Abstract
Nutrient composition of a diet (D) has been shown to interact with genetic predispositions (G) to affect various lipid phenotypes. Our aim in this study was to confirm GxD interaction and determine whether the interaction extends to other cardiometabolic risk factors such as glycemic measures and body weight. Subjects were vervet monkeys (Chlorocebus aethiops sabaeus) (n = 309) from a multigenerational pedigreed colony initially fed a plant-based diet, standard primate diet (18% calories from protein, 13% from fat, and 69% from carbohydrates) and subsequently challenged for eight weeks with a diet modeled on the typical American diet (18% calories from protein, 35% from fat, and 47% from carbohydrates). Our results showed that although exposure to the challenge diet did not result in significant changes in weight, most lipid and glycemic biomarkers moved in an adverse direction (p < 0.01). Quantitative genetic analyses showed that cardiometabolic phenotypes were significantly heritable under both dietary conditions (p < 0.05), and there was significant evidence of G x D interaction for these phenotypes. We observed significant differences in the additive genetic variances for most lipid phenotypes (p < 10−4), indicating that the magnitude of genetic effects varies by diet. Furthermore, genetic correlations between diets differed significantly from 1 with respect to insulin, body weight and some lipid phenotypes (p < 0.01). This implied that distinct genetic effects are involved in the regulation of these phenotypes under the two dietary conditions. These GxD effects confirm and extend previous observations in baboons (Papio sp.) and suggest that mimicking the typical human nutritional environment can reveal genetic influences that might not be observed in animals consuming standard, plant-based diets.
Keywords: Nutrient composition, Genetic predisposition, Quantitative genetic analysis
INTRODUCTION
The prevalence of obesity, diabetes, hypertension and other cardiometabolic disorders has risen steadily over the past few decades [Slomko et al., 2012; Temelkova-Kurkstchiev and Stefanov, 2012]. These disorders seem to parallel changes in diet and lifestyle reflecting a combination of increasingly easy access to calorie- rich foods [Poston and Foreyt, 1999; Schrauwen and Westerterp, 2000; Fujimoto et al., 2000; Roberts and Barnard, 2005] and decreasing opportunities for physical activity [Caldwell et al., 2006]. However, the effect of these environmental changes on cardiometabolic risk factors seems to be more pronounced in individuals who are genetically susceptible to them [Comuzzie et al., 2010; Temelkova-Kurkstchiev and Stefanov, 2012]. Therefore, the response of an individual’s genetic background to the changing environment determines his/her susceptibility to these metabolic disorders [Weinseir et al., 1998; Schrauwen and Westerterp, 2000; Comuzzie et al., 2010]. Most dietary intervention studies have shown inter-individual variability in metabolic responses [Ordovas and Shen, 2008; Lairon et al., 2009, Comuzzie et al., 2010] including family-based and randomized, crossover-based designs [Dreon et al., 1997; Dreon et al., 2000; Krauss 2001: Kraus, 2005]. However, these studies have focused mainly on lipid phenotypes.
The non-leaf eating Old World monkeys such as baboon, macaques and vervets resemble humans genetically and physiologically, and tend to show similar metabolic responses to changes in diet [McGill et al., 1981; Jen et al., 1985; Bodkin et al., 1993; Mahaney et al., 1998; Comuzzie et al., 2003; Cann et al., 2010; Shelton, Clarkson and Kaplan 2012]. Studies conducted in baboons (Papio hamadrayas) and rhesus monkeys (Macaca mulatta) have shown adverse metabolic responses to changes in diet compositions [Maheney et al., 1998; Higgins et al., 2010; Suzuki et al., 2006; McCurdy et al., 2009]. Higgins et al. [2010] showed that baboons, consuming a diet high in fat and simple sugars resulted in increased body fat, plasma triglycerides, adipokines and hemoglobin A1C in an eight-week period. Mahaney et al. [1998] showed significant effects of GxD interaction on the response of HDL-c cholesterol following a switch from a plant-based, low fat ‘standard primate’ diet to a more human-like, Western diet, high in cholesterol and saturated fat.
Vervet or African green monkeys (Chlorocebus aethiops sabaeus) also demonstrate vulnerability to obesity and cardiometabolic disorders such as insulin resistance, type 2 diabetes, elevated BP [Kavanagh et al., 2007a, Kavanagh et al., 2007b, Cann et al., 2010]. These adverse changes in body composition and other health indices occur even when animals consume a low fat, “standard” monkey diet. Under this dietary condition, these cardiometabolic traits were significantly heritable, with the estimates ranging between 20 and 45% [Kavanagh et al. 2007a]. However, these monkeys have also been characterized as good models for studying genetics of cardiometabolic disorders through dietary manipulations [Rudel et al., 1995; Kavanagh et al., 2007a]. When fed diets with 35% of energy derived from trans-monounsaturated fatty acids, as compared to cis-monounsaturated fatty acids, vervets gained significant weight and abdominal fat in spite of controlled food intake (Kavanagh et al.2007b).
Cardiometabolic phenotypes, mainly quantitative, often exhibit differences in their expressions due to genotype by environment (GxE) interaction. The GxE interaction can result in the same genotype giving rise to two different phenotypes in two different environments. These environments can be internal such as sex [Martin et al., 2002, Voruganti et al., 2011], adiposity [Diego et al., 2007] and disease states [Puppala et al., 2007] or external as might be represented by smoking status [Martin et al., 2003, Czerwinski et al., 2004] and alcohol intake [Arya et al., 2005]. This concept can be extended to analyze the effects of genotype by diet (GxD) interaction where exposure of the same individuals to different diets can represent different environments eliciting differential cardiometabolic responses [Maheney et al., 1999].
In the current study, we challenged for seven to eight weeks an entire pedigreed colony of vervet monkeys – initially consuming a low fat, standard primate diet – with a “typical American” diet (TAD) that was high in fat (37% of calories)[Wagner et al., 2009]. Although dietary challenges have been conducted in human subjects to identify GxD interactions (especially with respect to plasma lipids [Dreon et al., 2000], most studies have been done on an outpatient basis using individuals of unknown relatedness. Such investigations are limited by the inability to ascribe heritability to the outcomes and also by the lack of environmental control inherent in human studies. The challenge described here avoided these potential limitations by using a pedigreed population in which all animals lived in standardized housing and consumed only what was provided by the investigators. The three primary objectives of the study were to determine: 1) whether GxD interactions occur in this species; 2) whether such interactions extend beyond lipids to include glycemic and adiposity indices; and 3) whether the type of GxD observed interactions (i.e., extent of genetic influence vs. role of different genes) resembled those reported previously in either humans or baboons.
