. The medium was aspirated and changed 3 days after plating. Adherent proliferating cells were trypsinized for subculturing (defined as passage 1) approximately 7–8 days after plating. The subcultured cells were defined as mADSCs.
Flow cytometry
mADSCs at passage 2 were suspended and incubated with an Fc blocker (553141), followed by antibodies: fluorescein isothiocyanate-conjugated CD29 (CD29–FITC; 555005), phycoerythrin-conjugated CD44 (CD44–PE; 553134), CD90.2–PE (553005), CD31–PE (553373), CD45–PE (553081), isotype control–PE (553930), and isotype control–FITC (553960). All antibodies were obtained from BD Biosciences and the catalog numbers are shown in parentheses. The cells were analyzed with a flow cytometer (Gallios; Beckman Coulter, Brea, CA).
Osteogenic differentiation of mADSCs followed by alkaline phosphatase assay and alizarin red S staining
mADSCs (passage 2) were replated at 1×104 cells/cm2 in a 6-well plate for staining with alizarin red S, and 3.3×104 cells/cm2 were plated for an alkaline phosphatase (ALP) assay using minimum essential medium (MEM) α with GlutaMAX-I (32571; Invitrogen/Life Technologies) supplemented with 10% FBS. Twenty-four hours after cell plating, differentiation was initiated by incubating the cells with osteogenic differentiation medium: MEM α GlutaMAX-I with 10% FBS, containing β-glycerophosphate disodium salt hydrate (G9891; Sigma-Aldrich, St. Louis, MO) at 10 mmol/liter, ascorbic acid (323-44822; Wako, Osaka, Japan) at 50 μmol/liter, dexamethasone (Dex) (BG08A; Fuji-Seiyaku, Tokyo) at 100 nmol/liter; or commercially available osteogenic differentiation medium (hMSC osteogenic BulletKit, PT-3002; Lonza Japan, Tokyo, Japan). The osteogenic differentiation medium was changed every 3–4 days. Seven days after osteogenic induction, the ALP assay was performed on mADSCs, using a LabAssay ALP kit (291-58601; Wako) according to the manufacturer's instructions. Four weeks after osteogenic induction, mADSCs were fixed with 4% paraformaldehyde (PFA) (100412; Muto-kagaku, Tokyo, Japan), washed with purified water (Synthesis A10; Millipore, Billerica, MA), and stained at room temperature for 10 min with alizarin red S (011-01192; Wako) at 10 g/liter.
Adipogenic differentiation of mADSCs and oil red O staining
mADSCs (passage 2) were replated at 3×104 cells/cm2 with DMEM–F12 supplemented with 10% FBS and GlutaMAX-I (basic medium) and cultured until confluency. The basic medium was replaced with adipogenic differentiation medium: basic medium supplemented with isobutylmethylxanthine (IBMX) (I7018; Sigma-Aldrich) at 0.5 mmol/liter, indomethacin (095-02472; Wako) at 100 μmol/liter, Dex at 500 nmol/liter, and insulin (Wako) at 10 μg/ml. Seventy-two hours later, the adipogenic differentiation medium was removed and replaced with basic medium containing insulin (10 μg/ml). This latter medium was changed every 3–4 days. Two weeks after adipogenic induction, the mADSCs were fixed with 10% formalin, washed with phosphate-buffered saline (PBS) and isopropanol (Wako), and subsequently stained with oil red O solution (09091; Muto-kagaku) at room temperature for 10–20 min.
Preparation and titering of replication-defective SIV lentiviral vectors
Self-inactivating (SIN) simian immunodeficiency virus (SIV) vectors were used in this study as previously described (Ogata et al., 2004). Enhanced green fluorescent protein (EGFP) or the hFIX minigene (hFIX cDNA containing the first intron) were cloned 3′ of the cytomegalovirus (CMV) promoter. The SIV vector was pseudotyped with the G glycoprotein of the vesicular stomatitis virus (VSV-G) envelope, and the vector titers were determined as previously described (Nakajima et al., 2000; Ogata et al., 2004).
SIV lentiviral vector transduction of mADSCs in vitro
mADSCs were plated on a 6-well tissue culture plate at 4000 cells/cm2 with DMEM–F12 basic medium (2 ml/well). Twenty-four hours after plating, the medium was replaced with basic medium (1 ml/well) containing lentiviral vectors (at increasing multiplicities of infection [MOIs]) in the presence of Polybrene (hexadimethrine bromide, H-9268; Sigma-Aldrich) at 8 μg/ml. Twenty-four hours after the vectors were introduced, fresh basic medium (1 ml/well) was added without Polybrene. Afterward, the transduced cells were propagated with basic medium to perform ELISAs or clotting assays as described below. To induce osteogenic differentiation or adipogenic differentiation, the medium was changed to the appropriate differentiation medium (see previously) 96 hr after vector transduction, and the cells were cultured and assessed as described previously.
ELISA
The culture medium in a 6-well tissue culture plate was replaced with fresh medium (1 ml/well) 24 hr before medium collection. The culture medium was harvested and frozen at −80°C until assessment by ELISA. hFIX ELISA was performed with an Asserachrom IX:Ag kit (630423; Roche Diagnostics, Basel, Switzerland) according to the manufacturer's instructions.
Clotting assay
The culture medium was changed to fresh medium containing menaquinone (vitamin K2) (V-9378; Sigma-Aldrich) 24 hr before medium collection. Normal plasma (Coagtrol N, 13490; Sysmex, Hyogo, Japan) was mixed with FIX-deficient plasma (15450; Sysmex) and the clotting time was measured at 37°C to obtain a standard curve for calibration. Subsequently, the clotting time of culture medium mixed with FIX-deficient plasma was measured with a semiautomatic coagulation analyzer (KC4 Delta; Trinity Biotech, Wicklow, Ireland).
mADSC sheets
mADSCs (passage 2 or 96 hr after vector transduction) were replated on an UpCell temperature-responsive culture dish (CellSeed, Tokyo, Japan), at a density of 4.5×104 cells/cm2. After 5 days of culture at 37°C in 5% CO2, the temperature was changed from 37 to 20°C to initiate detachment of the mADSC cell sheet from the culture surface. The mADSCs were harvested as a monolayer cell sheet after an ∼20-min incubation at 20°C. Fluorescence images of EGFP-transduced mADSC sheets detaching from the temperature-responsive culture dishes were captured with an OV110 imager (Olympus, Tokyo, Japan). For ELISA, hFIX-transduced mADSC sheets were harvested and reattached onto collagen type IV-coated tissue culture dishes (Asahi Glass, Tokyo, Japan). After 24 hr of culture, the medium was collected for ELISAs to detect hFIX.
Transmission electron microscopy
Transmission electron microscopy (TEM) of mADSC sheets was performed by Tokai Electron Microscopy Analysis (Aichi, Japan). mADSC sheets were fixed with a solution comprising 2% PFA, 2% glutaraldehyde (GA) in 0.1 M PBS, and then incubated in 2% GA in 0.1 M PBS (supplied by the company).
