Abstract
Drug-induced liver injury (DILI) limits the development and utilization of numerous therapeutic compounds, and consequently presents major challenges to the pharmaceutical industry and clinical medicine1, 2. Acetaminophen (APAP) containing compounds are among the most frequently prescribed drugs, and also the most common cause of DILI3. Here we describe a pharmacological strategy that targets gap junction communication to prevent amplification of fulminant hepatic failure and APAP-induced hepatotoxicity. We report that connexin 32 (Cx32), a key hepatic gap junction protein, is an essential mediator of DILI by showing that mice deficient in Cx32 are protected against liver damage, acute inflammation, and death. We identified a small molecule inhibitor of Cx32 as a novel hepatoprotectant that achieves the same result in wildtype mice when coadministered with known hepatotoxic drugs. These findings demonstrate that gap junction inhibition is an effective therapy for limiting DILI, and suggest a novel pharmaceutical strategy to improve drug safety.
Drug safety is an important public health concern affecting the pharmaceutical industry, regulatory agencies, and physicians. The decision to develop, approve, and prescribe a drug requires that the therapeutic benefits of medications be continuously weighed against their potential toxicities1, 4. The liver represents an important target of drug toxicity because it metabolizes exogenous compounds into reactive intermediates, which can then cause progressive hepatocyte damage, fulminant hepatic failure, and death if liver transplantation is not performed5. Due to its association with significant patient morbidity and mortality, drug-induced liver injury (DILI) is the most frequently cited reason for abandoning compounds early in development or withdrawing them from the market after approval1, 6. Moreover, many clinically available drugs, most notably acetaminophen, are dose limited because of their potential to induce significant liver injury7.
The pathogenesis of DILI involves a multiphase process that exhibits marked spatial heterogeneity2, 8-10. The liver is functionally heterogeneous at baseline, with the majority of P450 enzyme expression and drug metabolism occurring in hepatocytes near the central veins11. In the initial phase of acute DILI, the perivenular cells are preferentially injured by reactive drug metabolites while the rest of the parenchyma remains viable11. The initial direct injury, which can be dose-dependent or idiosyncratic, subsequently progresses to involve the entire hepatic lobule through host immune mechanisms that remain poorly defined12. Interestingly, acetaminophen (APAP), the most common cause of DILI, results in hepatocellular damage that continues to occur even after the concentration of circulating hepatotoxin has declined to unmeasurable levels, suggesting an important role for propagation of preexisting injury rather than de novo direct injury13. Targeting propagation of direct hepatocyte injury represents a generalizable strategy for limiting the severity of dose-dependent and potentially idiosyncratic DILI. However, current efforts have been limited by inadequate knowledge of the molecular details underlying DILI propagation.
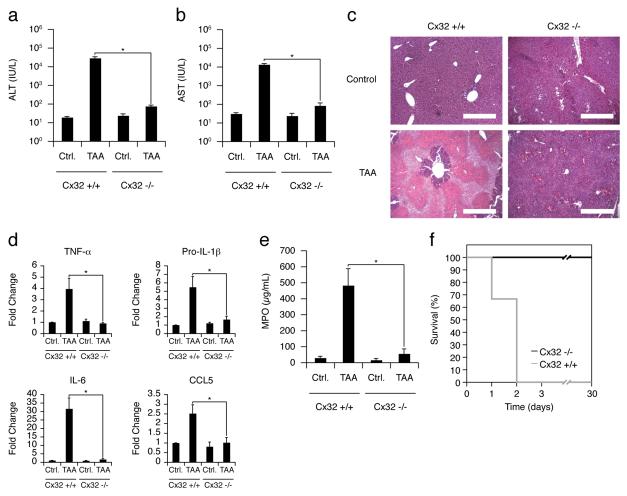
Recent work by our group and others have demonstrated a link between hepatic gap junctions, molecular channels composed of connexin proteins (Cx) that enable direct intercellular communication between coupled cells14, and the amplification of liver inflammation15, 16. Based on these findings, we hypothesized that liver-specific gap junction inhibitors function as a novel class of ‘hepatoprotectants’ that can be coformulated with hepatotoxic drugs to prevent progression of liver injury. We first examined the dependency of chemical-induced hepatotoxicity on connexin 32 (Cx32), the predominant gap junction protein expressed in the liver. Cx32-deficient (Cx32−/−) and wildtype (Cx32+/+) mice were treated with a single dose of thioacetamide (TAA), a classic hepatotoxin known to cause fulminant hepatic failure. After 24 hours, Cx32+/+ mice had significantly elevated serum ALT/AST levels, indicative of severe hepatocellular damage, whereas Cx32−/− mice exhibited near normal levels of ALT/AST and significantly reduced histological evidence of parenchymal damage in the liver (Fig. 1a-c). The livers of Cx32+/+ mice displayed a classic perivenular pattern of injury that extended into the hepatic lobule, while Cx32−/− mice exhibited only a small, localized perivenular ring of injury and necrosis that did not significantly radiate throughout the hepatic lobule (Fig. 1c). The overall inflammatory response of the liver and the associated recruitment of neutrophils were also attenuated in Cx32−/− mice (Fig. 1d, e). This decreased hepatotoxicity in Cx32−/− mice lead to a dramatic difference in TAA-induced mortality. Treatment of Cx32+/+ mice with a lethal dose of TAA resulted in 100% mortality, compared to a 100% survival in Cx32−/− mice (Fig. 1f). We confirmed that the protective effects of gap junction deficiency were not simply a result of defective drug metabolism by showing that serum concentrations of TAA and its toxic metabolite were the same in Cx32+/+ and Cx32−/− mice, as was phase I cytochrome P450 and phase II GST activity (Supplementary Fig. 1, 2). Together, these results demonstrate that Cx32 is an essential mediator of chemical-induced liver injury, and suggest that Cx32 inhibition might be a viable strategy for preventing hepatotoxic effects of drugs.