METHODS
Subjects
The subjects in this study were vervet/African green monkeys (Chlorocebus aethiops sabaeus) from the Vervet Research Colony (VRC). The VRC is a multi-generational, pedigreed and genotyped colony that was originally founded in 1975 at the Greater Los Angeles Department of Veterans Affairs with animals imported from the Caribbean (St. Kitts). Current colony members are all descendants of 57 founding animals introduced into the colony between 1975 and 1985. In early 2008, the Vervet Research Colony (VRC) was transferred from Los Angeles to the Wake Forest University Primate Center (WFUPC).
Housing
All subjects were housed in matrilineal breeding groups that occupied 16 identical, indoor-outdoor pens. Each pen consisted of a large outdoor area (~1200 square-feet) and a divided indoor area (300 square-feet). All pens were fitted with elevated perches, platforms, and climbing structures. Each breeding group contained 1–2 adult males along with varying numbers of adult females and immature offspring. The 16 groups contained between 11 and 36 animals (19.31±1.82) that had matured beyond the juvenile period (i.e., ≥ three years of age). In general, females remained in their natal groups. Males were removed at 4 years of age to prevent inbreeding and adult males were replaced every 3–5 years to maintain genetic diversity. Population size was controlled by vasectomizing males and periodic culling of animals.
Pregnancy status of adult females at each sampling period was determined by 1) ultrasound on the date of sampling and/or 2) if the animal gave birth within 165 days of the sampling date. Gestational age, approximately 165 days, was estimated by counting back from the date of birth. Pregnancy status (yes/mo) was accounted for in the genetic analyses.
Diet
Prior to 2004, the colony had been fed a standard primate diet (LabDiet 5038, Purina, St Louis MO). In 2004 this diet was replaced with a high-fiber, high-protein diet (LabDiet 5052) that was similarly low in fat and cholesterol [Fairbanks et al., 2010]. In January and February 2008 the colony was transferred to WFUPC and the diet was switched back to LabDiet 5038 (13% of calorie from fat, 18% from protein sources and 69% from carbohydrates ( ~42% from starches and ~ 5% from simple sugars) for an accommodation period of approximately 11 months. Then, as part of the diet challenge study described here, the diet was switched for six months to the TAD (LabDiet 5L0P). Following this, the animals again were fed LabDiet 5038. The challenge diet (TAD) was formulated to mimic the ‘typical’ American consumption deriving 37% of calories from fat, primarily saturated and monounsaturated fatty acids from animal fat, 18% from protein sources (mostly animal), 45% from carbohydrates (~20% starches, and ~19% simple sugars), and containing 0.18 mg/Kcal cholesterol. The diet formulations are described in Table 1. Complete diet formulas are provided in Supplementary Sheets 1 and 2. Throughout the study, all social groups were given environmental enrichment in the form of apples, oranges, or unflavored popcorn three times per week in quantities designed to provide a minimal percentage of the daily caloric intake. However, the caloric density of enrichment provided each day. All animals had ad libitum access to food, water, and opportunities to exercise. Prior observations in this population and in other species of Old World monkeys indicate that intra-animal variance of the measured phenotypes is relatively low in response to diets similar to those used here; this comparatively low intra-animal variance contrasts with often marked inter-individual variance observed in most primates, including humans [Shelton, Clarkson and Kaplan, 2012].
Table 1.
Diet formulations for the ‘standard’ diet and the challenge ‘high fat’ (TAD) diet
| Standard (Lab Diet 5038) | TAD (Lab Diet 5L0P) | |
|---|---|---|
| Fiber (Crude) | 5% | 9% |
| Calories by Protein | 18% | 18% |
| Calories by Fat | 13% | 37% |
| Calories by Carbohydrates | 69% | 45% |
| Metabolizable Energy (kcal/g) | 3.22 | 3.34 |
Experimental Procedures
All experimental procedures involving animals in this study were approved by the Institutional Animal Care and Use Committee of Wake Forest University, which is fully AAALAC accredited. This research adheres to the American Society of Primatologists principles for the ethical treatment of primates. Animals were sampled for cardiometabolic risk factors at two time points: 1) Baseline (11 months following their arrival at WFUPC and consuming LabDiet 5038); and 2) approximately 7–8 weeks after the animals began consuming the challenge (typical American) diet (LabDiet 5LOP). This time period was determined to be optimum for observing the effects of genotype by diet interaction on cardiometabolic factors based on a previous study by Mahaney et al [1999]. One or two entire social groups were sampled per day and each sampling period took place over 2–3 weeks. Consumption of the high fat challenge diet was staggered so that a similar amount of time elapsed between the start of the diet challenge and the collection of samples.
At each sampling period the animals were fasted overnight, captured and sedated with 15 mg/kg ketamine HCl intramuscularly. Animals were weighed and 6 ml of blood was collected via femoral venipuncture using EDTA vacutainers (BD Vacutainer®, Franklin Lakes, NJ). The blood samples were put on wet ice immediately after collection. After sampling was completed for the day the samples were transferred to the lab and samples were centrifuged for 25 minutes at 1,000Xg and aliquots of plasma were taken for lipid (600 μl) and glycemic (400 μl) assays. All samples were frozen at −20° C after initial processing and then transferred to −80° C freezer until assays were performed.
Lipoprotein cholesterol distributions were determined using size separations of lipoprotein classes via gel filtration chromatography [Garber et al., 2000]. Plasma glucose (Sigma-Aldrich, St. Louis, MO) and fructosamine (Roche Diagnostics, Mannheim, Germany) were assayed by enzymatic colorimetric methods. The inter-assay and intra-assay coefficients of variation (CV) % were <5% for glucose and <10% for fructosamine. Insulin concentrations were determined using ELISA (Mercodia, Uppsala, Sweden) with inter-assay and intra-assay CV being <10%.