Statistical analysis
Data are presented as means±standard deviation (SD). Group-wise comparisons were made by one-way analysis of variance (ANOVA) followed by the Tukey (HSD) post-hoc test, using SPSS statistics software (PASW statistics, version 18; SPSS, Chicago, IL). A value of p<0.05 was considered statistically significant.
Results
Characterization of mADSCs
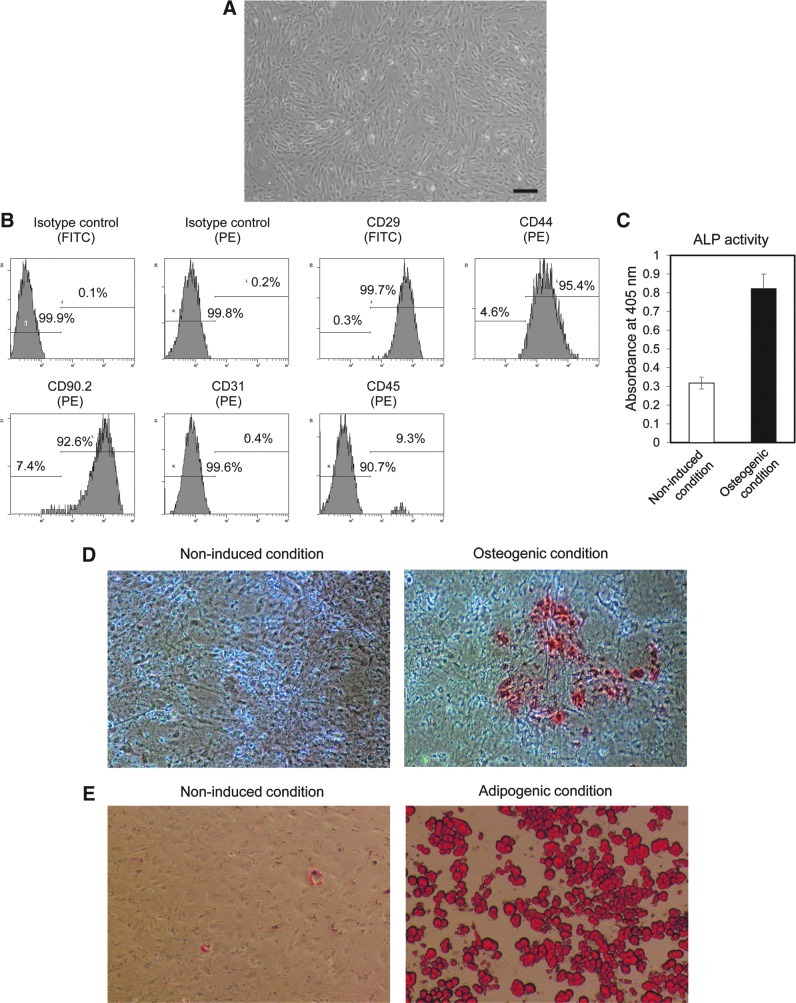
Figure 1A presents an optical microphotograph of mADSCs at passage 2. The cell-surface protein profiles of mADSCs were analyzed by flow cytometry. mADSCs at passage 2 expressed the mesenchymal stem cell markers (Mitchell et al., 2006) CD29 (99.7%), CD44 (95.4%), and CD90.2 (92.6%) (Fig. 1B). mADSCs were negative for CD31. A small population of the cells were CD45 positive (9.3%), but this may be due to contamination with hematopoietic cells.
FIG. 1.
Confirmation of the characteristics and bipotency of mouse adipose tissue-derived stem/stromal cells (mADSCs). (A) A microphotograph of mADSCs at passage 2, showing their spindle-like morphology. Scale bar: 50 μm. (B) Characterization of mADSCs by flow cytometry. mADSCs of passage 2 expressed the mesenchymal stem cell markers CD29, CD44, and CD90.2 and were negative for CD31 and CD45. FITC, fluorescein isothiocyanate; PE, phycoerythrin. (C–E) The osteogenic and adipogenic differentiation of mADSCs was verified by an alkaline phosphatase (ALP) assay and alizarin red S staining (markers of osteogenesis), and by oil red O staining (marker of adipogenesis), respectively. (C) The ALP assay was performed on day 7 of the culture of osteogenically differentiated mADSCs (n=3). (D) Alizarin red S staining was performed after 4 weeks of osteogenic induction culture conditions. The stain in the photograph of the induced condition indicates calcium deposition in the cells. (E) Oil red O staining was performed 2 weeks after the initiation of adipogenic induction culture conditions. Color images available online at www.liebertpub.com/hum
The mADSCs was verified as bipotent, demonstrating the ability to differentiate into their osteogenic and adipogenic lineages. Osteogenic induction increased ALP activity (Fig. 1C) and calcium accumulation as visualized by alizarin red S staining (Fig. 1D right). No calcium deposition was found in the noninduced condition (Fig. 1D left). Adipogenic differentiation was observed by the presence of lipid droplets detected by oil red O staining (Fig. 1E right). A few lipid droplets were observed in the noninduced condition (Fig. 1E left), suggesting that some mADSCs have the ability to spontaneously differentiate along the adipogenic lineage. These results confirmed that the mADSCs isolated in this study possessed the same characteristics as those of previously reported ADSCs (Liu et al., 2007).