Figure 1.
Chemically-induced hepatotoxicity is dependent on connexin 32. (a,b) Significantly lower serum transaminase levels in Cx32−/− compared to Cx32+/+ mice 24 hours after treatment with a single sub-lethal dose of TAA (200 mg/kg, *P<.01). (c) Less liver hemorrhaging, necrosis, and acute inflammation in Cx32−/− mice compared to Cx32+/+ mice 24 hours after TAA treatment (H&E staining; original magnification 10X; scale bar = 400 μm). (d) Decrease in total liver TNF-α, pro-IL-1β, IL-6, and CCL5 transcripts, as measured by Q-PCR, in Cx32−/− mice 12 hours after TAA, compared to Cx32+/+ mice (*P<.01). (e) Liver tissue myeloperoxidase activity (MPO) in Cx32+/+ and Cx32−/− mice 24 hours after treatment with TAA (*P<.01). (f) Kaplan-Meier survival curve for Cx32+/+ and Cx32−/− mice over 30 days after a single lethal dose of 500 mg/kg TAA (Cx32+/+ and Cx32−/−: n=12).
Given the unique spatial distribution of both the injury and protection observed in the livers of Cx32+/+ and Cx32−/− mice, respectively, we hypothesized that gap junction communication might promote the propagation and amplification of an injury signal after TAA treatment. Since oxidative stress is a well-established consequence of hepatotoxin exposure17, 18 and free radicals are known to propagate through gap junctions, we considered the possibility that gap junctions amplify liver injury by promoting the propagation of an oxidative stress signal throughout the parenchyma. We first demonstrated the dependence of DILI on oxidative stress (Supplementary Fig. 3), and then compared the burden of intracellular free radicals in the livers of Cx32+/+ and Cx32−/− mice. We found that livers of TAA-treated Cx32+/+ mice exhibited intense focal regions of ROS activity, while those of Cx32−/− mice showed comparatively little activity (Supplementary Fig. 4a). We next devised an in vitro co-culture system to test whether hepatotoxin-exposed cells require Cx32 to propagate an oxidative stress signal to surrounding unexposed neighbors (Supplementary Fig. 4b,c). Connexin-deficient HeLa cells (HeLa WT) and Cx32-expressing HeLa cells (HeLa Cx32) were stimulated with the reactive metabolite of TAA (Thioacetamide sulfoxide; TASO) in the presence or absence of free radical scavengers18, and plated onto the Cx32-expressing hepatocyte-derived H35 cells preloaded with a fluorescent ROS probe (Supplementary Fig. 4c). Using this co-culture system, we detected ROS in unexposed neighbors only when they came into contact with TASO-exposed cells expressing Cx32, and found that this effect is prevented by pretreating the TASO-exposed cells with free radical scavengers (Supplementary Fig. 4d). Together these results suggest that Cx32 gap junctions propagate hepatotoxin-induced oxidative stress between hepatocytes.