Statistical Analyses
Mean differences were estimated by likelihood ratio tests implemented in the software package Sequential Oligogenic Linkage Analysis Routines (SOLAR) [Almasy and Blangero, 1998]. Mean differences were considered significant at an alpha < 0.05
Quantitative genetic analyses
Estimation of heritability
To estimate the genetic component influencing variation in phenotypes, heritability was estimated using age, sex, sex-specific age and age squared terms and pregnancy status as covariates. Quantitative genetic analyses of all phenotypes were conducted utilizing the maximum likelihood-based variance components decomposition method implemented in SOLAR (Almasy & Blangero, 1998). Accordingly, the total phenotypic variance (σ2P) was decomposed into its additive genetic (σ2G) and environmental (σ2E) components. The heritability (h2) of a phenotype refers to the portion of phenotypic variance that can be attributed to its additive genetic effects (h2 = σ2G/σ2P).
Genotype by diet interaction
To examine whether genetic influences on the selected phenotypes varied by diet, we conducted a GxD interaction analysis. The approach for genotype by diet (GxD) interaction is an extension of the variance components decomposition approach [Blangero and Almasy, 1997; Almasy & Blangero, 1998] and tests two hypotheses: 1) whether variance due to genetic factors was significantly different in response to the two diets (i.e., whether the genetic influence was the same); and 2) whether the genetic correlation between the phenotypes measured during the two diet periods differed significantly from 1.0 (i.e., whether different genes contributed to the heritability). Additive genetic variance was then modeled as the product of the genetic correlations between diets and diet-specific genetic standard deviations:
For Ω = Covariance between family members, Φ = Kinship coefficient between the two individuals, ρG(G1, G2) = Genetic correlation between the expression of the trait in animals on two different diets, σg1 = Genetic standard deviation of the trait on diet1, σg2 = Genetic standard deviation of the trait on diet 2, I = Identity matrix, σe2 = Environmental variance. For a GxD interaction to be significant the genetic correlation between the two diets should be significantly less than 1.0 and/or the genetic variance for the two diets should be significantly different from each other. That is, rejection of either hypothesis by itself or both is taken as evidence of significant GxD interaction. For reasons discussed above, the likelihood ratio test (LRT) in the former case is distributed as a 50:50 mixture of a point-mass at 0 and a chi-square with 1 degrees of freedom and in the latter case as a chi-square with 1 degree of freedom [Self and Liang, 1987].
RESULTS
Descriptive statistics
A total of 309 animals (males = 51, females =258) were included in these analyses. Each of these animals was measured under both diet conditions (first the standard primate diet and then following consumption of TAD). Younger animals (age less than or equal to 3 years) and/or animals measured under only one diet condition were excluded from the analyses. Male vervets in this breeding colony had higher body weight and were younger, on average, compared to female monkeys (Table 2). Although there was no perceptible increase in weight (p=0.87), considerable increases were found in plasma values of glucose, high-density lipoprotein (HDL-c), low-density lipoprotein (LDL-c), very low-density lipoprotein (VLDL-c) and total cholesterol with the change to the typical American diet (Table 3). Plasma insulin concentrations and triglycerides showed significant increase in females only. Distributions of HDL-c and LDL-c on the two diets, separated by sex and pregnancy status, are shown in Figure 1. There were 31 females pregnant during the standard period while there were 83 females pregnant during the TAD challenge. As Figure 1 indicates, the HDL-c concentrations were reduced in pregnant females, with the most pronounced effect evident for the TAD challenge.
Table 2.
Sample size, baseline age, baseline weight, weight change and percent of weight change.
| Sex | N | Baseline Age | Baseline Weight (kg) | Weight Change (kg) | Percent of Weight Change |
|---|---|---|---|---|---|
| Female | 258 | 9.80 ± 0.32 | 5.09 ± 0.05 | 0.01 ± 0.03 | 0.9% ± 0.6% |
| Male | 51 | 5.51 ± 0.28 | 6.44 ± 0.19 | −0.10 ± 0.54 | −0.2% ± 1.2% |
| Total | 309 | 9.09 ± 0.28 | 5.31 ± 0.06 | 0.00 ± 0.03 | 0.7% ± 0.5% |
Table 3.
Descriptive statistics of cardiometabolic phenotypes.
| Phenotype | Female1 | Male1 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Standard | TAD | P value2 | Standard | TAD | P value2 | |
| Girth (cm) | 33.68 (4.5) | 33.88 (4.2) | 0.62 | 33.35 (4.) | 31.57 (3.4) | 0.25 |
| Glucose (mg/dl) | 61.06 (19.1) | 69.10 (24.3) | < 0.001 | 68.73 (13.6) | 74.78 (14.1) | <0.005 |
| Fructosamine | 141.17 (25.9) | 149.89 (30.1) | < 0.001 | 150.47 (25.3) | 160.65 (19.4) | < 0.005 |
| Insulin (uU/ml) | 17.48 (21.8) | 23.80 (31.3) | <0.005 | 26.58 (21.2) | 21.26 (21.5) | NS |
| Triglyceride (mg/dl) | 35.25 (15.1) | 40.54 (18.9) | < 0.001 | 24.39 (8.2) | 19.99 (6.3) | <0.005 |
| HDL cholesterol (mg/dl) | 78.59 (20.1) | 90.15 (40.8) | < 0.0001 | 88.19 (19.1) | 117.07 (35.4) | <0.0001 |
| LDL cholesterol (mg/dl) | 58.53 (16.9) | 95.98 (32.02) | < 0.0001 | 45.65 (12.1) | 104.27 (42.0) | <0.0001 |
| VLDL cholesterol (mg/dl) | 1.30 (1.1) | 2.26 (1.5) | < 0.001 | 0.84 (0.7) | 2.09 (1.6) | <0.0001 |
| Total cholesterol (mg/dl) | 138.43 (25.4) | 188.41 (52.1) | < 0.0001 | 134.68 (21.7) | 223.56 (52.2) | <0.0001 |
| Totchol/HDL-c | 1.83 (0.4) | 2.56 (1.4) | < 0.0001 | 1.55 (0.2) | 2.14 (1.4) | < 0.005 |
| VLDLc_LDLc (mg/dl) | 59.82 (17.2) | 98.23 (32.65) | < 0.0001 | 46.49 (12.1) | 106.36 (42.8) | < 0.0001 |
Phenotype values are shown as mean (standard deviation)
p value for significant differences between cardiometabolic phenotypes on standard diet and TAD
Figure 1.