Efficiency of lentiviral transduction of mADSCs
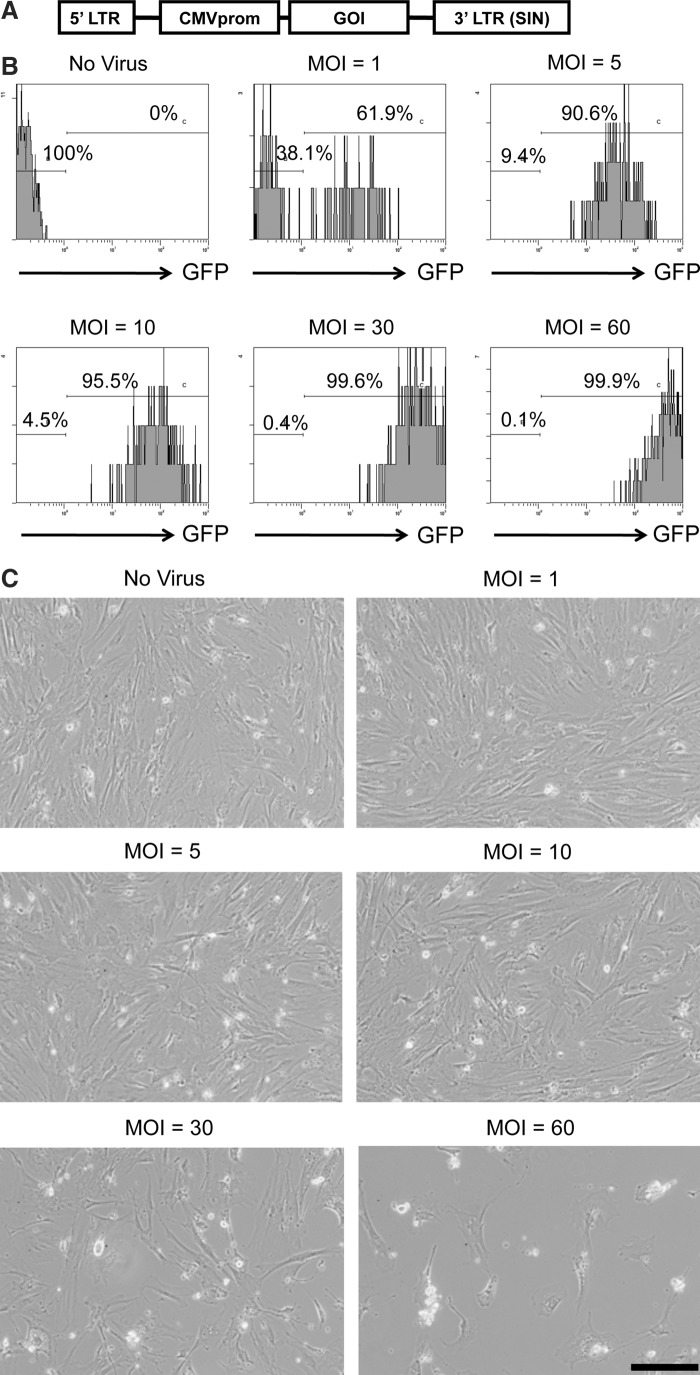
Figure 2A depicts the organization of the replication-defective SIV lentiviral vector, showing the location of the cytomegalovirus promoter (CMVprom) and the gene of interest (GOI). The GOIs used in this study were EGFP (SIV-EGFP) and human FIX (SIV-hFIX). To determine the optimal dose for transducing mADSCs using SIV lentiviral vectors, mADSCs at passage 2 were infected with SIV-EGFP at various multiplicities of infection (MOIs) from 1 to 60. Flow cytometric analysis was performed 4 days after infection to determine the number of GFP-positive cells. At an MOI of 5, ∼90% of the mADSCs were transduced with SIV-EGFP (Fig. 2B). Increasing the MOI up to 60 elevated the number of GFP-positive cells to nearly 100%. Moreover, the intensity of GFP fluorescence increased in a dose-dependent manner. However, the SIV-hFIX vector induced toxicity to the mADSCs at MOIs of 30 and higher (Fig. 2C). In addition, the SIV-transduced mADSCs had the same adipogenic and osteogenic differentiation abilities as nontransduced mADSCs (Supplementary Fig. S1; supplementary data are available online at www.liebertonline.com/hum). Taken together, these data show that SIV lentiviral vectors can be used over a fairly wide range of MOIs to genetically modify mADSCs without promoting cellular injury.
FIG. 2.
SIV lentivirus transduction of mouse adipose tissue-derived stem/stromal cells (mADSCs). (A) The SIV lentiviral vectors used in this study expressed the gene of interest (GOI) under the control of the cytomegalovirus promoter (CMVprom). 3′ LTR (SIN), self-inactivating 3′ long terminal repeat. (B) The efficiency of transduction was determined by measuring the proportion of EGFP fluorescence-positive cells by flow cytometry. mADSCs were transduced with SIV-EGFP at MOIs of 0, 1, 5, 10, 30, and 60. Transduction efficiencies were analyzed by flow cytometry 96 hr after transduction. (C) Microscopy images of mADSCs transduced with SIV-human coagulation factor IX (hFIX) at MOIs of 0, 1, 5, 10, 30, and 60. Scale bar: 50 μm. The experiments were repeated three times with similar results.
hFIX production by SIV-transduced mADSCs
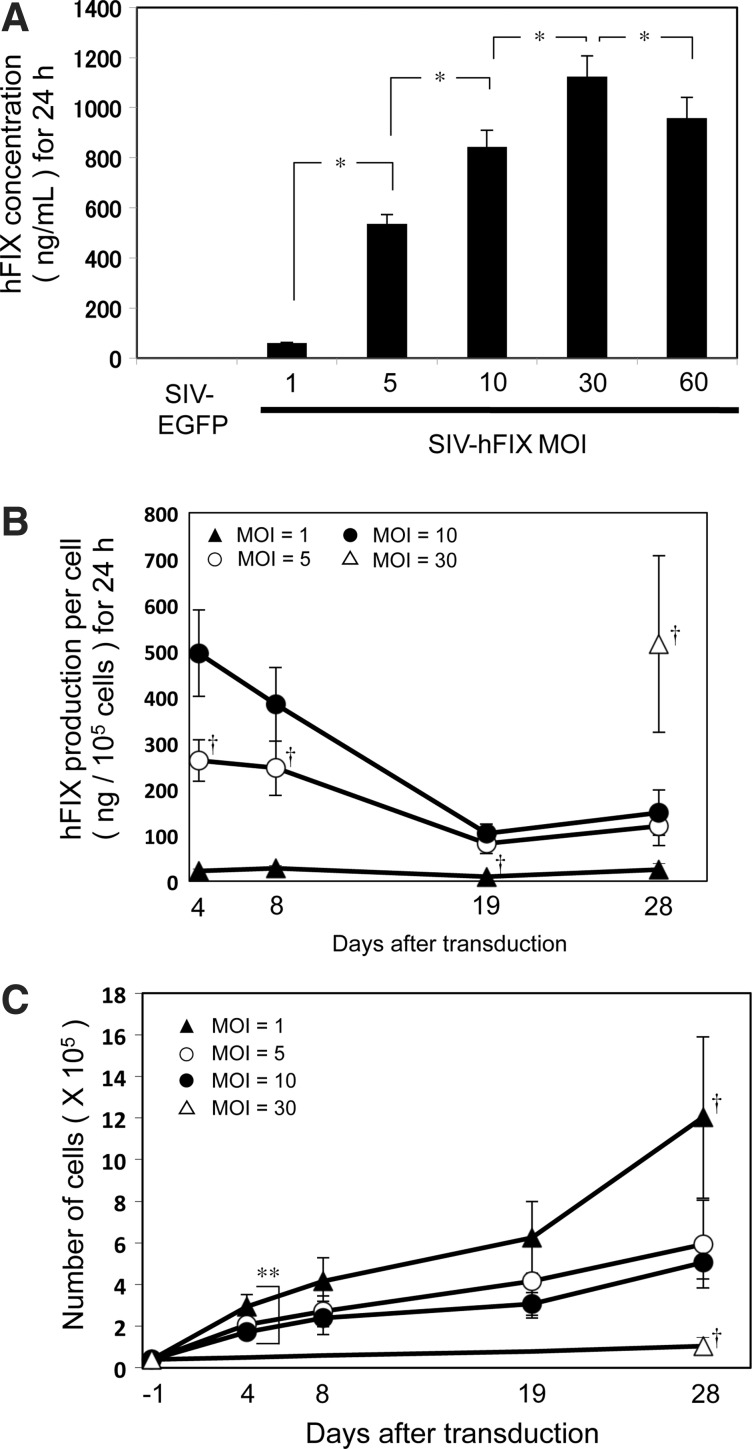
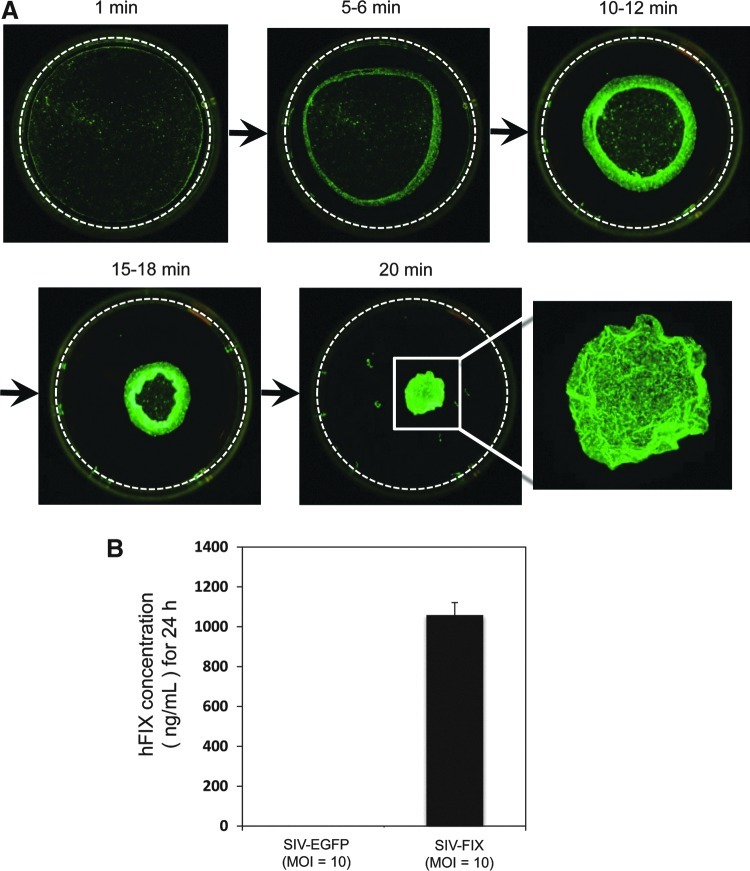
mADSCs at passage 2 were transduced with the SIV-hFIX lentivirus at various MOIs from 1 to 60. The culture medium was collected 96 hr after transduction, and the hFIX concentration was measured by ELISA (Fig. 3A). A dose-dependent increase in hFIX protein was observed from an MOI of 1 to 30. The amount of hFIX protein secreted at an MOI of 30 was ∼1100 ng/ml (Fig. 3A). hFIX production was lower at an MOI of 60 than at an MOI of 30, which could be due to the decreased cell number at the higher dose, likely resulting from the inherent toxicity of the VSV-G pseudotype (Fig. 2C).
FIG. 3.