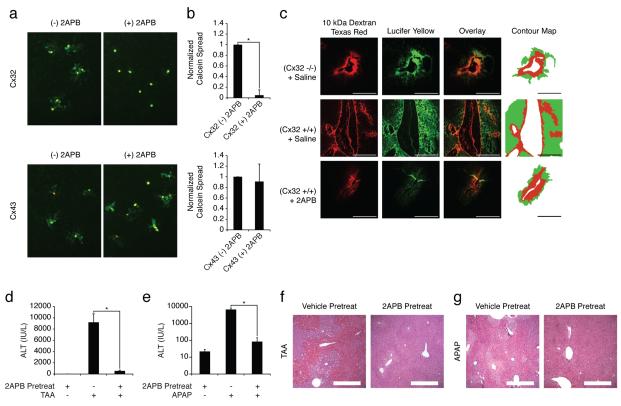
To investigate the therapeutic potential of Cx32 inhibition for preventing hepatotoxicity, we first screened chemical libraries for small molecule inhibitors of Cx32 that could be administered to wildtype mice to achieve the same hepatoprotection observed in the Cx32 deficient mice. We identified 2-aminoethoxydipenyl-borate (2APB), a compound previously shown to transiently inhibit Cx32 gap junctions in vitro19. We determined the specificity of 2APB for Cx32 gap junctions by performing a calcein-AM dye-coupling “parachute” assay using Cx32- and Cx43-expressing HeLa cells (HeLa Cx32, HeLa Cx43). Treatment with 2APB blocked the spread of calcein via Cx32 gap junctions, but had a modest effect on Cx43 gap junctions (Fig. 2a,b). We then adapted the classic “scrape and load” gap junction intercellular communication assay to explanted liver slices from Cx32+/+ and Cx32−/− mice, and demonstrated that 2APB effectively blocks hepatic gap junction communication in vivo (Fig. 2c). We next examined the effect of 2APB pretreatment of TAA- and APAP-induced liver injury. APAP is the active ingredient in several common over-the-counter and prescription pain medications. When wildtype mice were pretreated with a single dose of 2APB 60 minutes prior to challenge with TAA or APAP, the hepatoprotective response was strikingly similar to that observed in Cx32−/− mice. Compared to DMSO vehicle-treated mice, those that received 2APB exhibited significantly reduced serum ALT and dramatically reduced histological evidence of hepatocellular injury and necrosis (Fig. 2d-g). We confirmed that the hepatoprotective effect of 2APB was not a result of defective drug metabolism by showing that serum concentrations of TAA and TASO were similar in mice treated with DMSO vehicle or 2APB (Supplementary Fig. 1). These findings demonstrate that 2APB is an effective hepatoprotectant, and support the hypothesis that targeting the Cx32 gap junction pathway with small molecule inhibitors represents a viable strategy for limiting DILI.
Figure 2.
Small molecule inhibitors of Cx32 selectively block hepatic gap junction communication and prevent drug-induced hepatotoxicity. (a, b) A dye-coupling parachute assay was used to determine the specificity of 2APB for Cx32 gap junctions compared to Cx43 gap junctions. HeLa Cx32 or Cx43 cells were loaded with gap junction permeable calcein-AM (10 μM) and impermeable CM-DiI (10 μM), and seeded onto unlabeled recipient HeLa Cx32 or Cx43 cells, respectively, in the presence or absence of 2APB (25 μM). (a, b) After 4 hours, the co-culture was analyzed by fluorescence microscopy (n>5), and gap junction communication was assessed by calculating the average number of calcein positive cells per CM-Dil positive cell, normalized to control conditions without 2APB treatment (*P<.01). (c) A tissue version of the scrape and load test was developed to evaluate functional gap junction intercellular communication in liver tissue. Cx32+/+ and Cx32−/− mice were treated with saline or 2APB (20 mg/kg) for 3 hours. Livers were excised, cut into 2-3 mm slices, and a small area of each slice was mechanically damaged with the insertion of a 27 gauge needle coated with 0.5% Lucifer yellow (gap junction permeable) and 0.5% Texas red labeled dextran (gap junction impermeable). Slices were washed, fixed, cryosectioned (7 μm), and analyzed by fluorescence microscopy (n>5) and automated image analysis software to produce iso-intensity contour maps outlining spread of Lucifer yellow (green) and dextran-Texas red (red). Hepatic gap junction connectivity is demonstrated by the spread of gap junction permeable Lucifer yellow compared to gap junction impermeable dextran-Texas red. (d, e) Serum ALT levels and (f, g) H&E liver histology (original magnification 10X; scale bar = 400 μm) from wildtype mice pretreated with 2APB (20 mg/kg) or vehicle (DMSO, .1 mL/kg) 60 minutes prior to challenge with either TAA (200 mg/kg) or APAP (500 mg/kg), and then sacrificed 24 hours after TAA or 16 hours after APAP challenge (*P<.01).