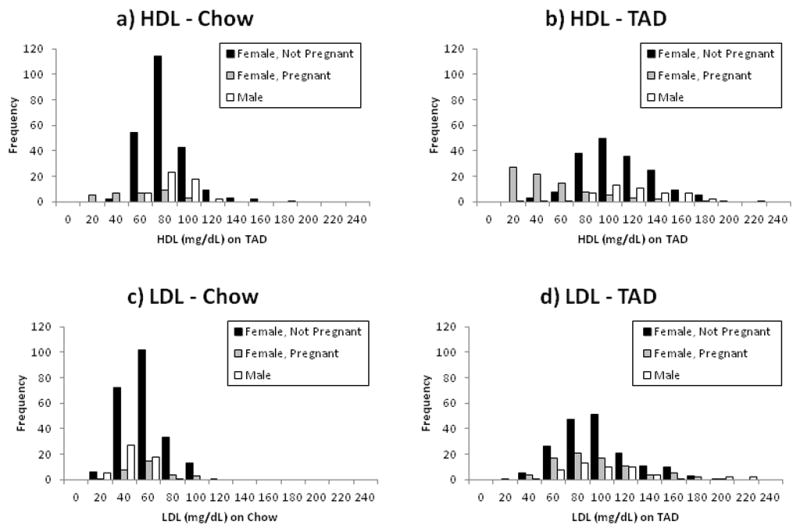
Frequency distribution of HDL-c (mg/dL) on a) chow and b) TAD.
Frequency distribution of LDL-c (mg/dL) on c) chow and d) TAD.
Heritabilities of cardiometabolic phenotypes
As can be seen in Table 4, all phenotypes measured in response to the TAD challenge were significantly heritable with the exception of glucose. In contrast, variation in fructosamine, HDL-c and VLDL-c cholesterol was not heritable in the same animals while consuming the standard primate diet. The mean individual differences in response to the diet change (TAD-standard) were also significantly heritable for morphometric and lipid phenotypes (Table 4). Since there were many more females than males in the colony, we also conducted female-specific heritability analyses. The results were similar to those in all animals, except for triglycerides whose heritabilities were not significant for standard diet or difference in diets in females (Table 5).
Table 4.
Heritabilities of cardiometabolic phenotypes
| Trait | Standard diet | TAD | Difference (TAD-standard) | |||
|---|---|---|---|---|---|---|
| h2 (SE) | P value | h2 (SE) | P value | h2 (SE) | P value | |
| Bodyweight | 0.83 (0.12) | < 0.0001 | 0.65 (0.13) | < 0.0001 | 0.56 (0.13) | < 0.0001 |
| Girth | 0.76 (0.14) | < 0.0001 | 0.57 (0.14) | < 0.0001 | 0.70 (0.14) | < 0.0001 |
| Glucose | 0.38 (0.17) | < 0.0001 | 0.04 (0.18) | 0.42 | 0.026 (0.10) | 0.43 |
| Fructosamine | 0.15 (0.16) | 0.28 | 0.25 (0.14) | < 0.05 | 0.098 (0.10) | 0.23 |
| Insulin | 0.61 (0.16) | < 0.0001 | 0.34 (0.15) | < 0.01 | 0.145 (0.14) | 0.07 |
| Triglycerides | 0.44 (0.21) | < 0.05 | 0.35 (0.16) | < 0.01 | 0.235 (0.16) | < 0.05 |
| Total cholesterol | 0.31 (0.14) | < 0.01 | 0.38 (0.13) | < 0.001 | 0.31 (0.14) | < 0.005 |
| HDL cholesterol | 0.17 (0.13) | 0.10 | 0.47 (0.14) | < 0.0001 | 0.40 (0.13) | < 0.0001 |
| LDL cholesterol | 0.46 (.15) | < 0.0001 | 0.30 (0.15) | < 0.05 | 0.135(0.15) | 0.11 |
| VLDL cholesterol | 0.36 (0.23) | 0.10 | 0.50 (0.20) | <0.005 | 0.50 (0.21) | < 0.0001 |
| Totchol/HDL-c | 0.31 (0.15) | < 0.05 | 0.40 (0.16) | < 0.005 | 0.39 (0.15) | < 0.0001 |
| VLDL-c_LDL-c | 0.46 (0.15) | < 0.001 | 0.29 (0.15) | < 0.05 | 0.138 (0.15) | 0.11 |
Table 5.