SIV lentivirus-mediated human coagulation factor IX (hFIX) production from mouse adipose tissue-derived stem/stromal cells (mADSCs). mADSCs were transduced with the SIV-hFIX vector at MOIs of 1, 5, 10, 30, and 60. (A) The culture medium was collected 96 hr after transduction, and the amount of hFIX protein secreted over 24 hr was assessed by ELISA. mADSCs transduced with SIV-EGFP were used as the negative control. (B) The hFIX secretion profile from mADSCs was observed over 4 weeks. The culture medium from a 24-hr period was collected on days 4, 8, 19, and 28 after transduction. The amount of hFIX protein per 1×105 cells was measured by ELISA. (C) mADSC cell numbers were counted on days 4, 8, 19, and 28 after transduction. Cells treated at an MOI of 30 were unable to reach confluency until day 28. *p<0.05 between groups. †p<0.05 versus the other MOI groups; **p<0.05 between MOIs 1 and 10 on day 4 (n=3).
Sustained hFIX production in lentiviral vector-transduced mADSCs
The persistence of hFIX production and secretion from lentiviral vector-transduced mADSCs (MOI, 1 to 30) were analyzed over a 4-week period. mADSCs transduced at an MOI of 60 were not used in our studies because of their morphological deterioration attributed to the administration of a large amount of vector (Fig. 2C). On days 4, 8, 19, and 28 after transduction, the culture medium was collected and cell numbers were counted by hemocytometry for cells transduced up to an MOI of 10 (Fig. 3B and Table 1). The amount of hFIX protein produced per cell at an MOI of 30 could be reliably calculated only on day 28 because the cells were not confluent at earlier time points (data not shown). hFIX production was sustained throughout the 28 days, even though the levels decreased to about one-third of the levels measured on day 4. mADSCs transduced at an MOI of 30 secreted the largest amount of hFIX per cell on day 28 (Fig. 3B and Table 1). However, considering the growth curve of the SIV-transduced mADSCs (Fig. 3C), mADSCs transduced with lower vector doses increased in cell number more rapidly than those administered the highest dose (MOI of 30). Although the hFIX production per cell was 3-fold higher for an MOI of 30 than an MOI of 10 on day 28, the cumulative amount of hFIX protein over the 28-day culture was highest for an MOI of 10 (762.32 ng) because there were 5-fold fewer cells at an MOI of 30 than at an MOI of 10 (Fig. 3C and Table 1). The cumulative amount of hFIX protein on day 8 was also highest for an MOI of 10 (885.83 ng). Taken together, the results showed that mADSC transduction at an MOI of 10 was the optimal condition to produce the greatest amount of hFIX.
Table 1.
Total Production of hFIX Protein for 24 Hours
| Days after transduction | MOI | hFIX protein (ng) per 105 cells | Cumulative number of cells (×105) | hFIX protein (ng) from cumulative cells |
|---|---|---|---|---|
| 4 | 1 | 21.28±5.30 | 2.93±0.60 | 60.37±3.24a |
| 5 | 261.57±45.23 | 2.08±0.33 | 535.49±37.69a | |
| 10 | 494.99±93.79 | 1.73±0.20 | 843.84±65.40a | |
| 30 | N/A | N/A | N/A | |
| 8 | 1 | 27.81±5.08 | 4.15±1.15 | 111.63±14.45a |
| 5 | 245.23±59.24 | 2.71±0.74 | 641.15±80.28a | |
| 10 | 383.78±80.05 | 2.40±0.80 | 885.83±138.03a | |
| 30 | N/A | N/A | N/A | |
| 19 | 1 | 9.57±2.58 | 6.26±1.72 | 57.87±11.86a |
| 5 | 81.26±22.14 | 4.15±1.77 | 316.31±93.21 | |
| 10 | 103.46±20.32 | 3.06±0.54 | 324.15±124.48 | |
| 30 | N/A | N/A | N/A | |
| 28 | 1 | 24.52±13.76 | 12.03±3.89 | 270.87±128.77 |
| 5 | 119.49±42.86 | 5.94±2.11 | 744.11±493.55 | |
| 10 | 148.97±49.51 | 5.05±0.78 | 762.32±326.91 | |
| 30 | 514.50±191.43 | 1.03±0.42 | 478.57±42.42 |
hFIX, human factor IX; MOI, multiplicity of infection; N/A, not applicable.