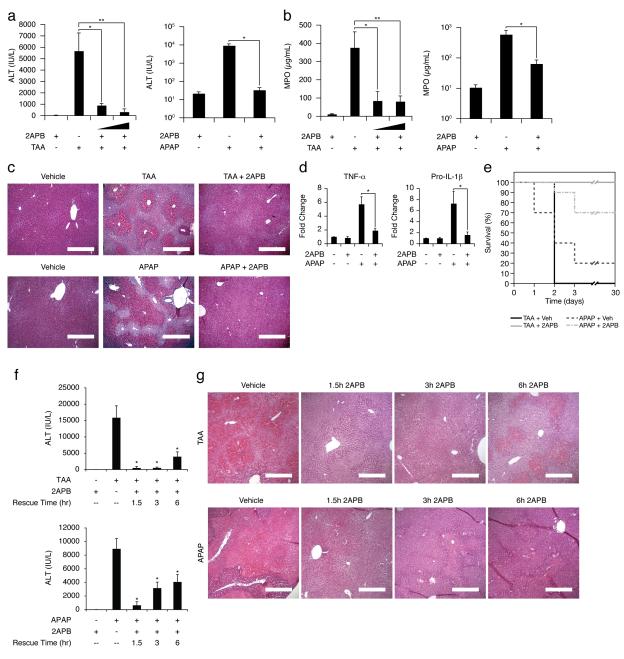
Unfortunately, pretreatment strategies for reducing drug hepatotoxicity have limited practical utility in both drug development and the clinic. Therefore, we considered a coadministration strategy, in which efficacious but potentially hepatotoxic drugs are coformulated with hepatoprotectants such as 2APB to improve their safety profiles. To develop this strategy, we used APAP, the most common cause of death due to acute liver failure20. We coadministered known hepatotoxic drugs (APAP or TAA) with the hepatoprotectant 2APB to wildtype mice, and compared their responses to mice receiving APAP or TAA with the appropriate DMSO vehicle. Coadministration of 2APB with either APAP or TAA dramatically reduced serum ALT, total hepatic free radicals levels, histological evidence of hepatic necrosis and hemorrhage, liver inflammation, and neutrophil infiltration to near normal levels (Fig. 3a-d and Supplementary Fig. 5). Remarkably, coadministration of 2APB reduced APAP-induced mortality from 80 to 30%, and TAA-induced mortality from 100 to 0% (Fig. 3e). Furthermore, we confirmed that this hepatoprotective effect of 2APB was not a result of its vehicle, DMSO, by changing the vehicle to ethanol (Supplementary Fig. 6). Together, these results suggest a novel strategy in which potential hepatotoxic drugs are coformulated with hepatoprotectants, such as Cx32 inhibitors, to improve their overall safety profile and reduce the clinical incidence of DILI.
Figure 3.
Coadministration of hepatotoxic compounds with Cx32 inhibitors reduces liver injury, limits inflammation, and enhances survival. (a) Serum ALT levels, (b) liver tissue MPO activity, and (c) H&E liver histology (original magnification 10X; scale bar = 400 μm) from wildtype mice after treatment with 2APB alone (20 mg/kg), TAA (200 mg/kg) plus vehicle (DMSO, .1 mL/kg), TAA (200 mg/kg) plus 2APB (1 or 20 mg/kg), APAP (500 mg/kg) plus vehicle (DMSO, .1 mL/kg), or APAP (500 mg/kg) plus 2APB (20 mg/kg). Livers and sera were collected 24 hours after TAA challenge, or 16 hours after APAP challenge (*P<.05, **P<.01). (d) Q-PCR for TNF-α and pro-IL-1β from whole livers of wildtype mice treated with APAP and 2APB as described above (*P<.01). (e) Kaplan-Meier survival curve for wildtype mice over 30 days after a single lethal dose of TAA (500 mg/kg) plus vehicle (DMSO, .1 mL/kg), TAA (500 mg/kg) plus 2APB (20 mg/kg), APAP (750 mg/kg) plus vehicle (DMSO, .1 mL/kg), or APAP (750 mg/kg) plus 2APB (20 mg/kg) (n=10). (f) Serum ALT levels and (j) H&E liver histology (original magnification 10X; scale bar = 400 μm) from wildtype mice after TAA (200 mg/kg) or APAP (500 mg/kg), with or without a rescue injection of 2APB (20 mg/kg) 1.5, 3 or 6 hours after hepatotoxin challenge. Livers and sera were collected 24 hours after TAA challenge, or 16 hours after APAP challenge (*P<.01). 2APB was dissolved in DMSO (200 mg/mL) for all experiments. All DMSO vehicle controls received this same amount of DMSO (.1 mL/kg) as the 2APB groups, with the appropriate volume of the hepatotoxin (TAA or APAP) dissolved in saline.
While coformulation of hepatotoxic compounds with gap junction inhibitors is an attractive strategy for future drug development, it is not directly applicable to the treatment of patients presenting after ingestion of compounds such as APAP at hepatotoxic doses. Therefore, we examined whether Cx32 inhibition could rescue from hepatotoxicity after drug ingestion. Single injections of 2APB or a DMSO vehicle were given at various times after challenge with either APAP or TAA. Mice treated with 2APB 1.5 hours after either APAP or TAA administration showed a nearly complete absence of hepatotoxicity (Fig. 3f, g). Even when administered 6 hours after hepatotoxin exposure, when hepatic necrosis is already evident and toxin metabolism is completed, 2APB rescue therapy reduced serum ALT levels and limited hepatocellular damage and necrosis (Fig. 3f, g). These findings demonstrate that hepatic gap junction inhibition with potent small compounds such as 2APB, successfully rescues from DILI and may provide a clinically useful means to treat liver injury associated with dose dependent hepatotoxic drugs such as APAP. However, the specificity of 2APB needs further investigation, as hepatoprotection might be occurring via various mechanisms, including but not limited to gap junction inhibition.