Sex-specific heritabilities (only females 4 years or older)
| Trait | Standard diet | TAD | Difference (TAD-standard) | |||
|---|---|---|---|---|---|---|
| h2 (SE) | P value | h2 (SE) | P value | h2 (SE) | P value | |
| Bodyweight | 0.80 (0.14) | < 0.0001 | 0.69 (0.15) | < 0.0001 | 0.37 (0.18) | < 0.05 |
| Girth | 0.81 (0.15) | < 0.0001 | 0.60 (0.16) | < 0.0001 | 0.62 (0.19) | <0.005 |
| Glucose | 0.38 (0.19) | < 0.05 | 0.13 (0.10) | 0.05 | 0.05 (0.16) | 0.50 |
| Fructosamine | 0.38 (0.16) | < 0.005 | 0.26 (0.17) | 0.05 | 0.065 (0.14) | 0.32 |
| Insulin | 0.65 (0.17) | < 0.0001 | 0.37 (0.19) | < 0.05 | 0.22 (0.13) | < 0.05 |
| Triglycerides | 0.31 (0.26) | 0.23 | 0.38 (0.18) | < 0.05 | 0.20 (0.16) | 0.07 |
| Total cholesterol | 0.33 (0.15) | < 0.01 | 0.44 (0.15) | < 0.0001 | 0.35 (0.14) | < 0.005 |
| HDL cholesterol | 0.14 (0.14) | 0.08 | 0.51 (0.15) | < 0.0001 | 0.48 (0.13) | < 0.0001 |
| LDL cholesterol | 0.52 (0.18) | < 0.001 | 0.34 (0.16) | < 0.01 | 0.11 (0.14)cd | 0.18 |
| VLDL cholesterol | 0.28 (0.23) | 0.09 | 0.38 (0.19) | < 0.05 | 0.45 (0.18) | < 0.005 |
| Totchol/HDL-c | 0.29 (0.17) | < 0.05 | 0.35 (0.16) | < 0.01 | 0.43 (0.18) | < 0.001 |
| VLDL-c_LDL-c | 0.52 (0.18) | < 0.005 | 0.33 (0.16) | < 0.01 | 0.11 (0.13) | 0.17 |
Genotype by diet (GxD) interaction
In evaluating GxD interaction, we tested a model with a null hypothesis of no interaction against a model where there was interaction. There were two types of interaction models. The first one tested the hypothesis that the variances due to the genetic effects in the phenotypes in the two diets were significantly different from each other. In our study we found genetic variances of triglycerides, HDL-c, VLDL-c and total cholesterol and ratio of total cholesterol to HDL-c were significantly different between the two diets (Table 6). The second hypothesis that we tested was that the genetic correlation between the phenotypes under the two diets is significantly different from 1.0. Genetic correlations between the two diets were significantly different from 1.0 for body weight and plasma concentrations of insulin, triglycerides, total, HDL-c and LDL-c cholesterol and the ratio of total cholesterol to HDL-c (Table 6).
Table 6.
Summary of the Genotype by diet interaction for cardiometabolic phenotypes
| Trait | σGd11 = σGd22 | ρG3(d1,d2) = 1 | ρG different from zero4 | ρG different from one5 |
|---|---|---|---|---|
|
| ||||
| P value | RhoG (SE) | P value | ||
| Body weight | 0.16 | 0.899 (0.04) | < 0.0001 | < 0.0005 |
| Glucose | 0.22 | 0.994 (0.20) | < 0.005 | 0.48 |
| Fructosamine | 0.98 | 0.814 (0.18) | < 0.05 | 0.46 |
| Insulin | 0.13 | 0.597 (0.17) | < 0.005 | < 0.005 |
| Triglyceride | < 0.0001 | 0.381 (0.26) | 0.227 | < 0.005 |
| Total cholesterol | < 0.0001 | 0.478 (0.22) | 0.053 | < 0.01 |
| HDL cholesterol | < 0.0001 | 0.344 (0.20) | 0.117 | < 0.0005 |
| LDL cholesterol | 0.08 | 0.823 (0.19) | < 0.005 | 0.35 |
| VLDL cholesterol | < 0.0001 | −0.064 (0.59) | 0.913 | 0.12 |
| VLDL-c-LDL-c | 0.09 | 0.847 (0.19) | < 0.005 | 0.41 |
| Total Chol/HDL-c | < 0.0001 | 0.616 (0.16) | < 0.005 | < 0.005 |
ρGd1: estimate for genetic variance on the standard primate diet
ρGd2: estimate for genetic variance on the challenge diet
ρG: estimate for genetic correlation
ρG different from zero indicates a significant genetic correlation between the two diet
ρG different from one indicates a significant GxD interaction between the two diets
DISCUSSION
A principal finding of this study was that significant increases in cardiometabolic disease risk factors occurred following a change from a standard primate diet (low fat and cholesterol) to a diet modeled on the typical American consumption (the TAD), high fat and cholesterol). This change occurred in the absence of any apparent gain in body weight over the relatively short period of this challenge (eight weeks). In addition, we also found a significant GxD interaction for these phenotypes showing that shared genetic factors might be regulating the variation in these phenotypes under the different diets and the response to diet changes.
In humans and animal models, changes in diet or eating patterns are believed to affect the risk for metabolic disorders. For example, some studies have shown adverse metabolic effects of increasing the fat in one’s diet [Lovejoy et al., 1998; Higgins et al., 2010; McCurdy et al., 2009; Sullivan et al., 2010; Coate et al., 2010; Rosenkranz et al., 2010], while other studies have shown the beneficial effects of either decreasing the fat content [Lane et al., 1999; Astrup et al., 2008] and/or changing the type of fat [Rudel et al., 1995; Mozaffarian et al., 2010] on metabolic disease risk. However, it should also be noted that durations of these diet challenges were considerably different in each study (ranging between 3weeks and 1 year and 8 weeks and 4 years in humans and nonhuman primates, respectively). In the current study in vervets, the change in diet did not result in an increase in body weight. However, the diet did result in a shift to more adverse lipid and glycemic profile. We observed significant increases in most lipid fractions, glucose and fructosamine, with insulin concentrations and triglycerides showing the shift only in females. Like baboons and rhesus monkeys, vervets develop obesity spontaneously and are known to develop adverse cardiovascular profile with dietary manipulations [Kavanagh et al., 2007a; Shelton, Clarkson and Kaplan, 2012]. As far as other non-human primates are concerned, not all species reacted in a similar fashion to comparable dietary challenges. A high-fat diet given to pregnant Japanese monkeys (Macaca fuscata sp.) resulted in weight gain and obesity [McCurdy et al., 2009]. However, in rhesus monkeys (Macaca mulatta sp.), a high fat diet did not result in weight gain but did result in increased concentrations of insulin and growth hormone [Schwartz et al., 1988]. In a study conducted in baboons (Papio hamadrayas sp), exposure to a high fat/high sugar diet for eight weeks did not elicit a significant increase in body weight but was effective in increasing fat mass, triglycerides, leptin and HbA1C [Higgins et al., 2010]. Increased intake of simple carbohydrates has been shown to increase triglycerides and general adiposity. In this study there was a considerable increase in simple carbohydrates (from 5% to 19%) in the TAD. Although, increase in triglycerides may also be due to the increase in saturated fats, simple carbohydrates have been shown to be more triglyceridemic than complex carbohydrates [Kritchevsky et al., 1974; Minehira et al., 2003; Adochio et al., 2009; Higgins et al., 2010].