Note: Data are expressed as mean values±SD.
p<0.05 versus the other groups in the same day after transduction (n=3).
Clotting assay
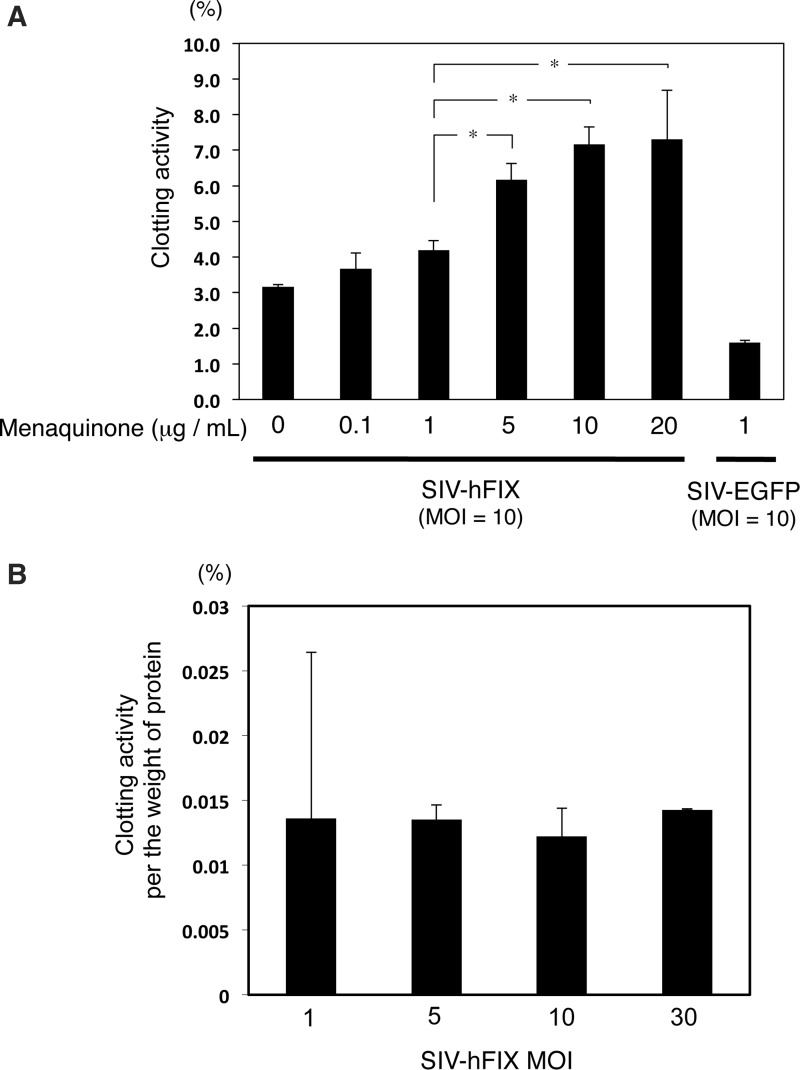
Because FIX proteins are intracellularly modified with the quinone vitamin K1 (phylloquinone) or vitamin K2 (menaquinone) (Blostein et al., 2008; Napolitano et al., 2010), the medium was supplemented with menaquinone (0–20 μg/ml) 72 hr after vector transduction. Twenty-four hours after the medium change, culture supernatant was collected and the clotting activity of hFIX secreted from the mADSCs (MOI, 10) was assessed. The clotting activity of the hFIX-containing medium depended on the menaquinone concentration (0 to 10 μg/ml) (Fig. 4A). The highest clotting activity, ∼7% of normal human plasma, was observed at a menaquinone concentration between 10 and 20 μg/ml. As menaquinone is dissolved in an organic solvent (i.e., 100% ethanol), which is harmful to most cultured cells, the remaining studies used menaquinone at 10 μg/ml. Figure 4B shows that the clotting ability per amount of hFIX protein did not depend on the vector dose.
FIG. 4.
Clotting activity of human coagulation factor IX (hFIX) produced by mouse adipose tissue-derived stem/stromal cells (mADSCs). (A) mADSCs were transduced with the SIV-hFIX lentivirus vector at an MOI of 10. Seventy-two hours after vector transduction, the medium was changed to fresh medium containing menaquinone (vitamin K2) at various concentrations (0, 0.1, 1, 5, 10, and 20 μg/ml). Twenty-four hours later, the culture supernatant was analyzed by a clotting assay. (B) The clotting activities of hFIX from mADSCs transduced at MOIs of 1, 5, 10, and 30 were analyzed 28 days after vector transduction. The medium was changed to fresh medium containing menaquinone (10 μg/ml) 24 hr before medium collection. *p<0.05 between groups (n=3).
mADSC sheets
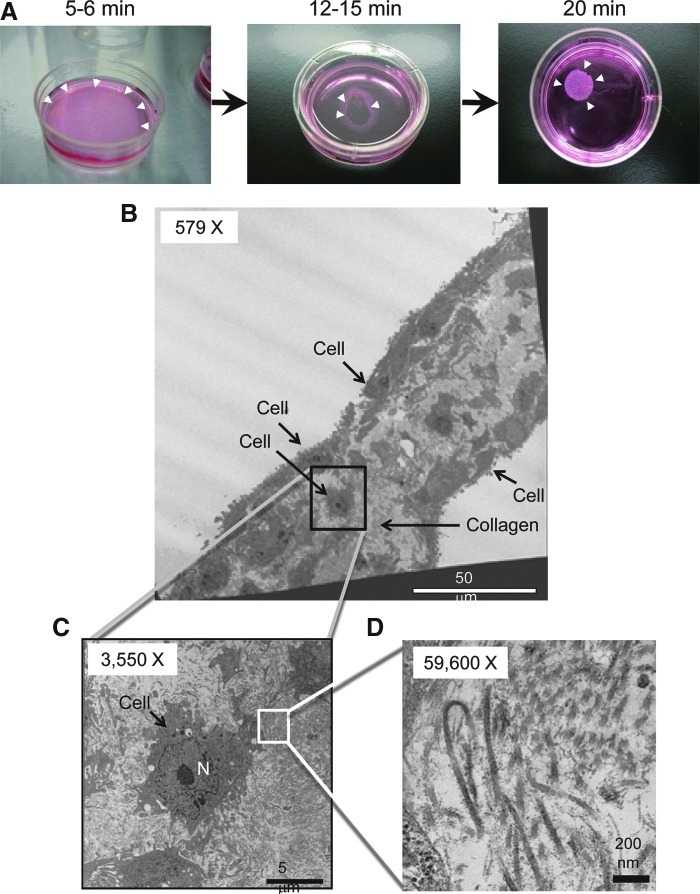
To bioengineer cell sheets, we used our standard protocol developed with other cell types (Miyahara et al., 2006; Ohashi et al., 2007) to assess whether mADSCs (passage 2) were capable of forming cell sheets. Reducing the culture temperature from 37 to 20°C for 20 min successfully released mADSCs cultured on temperature-responsive culture dishes as an intact cell sheet (Fig. 5A). Transmission electron microscopy (TEM) analysis revealed that (1) mADSC sheets consisted of two or three cell layers and (2) the spaces between cells were filled with collagen fibers (Fig. 5B–D), suggesting that mADSCs cultured on a temperature-responsive culture dish proliferate and produce extracellular matrix. Low-power fluorescence microscopy analysis was used to visualize the process of detachment of EGFP-transduced mADSCs from the temperature-responsive culture dish as a cell sheet (Fig. 6A). Furthermore, hFIX-transduced mADSC sheets were harvested and reattached on a plastic dish coated with collagen type IV. The transferred hFIX-transduced mADSC sheets produced significant levels of hFIX (Fig. 6B). These results suggest that cell sheets of gene-transduced mADSCs are functional materials that could improve the clotting ability of patients with hemophilia after transplantation.