Drug-induced hepatotoxicity is classically divided into dose-dependent and idiosyncratic liver injury. The prototypical dose-dependent hepatotoxin, APAP, is the most common cause of DILI and is responsible for approximately half of the cases of acute liver failure3, 20. In contrast, idiosyncratic DILI affects over 900 clinically available drugs, but does so at a low incidence and in an unpredictable fashion2. Consequently, there are no prototypical idiopathic hepatotoxins and no experimental animal models of idiopathic DILI with which to study its pathogenesis or develop new therapies21. The strategy described here was developed in the context of dose-dependent hepatotoxins such as APAP, because of their clinical importance as well as their experimental accessibility. Given that the strategy targets the propagation phase of DILI, the possibly exists that the findings have applicability in cases of idiosyncratic DILI. Such generalizability was previously demonstrated for N-acetylcysteine (NAC), which was originally developed as an antidote for dose-dependent APAP hepatotoxicity, but has also shown benefit in the treatment of patients with non-APAP-induced acute liver failure 22. Additionally, recent evidence suggests that dose-dependent and idiosyncratic DILI may share some common modes of action and therefore potentially benefit from similar therapies 23.
Applications for hepatoprotectants such as 2APB range from early drug development to clinical medicine. In the pharmaceutical industry, coformulation and coadministration has previously been used to reduce gastrointestinal24 and renal toxicity25 but it has yet to be applied to hepatotoxicity, the most common reason for abandoning efficacious compounds during preclinical and clinical trials. Coformulation of hepatoprotectants such as 2APB with potential hepatotoxins represents a promising strategy for rescuing compounds in the drug development pipeline by improving their safety profiles. In clinical medicine, the hepatoprotectant coadministration strategy might be used to allow continuation of medications such as statins, antibiotics, anti-epileptics, or anti-TB drugs when they provoke significant elevations in liver enzymes or when they must be given to patients with preexisting liver disease. Finally, the most immediate clinical application of hepatoprotectants such as 2APB is in the acute management of acetaminophen overdose. Currently, the only available therapies are NAC, supportive care, and liver transplantation. We demonstrated the that 2APB can prevent and even rescue mice from death due to APAP toxicity, adding to the therapeutic armamentarium for treatment of this frequently fatal condition. It remains unclear to what extent 2APB will be helpful in idiosyncratic DILI since there are no animals models, however early success treating non-APAP DILI with NAC suggests the potential for generalization from dose-dependent to idiosyncratic DILI 22. These applications represent promising areas for further research.
Gap junctions represent an elegant mechanism for enabling direct communication between neighboring cells. In some organs such as the heart, gap junctions have a clearly identified physiological role26 and clinical trials of modulators are currently in progress such as a Phase II trial of a gap junction inhibitor intended to prevent life threatening arrhythmias following myocardial infarction27. In the liver however, the role of gap junctions remains poorly understood. Here we show that progression of DILI is gap junction-dependent and that Cx32 plays an essential role in amplifying injury, making it an ideal therapeutic target for hepatoprotection.
Methods
Animals and cell lines
C57BL/6 mice were purchased from Jackson Laboratory. Cx32−/− mice were a generous gift (see Acknowledgements). All animal protocols were approved by Massachusetts General Hospital Subcommittee on Research Animal Care. For survival experiments, animals were euthanized when they became moribund according to the criteria of lack of response to stimuli or lack of righting reflex. H35 hepatocyte-derived cells were maintained as previously described28. Connexin 26, 32, and 43 expressing HeLa cells were gifts (see Acknowledgements).
TAA-induced hepatotoxicity
TAA (Sigma Aldrich) solution was made fresh for each experiment in 0.9% saline at 20 mg/ml. TAA was dosed at 200, 500 or 1000 mg/kg, depending on the experiment, and injected intraperitoneally. Control mice received the appropriate volume of 0.9% saline. Animals were euthanized by ketamine/xylazine injection at 24 hours for collection of serum and liver tissue for qPCR, GSH/GST assay, MPO activity assay, and histology. For survival experiments, animals were observed every 24 hours for 30 days.
APAP-induced hepatotoxicity
APAP (Sigma Aldrich) solution was made fresh for each experiment in 0.9% saline at 20 mg/ml and heated until dissolved. APAP was dosed at 500 or 750 mg/kg, and injected intraperitoneally after 15 hours of starvation. Animals were euthanized by ketamine/xylazine injection at 16 hours for collection of serum and liver tissue for MPO activity assay and histology.