In a study in cynomolgus monkeys (imported as adults from Malaysia and Philippine islands), a high fat diet resulted in significant increase in both HDL-c and total cholesterol [Kaplan et al., 1991]. A high fat atherogenic diet was also responsible for the development of myocardial lesions in rhesus and cynomolgus (Malaya and Philippines) monkeys [Bond et al., 1980]. In a previous study in vervets, animals fed trans-fat gained body weight with concomitant increase in intra-abdominal fat and fructosamine concentrations [Kavanagh et al., 2007b]. All these studies showed that a variety of Old World nonhuman primates exhibit similar changes in metabolic risk as do humans in response to diets high in fats, simple sugars, and cholesterol and therefore indicating their usefulness for investigating human metabolic diseases through dietary interventions.
To assess the genetic contribution to differences in response to diet, we conducted genotype by diet interaction analyses. These analyses revealed that there are substantial differences in the additive genetic variances among the studied phenotypes between the two diets. As a first step in these analyses, we estimated heritabilities for metabolic traits under both diets. The heritability estimates ranged from 0.15 to 0.83 which are in the same range as those reported by previously by Kavanagh et al. [2007a] in vervets and Comuzzie et al.[2003] and Cai et al. [2004] in baboons. Significant differences in the additive genetic variances for lipid traits showed that even though a common set of genes influence them under the two diets, the magnitude or the intensity of the effect is different between them. The genetic correlations between the two diets for insulin, weight and lipid related traits being significantly different from 1.0 implied that distinct genetic effects are involved in the regulation of these traits when the diets were switched. Maheney et al [1998] have previously reported significant GxD interaction effects, in baboons (Papio hamadrayas sp.), on HDL-c and LDL-c responses to a high-cholesterol, high saturated effects. Our results replicated the GxD effects on lipids and extended these results to weight and insulin phenotypes in another non-human primate model.
Studies investigating effects of high-fat diets on metabolic risk factors in humans have shown similar results. In a study conducted by Rosenkranz et al.[2010], a high-fat meal resulted in higher concentrations of triglycerides and total cholesterol in healthy men and women. A high fat diet also resulted in a reduction in insulin sensitivity and an increase in leptin concentrations in African American women in a controlled diet study [Lovejoy et al., 1998]. Human studies have also shown similar heritability estimates for body weight, lipids and glycemic-related traits [Comuzzie et al., 1995; Voruganti et al., 2006; Comuzzie et al., 2010]. Similarly, they have also shown that not all individuals respond in a similar fashion to changes in diet and that genetic factors seem to modulate these responses [Lairon et al., 2009; Johnson et al., 2009; Hasselbalch, 2010]. In a dietary intervention study in 169 participants, where participants were switched from a typical western diet to a Mediterranean diet, significant evidence of interaction was shown between gene polymorphisms and metabolic responses to Mediterranean diet [Lairon et al., 2009]. A study conducted in children [Dreon et al., 2000] who switched from their usual diet to a low-fat (10%) high carbohydrate diet showed a differential lipoprotein response based on their parents genotypes with the shift in the diet. In an earlier study in premenopausal women, Dreon et al [1997] showed that genetic factors underlying small, dense LDL-c particles may have an effect on LDL-c and triglyceride response when their diets are shifted from predominant fats to carbohydrates.
CONCLUSIONS
i) we show for the first time that the entire multigenerational, pedigreed and genotyped vervet colony, when fed a human-like diet, exhibits significant effects of GxD interactions on cardiometabolic risk factors which extend, beyond lipids, to weight and insulin phenotypes; and ii) these results not only replicated the suspected effect of genes on diet responses in humans, but also the GxD interaction effects on lipids reported in baboons.
The knowledge gained here offers important insights into the differential genetic effects of the two (standard and TAD) diets on metabolic responses. Thus, future studies aimed at understanding how GxD interaction modulates cardiometabolic response should mimic human nutritional environment. Doing otherwise could mask or cause misidentification of genetic contributions.
Supplementary Material
Acknowledgments
The study was supported by National Institute of Health grant RR019963//OD010965 for the VRC as Biomedical Resource and statistical analyses were supported by the National Institutes of Health grant MH59490.
List of abbreviations
- GxD
Genotype by diet interaction
- TAD
Typical American Diet
- HDLc
High density lipoprotein cholesterol
- VLDL-c
Very low density lipoprotein cholesterol
- LDL-c
low-density lipoprotein cholesterol
Footnotes
AUTHOR CONTRIBUTIONS
VSV performed statistical analyses and wrote the manuscript. MJJ participated in data collection and helped with data management and statistical analysis. Manuscript edits were performed by MJJ, JRK, KK, LLR, RT, LAF and AGC. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST: None
References
- Adochio RL, Leitner JW, Gray K, Draznin B, Cornier MA. Early response of insulin signaling to high- carbohydrate and high-fat overfeeding. Nutrition and Metabolism. 2009;6:37. doi: 10.1186/1743-7075-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. American Journal of Human Genetics. 1998;62:1198–1121. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya R, Dyer TD, Warren DM, et al. Effect of genotypes x alcoholism interaction on linkage analysis of an alcoholism-related quantitative phenotype. Bio Med Central Genetics. 2005;6(Suppl 1):S120. doi: 10.1186/1471-2156-6-S1-S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A, Dyerberg J, Selleck M, Stender S. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obesity Reviews. 2008;9(Suppl1):48–52. doi: 10.1111/j.1467-789X.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- Blangero J, Almasy L. Multipoint oligogenic linkage analysis of quantitative traits. Genetic Epidemiology. 1997;14:959–964. doi: 10.1002/(SICI)1098-2272(1997)14:6<959::AID-GEPI66>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bodkin NL, Hannah JS, Ortmeyer HK, Hansen BC. Central obesity in rhesus monkeys: association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? International Journal of Obesity. 1993;17:53–61. [PubMed] [Google Scholar]