FIG. 5.
Creation of cell sheets of mouse adipose tissue-derived stem/stromal cells (mADSCs). (A) mADSCs were cultured on a temperature-responsive culture surface (UpCell 35-mm dish). Lowering the temperature to 20°C allowed mADSC sheets to detach from the edge (arrowheads, left, at 5–6 min), shrink in size (arrowheads, middle, at 12–15 min), and float to the top of the medium (arrowheads, right, at 20 min). (B) Transmission electron microscopy (TEM) image showing two or three layers of mADSCs and collagen fibers in a cell sheet (original magnification,×579). (C) Magnified TEM image of the outlined region shown in (B) (original magnification,×3550). N, nucleus. (D) Magnified TEM image of the outlined region in (C) (original magnification,×59,600). Numerous striped fibers were observed, the typical feature of collagen. Scale bars: (B) 50 μm; (C) 5 μm; (D) 200 nm. Color images available online at www.liebertpub.com/hum
FIG. 6.
Cell sheets of lentivirus-transduced mouse adipose tissue-derived stem/stromal cells (mADSCs). (A) mADSCs were transduced with SIV-EGFP lentiviral vector and replated on a temperature-responsive culture surface (UpCell 35-mm dish) 96 hr after transduction. After reaching confluency on day 4 of culture, the culture temperature was lowered to 20°C to observe cell sheet harvesting. EGFP-transduced mADSCs started to detach from the edge as a cell sheet, shrank in size, detached, and floated in the medium after 20 min. Bottom right: High-magnification image of a floating EGFP-transduced cell sheet. The dashed circle represents the edge of the temperature-responsive culture dish (diameter, 35 mm). (B) SIV-hFIX-transduced mADSCs were replated on a temperature-responsive culture dish 96 hr after transduction. After 4 days of culture, the hFIX-transduced mADSCs were harvested as a cell sheet by lowering the culture temperature to 20°C and were reattached onto another culture dish. After 24 hr of culture, the medium was collected to measure hFIX protein levels by ELISA.
Discussion
This study demonstrates a relatively simple and efficient protocol to genetically modify mADSCs, using lentiviral vectors derived from SIV. As previously reported, coagulation factor IX (FIX) gains its full biological function after intracellular modification by a group of enzymes including γ-glutamyl carboxylase, vitamin K epoxide reductase, quinone reductase, and paired basic amino acid cleaving enzyme (PACE)/furin (Blostein et al., 2008; Napolitano et al., 2010; Tie et al., 2011). Our results showed that the clotting activity of de novo-synthesized hFIX was modulated by menaquinone (vitamin K2) in a dose-dependent manner. These results provide evidence that mouse ADSCs possess the posttranslational modification mechanisms required to produce biologically active FIX. Furthermore, mADSCs could form cell sheets that may be applicable for use in transplantation therapy.
The liver produces the majority of coagulation factors in humans. Although liver organ transplantation has cured patients with hemophilia A and B (Gordon et al., 1998), shortages of available livers have greatly limited the ability of liver transplantation to become a standard treatment. In earlier proof-of-concept studies, our laboratory has demonstrated the therapeutic value of cell-based approaches for hemophilia, including hepatocyte transplantation and liver tissue engineering (Tatsumi et al., 2008b; Ohashi et al., 2010). These successful experiments prompted us to generate FIX-producing cells from an autologous tissue origin. Autologous cells would present advantages to patients including (1) the reduction or complete avoidance of immunosuppressive regimens, and (2) the minimization of ethical problems by using the patient's own cells. Regardless of the cell type used for transplantation, the native cells that differentiate after implantation are generally insufficient to recapitulate normal function. For this reason, genetic methods are required to safely transport genes of interest into the cells to mass produce intracellular or secreted proteins or to inhibit the transcription/translation of deleterious genes. Earlier studies have shown some promise in the use of genetically modified bone marrow-derived mesenchymal stem cells (BM-MSCs) as a possible cell-based treatment modality for hemophilia (Oh et al., 2006; Coutu et al., 2011). Compared with BM-MSCs, ADSCs may have an advantage in that they are abundant and can be obtained by less invasive procedures (Kern et al., 2006). Various vectors have been used in investigations of methods to modify cells to express genes of interest, including nonviral systems such as nucleic acid transfection and viral vectors based on adenoviruses, adeno-associated viruses (AAVs), Sendai virus, or simple and complex retroviruses (Coffin et al., 1997; Walther and Stein, 2000; Anjos-Afonso et al., 2004; Haleem-Smith et al., 2005; Oh et al., 2006; Talens-Visconti et al., 2006; Zaragosi et al., 2007; Li and Lu, 2009; Sugii et al., 2010; Coutu et al., 2011; Kim et al., 2011; Li et al., 2011b).
In our study, we used SIV-based lentiviral vectors to genetically manipulate mADSCs. Although the SIV vectors were able to transduce mADSC cells to produce hFIX, the level of expression tended to decrease over the duration of the experiment. It is not clear from our studies why this occurred, but the main reason may be the choice of promoter in our vector system. We used the strong CMV promoter, which is able to produce enormous amounts of transgene products but has a propensity to be silenced over time (Prosch et al., 1996; Mehta et al., 2009; Duan et al., 2012). Thus, some small molecules such as DNA methyltransferase inhibitors or histone deacetylase inhibitors might be able to sustain hFIX gene expression. In addition, other mammalian promoters need to be studied in the context of these SIV vectors.
Another issue with the SIV vectors is the toxicity to mADSCs observed at MOIs greater than 30. It is likely that the pseudotype envelope protein VSV-G was the root cause of this problem, as observed in previous studies using HIV-based vectors coated with VSV-G. Additional effort could be made to minimize the toxicity and improve the safety profile of this vector. One method would be to perform “spinfection,” in which the cells of interest are centrifuged with the vector medium to improve the efficiency of viral infection (Li and Lu, 2009). Another possibility would be to replace the wild-type FIX cDNA with a hyperactive form of FIX. Researchers have described that the R338L mutation resulted in 8-fold higher biological activity, whereas V86A, E277A, and R338A resulted in 13-fold higher biological activity than wild-type FIX (Simioni et al., 2009; Lin et al., 2010).