2-Aminoethoxydiphenyl Borate treatment
2APB (Sigma Aldrich) was made fresh for each experiment in DMSO (200 mg/ml) or 99% ethanol (100 mg/ml) as a vehicle. 2APB was dosed at 1 or 20 mg/kg, and was administered before (60 minutes), with, or after (1.5, 3 or 6 hours) the appropriate dose of TAA or APAP. All vehicle control mice received the same volume of DMSO (.1 mL/kg) or ethanol (.2 mL/kg) used to dissolve 2APB, mixed with the appropriate volume of the hepatotoxin (TAA or APAP) dissolved in saline.
DMSO treatment
Fresh anhydrous DMSO (Sigma Aldrich) was used for each experiment. DMSO was dosed at 0.1 or 1 ml/kg, and coadministered with 200 mg/kg TAA or saline.
N-Acetylcysteine treatment
NAC (Sigma Aldrich) was made fresh for each experiment in 0.9% saline at 20 mg/ml. NAC was doses at 200 mg/kg, and injected intravenously in the tail vein of mice after 15 hours of starvation.
Myeloperoxidase (MPO) activity assay
Mouse liver tissues were homogenized in MPO buffer (0.5% hexadecyl trimethyl ammonium bromide, 10 mM EDTA, 50 mM Na2HPO4, pH 5.4) using a Polytron homogenizer. Liver homogenates were then subject to three freeze-thaw cycles and cleared by centrifugation. MPO reaction was carried out using the Invitrogen EnzChek Myeloperoxidase Activity Assay Kit according to the manufacturer’s protocol.
Quantitative RT-PCR
Mouse liver tissues were crushed to a powder in liquid nitrogen, and total RNA was extracted using the Invitrogen Trizol RNA extraction kit, and then purified using the RT2 qPCR-Grade RNA Isolation Kit (SA Biosciences), according to the manufacturer’s protocol. Total RNA (500 ng) was converted into cDNA using the RT2 First Strand Kit (SA Biosciences). Quantitative RT-PCR was performed using the Stratagene Mx3000P QPCR System and the RT2 qPCR Master Mix Kit (SA Biosciences). Quantitative RT-PCR was performed for mRNA expression of Gapdh, TNFα, pro-IL-1β, IL6, CCL5, Cyp1a1, Cyp1a2, Cyp2e1, Cyp2b10, and Cyp3a using primers designed by SA Biosciences. Expression of Gapdh was used to standardize the samples, and the results were expressed as a ratio relative to control.
Dye-coupling Parachute Assay for Gap junction communication
HeLa Cx32 and HeLa Cx43 cells were grown to confluence, and then double-labeled with 10 μM CM-DiI, a membrane dye that does not spread via gap junctions, and 10 μM calcein-AM, which is converted intracellularly into the gap junction-permeable dye calcein. The labeled cells were then trypsinized, washed, and seeded onto confluent unlabeled recipient HeLa Cx32 or Cx43 cells, respectively, at a 1:200 ratio, in the presence or absence of 2APB (25 μM). The labeled cells were allowed to attach to the monolayer of unlabeled cells and form gap junctions for 4 hours at 37°C and then examined by fluorescence microscopy. For each 2APB experimental condition, the number of unlabeled recipient cells positive for calcein and negative for DiI was determined and normalized to no 2APB control conditions.
Tissue scrape and load assay for Gap junction communication
Mice were treated i.p. with saline or 2-APB (20 mg/kg), and 3 hours later livers were excised and freshly sliced. A 27-gauge needle was dipped into a solution containing 0.5% Lucifer Yellow (Invitrogen) and 0.5% 10kDa dextran-Texas Red (Invitrogen), and the needle was used to both mechanically damage a small area of each slice and apply the dyes. The liver slices were incubated with the dye solution for 5 minutes, rinsed in saline, fixed in 4% paraformaldehyde for 30 minutes, frozen in OCT compound, cyro-sectioned into 7 μm sections, rinsed in saline, mounted, and imaged by fluorescence microscopy.
H2DCFH-DA and dihydroethidine hydrochloride (DEH) staining
Freshly cut frozen liver sections (7 μm) were stained with 10 μM H2DCFH-DA (Invitrogen) or 2 μM DEH (Invitrogen) for 30 minutes at 37°C, and imaged by fluorescence microscopy as previously described29.
Fluorescence microscopy
Fluorescence images were captured on a Zeiss 200 Axiovert microscope at a fixed exposure and gain. Images for the tissue scrape load assay were quantified using custom image analysis routines written in MATLAB15. Briefly, images were median filtered, auto-thresholded, and segregated to identify discreet closed regions representing the spread of Lucifer Yellow dye and dextran-Texas Red. Regional outlines were plotted as contour maps by displaying iso-intensity lines at the determined threshold level.