- Bond MG, Bullock BC, Bellinger DA, Hamm TE. Myocardial infarction in a large colony of nonhuman primates with coronary artery atherosclerosis. American Journal of Pathology. 1980;101:675–692. [PMC free article] [PubMed] [Google Scholar]
- Caldwell D, Dunn C, Keene A, et al. Eat Smart, Move more: North Carolina’s Plan to Prevent Overweight, Obesity, and Related Chronic Disease. Eat Smart Move More Leadership Team; Raleigh, NC: 2006. http://www.eatsmartmovemorenc.com/ESMMPlan/Texts/ESMMPlan_Desktop.pdf. [Google Scholar]
- Cai G, Cole SA, Tejero ME, et al. Pleiotropic effects of genes for insulin resistance on adiposity in baboons. Obesity Research. 2004;12:1766–1772. doi: 10.1038/oby.2004.219. [DOI] [PubMed] [Google Scholar]
- Cann JA, Kavanagh K, Jorgensen MJ, et al. Clinicopathologic characterization of naturally occurring diabetes mellitus in vervet monkeys. Veterinary Pathology. 2010;47:713–8. doi: 10.1177/0300985810370011. [DOI] [PubMed] [Google Scholar]
- Coate KC, Scott M, Farmer B, et al. Chronic consumption of a high-fat/high-fructose diet renders the liver incapable of net hepatic glucose uptake. American Journal of Physiology-Endocrinology and Metabolism. 2010;299:E887–98. doi: 10.1152/ajpendo.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comuzzie AG, Blangero J, Mahaney MC, et al. Major gene with sex-specific effects influences fat mass in Mexican Americans. Genetic Epidemiology. 1995;12:475–88. doi: 10.1002/gepi.1370120505. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Cole SA, Martin L, et al. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obesity Research. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Higgins PB, Voruganti S, Cole S. Cutting the fat: the genetic dissection of body weight. Prog Mol Biol Transl Sci. 2010;94:197–212. doi: 10.1016/s1877-1173(10)94007-6. [DOI] [PubMed] [Google Scholar]
- Czerwinski SA, Mahaney MC, Rainwater DL, et al. Gene by smoking interaction: evidence of effects on low-density lipoprotein size and plasma levels of triglyceride and high-density lipoprotein cholesterol. Human Biology. 2004;76(6):863–876. doi: 10.1353/hub.2005.0014. [DOI] [PubMed] [Google Scholar]
- Diego VP, Rainwater DL, Wang XL, et al. Genotype x adiposity interaction linkage analyses reveal a locus on chromosome 1 for lipoprotein-associated phospholipase A2, a marker of inflammation and oxidative stress. American Journal of Human Genetics. 2007;80(1):168–177. doi: 10.1086/510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreon DM, Fernstrom HA, Williams PT, Krauss RM. LDL subclass patterns and lipoprotein response to a low-fat, high-carbohydrate diet in women. Arteriosclerosis Thrombosis Vascular Biology. 1997;17:707–714. doi: 10.1161/01.atv.17.4.707. [DOI] [PubMed] [Google Scholar]
- Dreon DM, Fernstrom HA, Williams PT, Krauss RM. Reduced LDL particle size in children consuming a very low-fat diet is related to parental LDL-subclass patterns. American Journal of Clinical Nutrition. 2000;71:1611–1616. doi: 10.1093/ajcn/71.6.1611. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Blau K, Jorgensen MJ. High-fiber diet promotes weight loss and affects maternal behavior in vervet monkeys. American Journal of Primatology. 2010;72:234–241. doi: 10.1002/ajp.20772. [DOI] [PubMed] [Google Scholar]
- Fujimoto WY, Bergstrom RW, Boyko EJ, et al. Preventing diabetes-applying pathophysiological and epidemiological evidence. British Journal of Nutrition. 2000;84(S2):S173–176. doi: 10.1079/096582197388635. [DOI] [PubMed] [Google Scholar]
- Garber DW, Kulkarni KR, Anantharamaiah GM. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. Journal of Lipid Research. 2000;41:1020–1026. [PubMed] [Google Scholar]
- Hasselbalch AL. Genetics of dietary habits and obesity – a twin study. Danish Medical Bulletin. 2010;57:B4182. [PubMed] [Google Scholar]
- Higgins PB, Bastarrachea RA, Lopez-Alvarenga JC, et al. Eight week exposure to a high sugar high fat diet results in adiposity gain and alterations in metabolic biomarkers in baboons (Papio hamadryas sp.) Cardiovascular Diabetology. 2010;29;9:71. doi: 10.1186/1475-2840-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen KL, Hansen BC, Metzger BL. Adiposity, anthropometric measures, and plasma insulin levels of rhesus monkeys. International Journal of Obesity. 1985;9:213–224. [PubMed] [Google Scholar]
- Johnson L, van Jaarsveld CH, Emmett PM, et al. Dietary energy density affects fat mass in early adolescence and is not modified by FTO variants. PLoS One. 2009;4:e594. doi: 10.1371/journal.pone.0004594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Shively C. The effects of fat and cholesterol on social behavior on monkeys. Psychosomatic Medicine. 1991;53:634–642. doi: 10.1097/00006842-199111000-00005. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Fairbanks LA, Bailey JN, et al. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity. 2007a;15:1666–1674. doi: 10.1038/oby.2007.199. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Jones KL, Sawyer J, et al. Trans fat diet induce abdominal obesity and changes in insulin sensitivity in monkeys. Obesity. 2007b;15:1675–1684. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- Krauss RM. Atherogenic lipoprotein phenotype and diet-gene interactions. Journal of Nutrition. 2001;131:340S–343S. doi: 10.1093/jn/131.2.340S. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D, Davidson LM, Shapiro IL, et al. Lipid metabolism and experimental atherosclerosis in baboons: Influence of cholesterol-free, semi-synthetic diets. American Journal of Clinical Nutrition. 1974;27:29–50. doi: 10.1093/ajcn/27.1.29. [DOI] [PubMed] [Google Scholar]
- Krauss RM. Dietary and genetic probes of atherogenic dyslipidemia. Arteriosclerosis Thrombosis Vascular Biology. 2005;25:2265–2272. doi: 10.1161/01.ATV.0000186365.73973.f0. [DOI] [PubMed] [Google Scholar]