For genetically modified ADSCs to become valuable as a therapy for hemophilia, further optimization is required to achieve efficient cell engraftment. Intravenous infusion of ADSCs has been associated with negative events including thromboembolism (Yukawa et al., 2009). To circumvent this issue, site-specific engraftment would be preferable to avoid these effects from intravenous administration. As a proof of principle, Coutu and colleagues (2011) created a transplantable cellular composite for hemophilia consisting of genetically modified BM-MSCs seeded on a biodegradable polymer scaffold. The cell sheet tissue-engineering technology developed in our laboratory (Shimizu et al., 2003; Kikuchi and Okano, 2005; Yang et al., 2005; Yamato et al., 2007) was successfully used in the current study to create mADSC sheets that should be sufficiently durable and viable for transplantation applications, similar to previous studies in which cell sheets secreted α1-antitrypsin or insulin (Ohashi et al., 2007; Shimizu et al., 2009).
In conclusion, this study demonstrated that SIV lentiviral vectors can efficiently transduce mADSCs and that hFIX expressed de novo from mADSCs is posttranslationally modified and able to form clots. Moreover, the genetically modified mADSCs were engineered as a cell sheet, indicating that they could become a valuable cell source for genetically engineered cell therapy to treat hemophilia, and possibly other genetic and nongenetic diseases in which patients lack secreted proteins, such as in diabetes.
Supplementary Material
Acknowledgments
The authors thank Mr. Takahiro Ohno, Mr. Yoshinori Matsubara, and Ms. Ayako Kohori (Olympus Corporation) for technical advice. The authors are grateful to Drs. Takanori Iwata, Soichi Takagi, Tamako Isaka, and Stefano Pietronave, and to Ms. Kyungsook Kim (Tokyo Women's Medical University), for helpful discussions. The authors appreciate Dr. Frank Park (Medical College of Wisconsin) and Dr. Norio Ueno (Tokyo Women's Medical University) for critical review of the manuscript. This work was supported by the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems, called the Cell Sheet Tissue Engineering Center (CSTEC); the Global COE Program; the Multidisciplinary Education and Research Center for Regenerative Medicine (MERCREM); a grant-in-aid (no. 24300174) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan; a Health Labor Sciences Research Grant for Research on HIV/AIDS from the Ministry of Health, Labor, and Welfare (MHLW), Japan; and a Bayer Hemophilia Award Program.
Author Disclosure Statement
Teruo Okano is an investor in CellSeed (JAPAN) and an inventor/developer designated on patents for the temperature-responsive culture surfaces described in this paper (patent nos. JP1972502, US5284766, FR0382214, NL0382214, DE0382214, GB0382214, SE0382214, CH0382214, and CH0382214).
References
- Anjos-Afonso F. Siapati E.K. Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J. Cell Sci. 2004;117:5655–5664. doi: 10.1242/jcs.01488. [DOI] [PubMed] [Google Scholar]
- Aurich I. Mueller L.P. Aurich H., et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56:405–415. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas A. Teratani T. Yamamoto Y., et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- Blostein M. Cuerquis J. Landry S., et al. The carboxylation efficiency of the vitamin K-dependent clotting factors: Studies with factor IX. Haemophilia. 2008;14:1063–1068. doi: 10.1111/j.1365-2516.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- Bolton-Maggs P.H. Pasi K.J. Haemophilias A and B. Lancet. 2003;361:1801–1809. doi: 10.1016/S0140-6736(03)13405-8. [DOI] [PubMed] [Google Scholar]
- Chuah M.K. Collen D. Vandendriessche T. Preclinical and clinical gene therapy for haemophilia. Haemophilia. 2004;10(Suppl. 4):119–125. doi: 10.1111/j.1365-2516.2004.00984.x. [DOI] [PubMed] [Google Scholar]
- Coffin J.M. Hughes S.H. Varmus H.E. The interactions of retroviruses and their hosts. In: Coffin J.M., editor; Hughes S.H., editor; Varmus H.E., editor. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. [PubMed] [Google Scholar]
- Coutu D.L. Cuerquis J. El Ayoubi R., et al. Hierarchical scaffold design for mesenchymal stem cell-based gene therapy of hemophilia B. Biomaterials. 2011;32:295–305. doi: 10.1016/j.biomaterials.2010.08.094. [DOI] [PubMed] [Google Scholar]
- Duan B. Cheng L. Gao Y., et al. Silencing of fat-1 transgene expression in sheep may result from hypermethylation of its driven cytomegalovirus (CMV) promoter. Theriogenology. 2012;78:793–802. doi: 10.1016/j.theriogenology.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Follenzi A. Benten D. Novikoff P., et al. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J. Clin. Invest. 2008;118:935–945. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F.H. Mistry P.K. Sabin C.A., et al. Outcome of orthotopic liver transplantation in patients with haemophilia. Gut. 1998;42:744–749. doi: 10.1136/gut.42.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleem-Smith H. Derfoul A. Okafor C., et al. Optimization of high-efficiency transfection of adult human mesenchymal stem cells in vitro. Mol. Biotechnol. 2005;30:9–20. doi: 10.1385/MB:30:1:009. [DOI] [PubMed] [Google Scholar]
- Honjo S. Narita T. Kobayashi R., et al. Experimental infection of African green monkeys and cynomolgus monkeys with a SIVAGM strain isolated from a healthy African green monkey. J. Med. Primatol. 1990;19:9–20. [PubMed] [Google Scholar]
- Jin M.J. Hui H. Robertson D.L., et al. Mosaic genome structure of simian immunodeficiency virus from West African green monkeys. EMBO J. 1994;13:2935–2947. doi: 10.1002/j.1460-2075.1994.tb06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuda S. Kubo A. Sakurai Y., et al. Establishment of embryonic stem cells secreting human factor VIII for cell-based treatment of hemophilia A. J. Thromb. Haemost. 2008;6:1352–1359. doi: 10.1111/j.1538-7836.2008.03022.x. [DOI] [PubMed] [Google Scholar]
- Kern S. Eichler H. Stoeve J., et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kikuchi A. Okano T. Nanostructured designs of biomedical materials: Applications of cell sheet engineering to functional regenerative tissues and organs. J. Control. Release. 2005;101:69–84. doi: 10.1016/j.jconrel.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Kikuchi J. Mimuro J. Ogata K., et al. Sustained transgene expression by human cord blood derived CD34+ cells transduced with simian immunodeficiency virus agmTYO1-based vectors carrying the human coagulation factor VIII gene in NOD/SCID mice. J. Gene Med. 2004;6:1049–1060. doi: 10.1002/jgm.609. [DOI] [PubMed] [Google Scholar]
- Kim J.H. Shin K.H. Li T.Z., et al. Potential of nucleofected human MSCs for insulin secretion. J. Tissue Eng. Regen. Med. 2011;5:761–769. doi: 10.1002/term.371. [DOI] [PubMed] [Google Scholar]