Flow cytometry
Cultured H35 hepatocyte-derived cells were loaded with 10 μM H2DCFH-DA for 30 minutes at 37°C. This cell-permeable compound is converted into a non-fluorescent product (H2DCF), and oxidized by free radicals to the highly fluorescent dichlorofluoresceine (DCF). Cells were washed in PBS three times, and then treated with saline, TAA (25 μM), TASO (5 μM), or H2O2 (100 μM) for 2 hours, or subject to the transplant co-culture assay. After treatment, cells were trypsinized, washed in PBS, and analyzed by flow cytometry.
Transplant co-culture assay
Connexin 32 expressing HeLa (HeLa Cx32) and connexin 43 expressing HeLa (HeLa Cx43) cells were stimulated with saline, TASO (5 μM), or H2O2 (100 μM) in suspension for 2 hours, in the presence or absence of cell permeable anti-oxidant MnTMPyP (Calbiochem). Two hours after treatment, HeLa cells were washed 3 times in PBS, counted, and plated onto a sub-confluent layer of H35 cells, preloaded with H2DCFH-DA, at a cell ratio of 2:1. After 4 hours of co-culture, cells were trypsinized and H35 cells were analyzed for ROS activity, as indicated by H2DCFH-DA fluorescence, by flow cytometry.
HPLC-based quantification of TAA and TASO
To quantify TAA and TASO in serum of mice, a reverse-phase HPLC assay was used, as previously described. Briefly, 7% acetonitrile, 50 mM sodium sulfate, and 50 mM potassium phosphate buffer was used as the mobile phase. An SPS-ODS column (5 μm; Regis Technologies) was used to separate the components at 1 ml/min. TAA was detected by UV absorption at 212 nm, and TASO at 290 nm, using a photodiode array detector. Retention times for TAA and TASO were approximately 4.1 and 3 min, respectively. Standards were prepared by including known amounts of TAA and TASO in serum from untreated mice.
Synthesis of TASO
Thioacetamide S-Oxide (TASO) was synthesized as previously described30. Briefly, thioacetamide (TAA) was dissolved in acetone and chilled to -5°C. Then 30% H2O2 was added rapidly, the mixture was agitated thoroughly, and stored at 4°C for 24 hours until the product crystallized. The product, TASO, was recovered by filtration and washed with 5 portions of cold acetone. The purity was examined by HPLC, as previously described30.
Analysis of GST activity and total GSH content
Mouse liver tissues were lysed in 100 mM potassium phosphate, containing 2 mM EDTA, and total protein content was determined. Enzymatic activity toward 1-chloro-2,4-dinitrobenzene (CNDB) (Sigma Aldrich) was assayed in a buffer containing 100 mM potassium phosphate, 0.1% Triton X-100, 1 mM glutathione and 1mM CNDB. Formation of glutathione/CNDB conjugate was measured in a spectrophotometer at 340 nm, as an indicator of GST activity. Total GSH content was measured using the Glutathione Assay Kit (Sigma Aldrich), as per the manufacturer’s protocol. Briefly, mouse liver tissues were lysed and total protein content was determined. Samples were deproteinized with 5% 5-sulfosalicylic acid, and glutathione content of the samples was assayed using a kinetic assay in which catalytic amounts of glutathione cause a continuous reduction of 5,5′-dithiobis-(2-nitrobenzoic) acid (DTNB) to TNB. TNB was measured colorimetrically at 412 nm, as an indicator of total GSH content.
Statistical Analysis
Results are reported as mean plus/minus standard deviation. Statistical analysis was performed using the Student’s t-test, with P<.05 considered significant.
Supplementary Material
Acknowledgements
The authors thank K. Willecke (University of Bonn) and D. Paul (Harvard University) for the generous gift of Cx32−/− mice, K. Willecke (University of Bonn) for connexin expressing HeLa cells, H. Duffy (Harvard Medical School) for development of the tissue scrape and load assay for GJIC, and M. Izamis (Harvard Medical School) for HPLC assistance. S.J.P. was supported in part by a Department of Defense CDMRP Prostate Cancer Predoctoral Training Award and a Shriner Hospital for Children Postdoctoral Fellowship Award. The work was supported by grants from the US National Institutes of Health (DK059766 and P41 EB-002503) and from the Shriners Hospital for Children.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- Cx32
connexin 32
- TASO
thioacetamide S-oxide
- ROS
reactive oxygen species
- DMSO
dimethyl sulfoxide
- DILI
drug-induced liver injury
- 2APB
2-aminoethyoxydiphenyl-borate
- TAA
thioacetamide
- APAP
acetaminophen
- NAC
N-Acetylcysteine
Footnotes
Author Contributions. S.J.P. initiated the project, performed the experiments, analyzed data, and wrote the manuscript. J.M.M. and K.R.K. performed the experiments, provided experimental advice, and wrote the manuscript. S.B., M.L., A.I., and A.V. performed the experiments and analyzed data. R.J. and B.P. provided conceptual and editorial support. M.L.Y. supervised the project.
Competing Interests Statement. The authors have competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and/or discussion reported in this article.
References
- 1.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 2.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 3.Hutson S. Painkiller concerns grow ahead of new guidelines. Nat Med. 16:10. doi: 10.1038/nm0110-10a. [DOI] [PubMed] [Google Scholar]
- 4.Platt R, Madre L, Reynolds R, Tilson H. Active drug safety surveillance: a tool to improve public health. Pharmacoepidemiol Drug Saf. 2008;17:1175–1182. doi: 10.1002/pds.1668. [DOI] [PubMed] [Google Scholar]
- 5.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 6.Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969-2002: the importance of reporting suspected reactions. Arch Intern Med. 2005;165:1363–1369. doi: 10.1001/archinte.165.12.1363. [DOI] [PubMed] [Google Scholar]
- 7.Lee WM. Acetaminophen toxicity: changing perceptions on a social/medical issue. Hepatology. 2007;46:966–970. doi: 10.1002/hep.21926. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 9.Chen CJ, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 10.Imaeda AB, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindros KO. Zonation of cytochrome P450 expression, drug metabolism and toxicity in liver. Gen Pharmacol. 1997;28:191–196. doi: 10.1016/s0306-3623(96)00183-8. [DOI] [PubMed] [Google Scholar]
- 12.Tujios S, Fontana RJ. Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol. 8:202–211. doi: 10.1038/nrgastro.2011.22. [DOI] [PubMed] [Google Scholar]
- 13.Bartolone JB, Cohen SD, Khairallah EA. Immunohistochemical localization of acetaminophen-bound liver proteins. Fundam Appl Toxicol. 1989;13:859–862. doi: 10.1016/0272-0590(89)90339-4. [DOI] [PubMed] [Google Scholar]
- 14.Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Patel SJ, King KR, Casali M, Yarmush ML. DNA-triggered innate immune responses are propagated by gap junction communication. Proc Natl Acad Sci U S A. 2009;106:12867–12872. doi: 10.1073/pnas.0809292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naiki-Ito A, et al. Gap junction dysfunction reduces acetaminophen hepatotoxicity with impact on apoptotic signaling and connexin 43 protein induction in rat. Toxicol Pathol. 38:280–286. doi: 10.1177/0192623309357951. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke H, et al. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 18.Ferret PJ, et al. Detoxification of reactive oxygen species by a nonpeptidyl mimic of superoxide dismutase cures acetaminophen-induced acute liver failure in the mouse. Hepatology. 2001;33:1173–1180. doi: 10.1053/jhep.2001.24267. [DOI] [PubMed] [Google Scholar]
- 19.Tao L, Harris AL. 2-aminoethoxydiphenyl borate directly inhibits channels composed of connexin26 and/or connexin32. Mol Pharmacol. 2007;71:570–579. doi: 10.1124/mol.106.027508. [DOI] [PubMed] [Google Scholar]
- 20.Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342–349. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 21.Shenton JM, Chen J, Uetrecht JP. Animal models of idiosyncratic drug reactions. Chem Biol Interact. 2004;150:53–70. doi: 10.1016/j.cbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Lee WM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. 864, e851. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth RA, Ganey PE. Intrinsic versus idiosyncratic drug-induced hepatotoxicity--two villains or one? J Pharmacol Exp Ther. 332:692–697. doi: 10.1124/jpet.109.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taha AS, et al. Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugs. N Engl J Med. 1996;334:1435–1439. doi: 10.1056/NEJM199605303342204. [DOI] [PubMed] [Google Scholar]
- 25.Birnbaum J, Kahan FM, Kropp H, MacDonald JS. Carbapenems, a new class of beta-lactam antibiotics. Discovery and development of imipenem/cilastatin. Am J Med. 1985;78:3–21. doi: 10.1016/0002-9343(85)90097-x. [DOI] [PubMed] [Google Scholar]
- 26.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–322. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 27.Wit AL, Duffy HS. Drug development for treatment of cardiac arrhythmias: targeting the gap junctions. Am J Physiol Heart Circ Physiol. 2008;294:H16–18. doi: 10.1152/ajpheart.01031.2007. [DOI] [PubMed] [Google Scholar]
- 28.King KR, et al. A high-throughput microfluidic real-time gene expression living cell array. Lab Chip. 2007;7:77–85. doi: 10.1039/b612516f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- 30.Porter WR, Neal RA. Metabolism of thioacetamide and thioacetamide S-oxide by rat liver microsomes. Drug Metab Dispos. 1978;6:379–388. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.