- Lee K.D. Kuo T.K. Whang-Peng J., et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Li G.B. Lu G.X. Gene delivery efficiency in bone marrow-derived dendritic cells: Comparison of four methods and optimization for lentivirus transduction. Mol. Biotechnol. 2009;43:250–256. doi: 10.1007/s12033-009-9197-1. [DOI] [PubMed] [Google Scholar]
- Li H. Haurigot V. Doyon Y., et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011a;475:217–221. doi: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Zhang B. Lu Y., et al. Adipose tissue-derived mesenchymal stem cell-based liver gene delivery. J. Hepatol. 2011b;54:930–938. doi: 10.1016/j.jhep.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.N. Kao C.Y. Miao C.H., et al. Generation of a novel factor IX with augmented clotting activities in vitro and in vivo. J. Thromb. Haemost. 2010;8:1773–1783. doi: 10.1111/j.1538-7836.2010.03913.x. [DOI] [PubMed] [Google Scholar]
- Lin K. Matsubara Y. Masuda Y., et al. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008;10:417–426. doi: 10.1080/14653240801982979. [DOI] [PubMed] [Google Scholar]
- Liu T.M. Martina M. Hutmacher D.W., et al. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- Mehta A.K. Majumdar S.S. Alam P., et al. Epigenetic regulation of cytomegalovirus major immediate-early promoter activity in transgenic mice. Gene. 2009;428:20–24. doi: 10.1016/j.gene.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Mitchell J.B. McIntosh K. Zvonic S., et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Miyahara Y. Nagaya N. Kataoka M., et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- Nakajima T. Nakamaru K. Ido E., et al. Development of novel simian immunodeficiency virus vectors carrying a dual gene expression system. Hum. Gene Ther. 2000;11:1863–1874. doi: 10.1089/10430340050129486. [DOI] [PubMed] [Google Scholar]
- Napolitano M. Mariani G. Lapecorella M. Hereditary combined deficiency of the vitamin K-dependent clotting factors. Orphanet J. Rare Dis. 2010;5:21. doi: 10.1186/1750-1172-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K. Yamato M. Hayashida Y., et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- Obokata H. Yamato M. Tsuneda S., et al. Reproducible subcutaneous transplantation of cell sheets into recipient mice. Nat. Protoc. 2011;6:1053–1059. doi: 10.1038/nprot.2011.356. [DOI] [PubMed] [Google Scholar]
- Ogata K. Mimuro J. Kikuchi J., et al. Expression of human coagulation factor VIII in adipocytes transduced with the simian immunodeficiency virus agmTYO1-based vector for hemophilia A gene therapy. Gene Ther. 2004;11:253–259. doi: 10.1038/sj.gt.3302174. [DOI] [PubMed] [Google Scholar]
- Oh T. Peister A. Ohashi K., et al. Transplantation of murine bone marrow stromal cells under the kidney capsule to secrete coagulation factor VIII. Cell Transplant. 2006;15:637–645. doi: 10.3727/000000006783981620. [DOI] [PubMed] [Google Scholar]
- Ohashi K. Yokoyama T. Yamato M., et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007;13:880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- Ohashi K. Tatsumi K. Utoh R., et al. Engineering liver tissues under the kidney capsule site provides therapeutic effects to hemophilia B mice. Cell Transplant. 2010;19:807–813. doi: 10.3727/096368910X508924. [DOI] [PubMed] [Google Scholar]
- Peister A. Mellad J.A. Larson B.L., et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Pipe S.W. High K.A. Ohashi K., et al. Progress in the molecular biology of inherited bleeding disorders. Haemophilia. 2008;14(Suppl. 3):130–137. doi: 10.1111/j.1365-2516.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- Prosch S. Stein J. Staak K., et al. Inactivation of the very strong HCMV immediate early promoter by DNA CpG methylation in vitro. Biol. Chem. Hoppe Seyler. 1996;377:195–201. doi: 10.1515/bchm3.1996.377.3.195. [DOI] [PubMed] [Google Scholar]
- Seo M.J. Suh S.Y. Bae Y.C., et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- Shimizu H. Ohashi K. Utoh R., et al. Bioengineering of a functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials. 2009;30:5943–5949. doi: 10.1016/j.biomaterials.2009.07.042. [DOI] [PubMed] [Google Scholar]
- Shimizu T. Yamato M. Kikuchi A., et al. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309–2316. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- Simioni P. Tormene D. Tognin G., et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua) N. Engl. J. Med. 2009;361:1671–1675. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- Sugii S. Kida Y. Kawamura T., et al. Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3558–3563. doi: 10.1073/pnas.0910172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talens-Visconti R. Bonora A. Jover R., et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J. Gastroenterol. 2006;12:5834–5845. doi: 10.3748/wjg.v12.i36.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K. Ohashi K. Kataoka M., et al. Successful in vivo propagation of factor IX-producing hepatocytes in mice: Potential for cell-based therapy in haemophilia B. Thromb. Haemost. 2008a;99:883–891. doi: 10.1160/TH07-09-0559. [DOI] [PubMed] [Google Scholar]
- Tatsumi K. Ohashi K. Shima M., et al. Therapeutic effects of hepatocyte transplantation on hemophilia B. Transplantation. 2008b;86:167–170. doi: 10.1097/TP.0b013e31817b9160. [DOI] [PubMed] [Google Scholar]
- Tie J.K. Jin D.Y. Straight D.L., et al. Functional study of the vitamin K cycle in mammalian cells. Blood. 2011;117:2967–2974. doi: 10.1182/blood-2010-08-304303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther W. Stein U. Viral vectors for gene transfer: A review of their use in the treatment of human diseases. Drugs. 2000;60:249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- Wang P.P. Wang J.H. Yan Z.P., et al. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem. Biophys. Res. Commun. 2004;320:712–716. doi: 10.1016/j.bbrc.2004.05.213. [DOI] [PubMed] [Google Scholar]
- Yamato M. Akiyama Y. Kobayashi J., et al. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog. Polymer Sci. 2007;32:1123–1133. [Google Scholar]
- Yang J. Yamato M. Kohno C., et al. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- Yukawa H. Noguchi H. Oishi K., et al. Cell transplantation of adipose tissue-derived stem cells in combination with heparin attenuated acute liver failure in mice. Cell Transplant. 2009;18:611–618. doi: 10.1177/096368970901805-617. [DOI] [PubMed] [Google Scholar]
- Zaragosi L.E. Billon N. Ailhaud G., et al. Nucleofection is a valuable transfection method for transient and stable transgene expression in adipose tissue-derived stem cells. Stem Cells. 2007;25:790–797. doi: 10.1634/stemcells.2006-0235. [DOI] [PubMed] [Google Scholar]
- Zuk P.A. Zhu M. Mizuno H., et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.