- Lairon D, Defoort C, Martin JC, et al. Nutrigenetics: links between genetic background and response to Mediterranean-type diets. Public Health Nutrition. 2009;12:1601–1606. doi: 10.1017/S1368980009990437. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk. Toxicological Sciences. 1999;52(S2):41–48. doi: 10.1093/toxsci/52.2.41. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Windhauser MM, Rood JC, de la Bretonne JA. Effect of a controlled high-fat versus low-fat diet on insulin sensitivity and leptin levels in African-American and Caucasian women. Metabolism. 1998;47:1520–1524. doi: 10.1016/s0026-0495(98)90080-4. [DOI] [PubMed] [Google Scholar]
- Mahaney MC, Blangero J, Rainwater DL, et al. Pleiotropy and genotype by diet interaction in a baboon model for atherosclerosis: a multivariate quantitative genetic analysis of HDL subfractions in two dietary environments. Arteriosclerosis Thrombosis Vascular Biology. 1999;19:1134–1141. doi: 10.1161/01.atv.19.4.1134. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Mahaney MC, Almasy L, et al. Leptin’s sexual dimorphism results from genotype by sex interactions medicated by testosterone. Obesity Research. 2002;10:14–21. doi: 10.1038/oby.2002.3. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Kissebah AH, Sonnenberg GE, Blangero J, Comuzzie AG Metabolic Risk Complications of Obesity Genes Project. Genotype-by-smoking interaction for leptin levels in the Metabolic Risk Complications of Obesity Genes project. International Journal of Obesity Related Metabolic Disorders. 2003;27(3):334–340. doi: 10.1038/sj.ijo.0802232. [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. Journal of Clinical Investigation. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill HC, Jr, McMahan CA, Kruski AW, Kelley JL, Mott GE. Responses of serum lipoproteins to dietary cholesterol and type of fat in the baboon. Arteriosclerosis. 1981;1:337–344. doi: 10.1161/01.atv.1.5.337. [DOI] [PubMed] [Google Scholar]
- Minehira K, Bettschart V, Vidal H, et al. Effect of carbohydrate overfeeding on whole body and adipose tissue metabolism in humans. Obesity Research. 2003;11:1096–1103. doi: 10.1038/oby.2003.150. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Medicine. 2010;23;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas JM, Shen J. Gene-environment interactions and susceptibility to metabolic syndrome and other chronic disease. Journal of Periodontology. 2008;79:1508–1513. doi: 10.1902/jop.2008.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston WS, 2nd, Foreyt JPO. Obesity is an environmental issue. Atherosclerosis. 1999;146:201–209. doi: 10.1016/s0021-9150(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Puppala S, Arya R, Thameem F, et al. Genotype by diabetes interaction effects on the detection of linkage of glomerular filtration rate to a region on chromosome 2q in Mexican Americans. Diabetes. 2007;56(11):2818–2828. doi: 10.2337/db06-0984. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Applied Physiology. 2005;98:3–30. doi: 10.1152/japplphysiol.00852.2004. [DOI] [PubMed] [Google Scholar]
- Rosenkranz SK, Townsend DK, Steffens SE, Harms CA. Effects of a high-fat meal on pulmonary function in health subjects. European Journal of Applied Physiology. 2010;109:499–506. doi: 10.1007/s00421-010-1390-1. [DOI] [PubMed] [Google Scholar]
- Rudel LL, Parks JS, Sawyer JK. Compared with dietary monounsaturated and saturated fat, polyunsaturated fat protects African green monkeys from coronary artery atherosclerosis. Arteriosclerosis Thrombosis Vascular Biology. 1995;15:2101–2110. doi: 10.1161/01.atv.15.12.2101. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Westerterp KR. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr. 2000;84:417–427. doi: 10.1017/s0007114500001720. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Wilson ME, Walker ML, Collins DC. Dietary influences on growth and sexual maturation in premenarchial rhesus monkeys. Hormones and Behavior. 1988;22:231–251. doi: 10.1016/0018-506x(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Self S, Liang K. Asymptotic properties of maximum likelihood-ratio tests under nonstandard conditions. Journal of American Statistical Association. 1987;82:605–610. [Google Scholar]
- Shelton KA, Clarkson TB, Kaplan JR. Nonhuman primate models of atherosclerosis. In: Abee CR, Mansfield K, Tardif S, Morris T, editors. Nonhuman primates in biomedical research, vol 2: diseases. 2. Boston: Elsevier Academic Press; 2012. pp. 385–411. (American college of laboratory animal medicine series) [Google Scholar]
- Slomko H, Heo HJ, Einstein FH. Minireview: Epigenetics of obesity and diabetes in humans. Endocrinology. 2012;153(3):1025. doi: 10.1210/en.2011-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. Journal of Neuroscience. 2010;30:3826–30. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Yamamoto D, Suzuki T, Fujii M, Suzuki N, Fujishiro M, Sakurai T, Yamada K. High fat and high fructose diet induced intracranial atherosclerosis and enhanced vasoconstrictor responses in non-human primate. Life Sciences. 2006;80:200–204. doi: 10.1016/j.lfs.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Temelkova-Kurktschiev T, Stefanov T. Lifestyle and genetics in obesity and type 2 diabetes. Experimental and Clinical Endocrinology and Diabetes. 2012;120(01):1–6. doi: 10.1055/s-0031-1285832. [DOI] [PubMed] [Google Scholar]
- Voruganti VS, Göring HH, Diego VP, et al. Genome-wide scan for serum ghrelin detects linkage on chromosome 1p36 in Hispanic children: results from the Viva La Familia study. Pediatric Research. 2007;62:445–50. doi: 10.1203/PDR.0b013e31813cbf02. [DOI] [PubMed] [Google Scholar]
- Voruganti VS, Diego VP, Haack K, et al. A QTL for genotype by sex interaction for anthropometric measurements in Alaskan Eskimos (GOCADAN Study) on chromosome 19q12-q13. Obesity. 2011;19:1840–1846. doi: 10.1038/oby.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JD, Jorgensen MJ, Cline JM, Lees CJ, Franke AA, Zhang L, Ayers MR, Schultz C, Kaplan JR. Effects of soy vs. casein protein on body weight and glycemic control in female monkeys and their offspring. American Journal of Primatology. 2009;71:802–811. doi: 10.1002/ajp.20716. [DOI] [PubMed] [Google Scholar]
- Weinsier RL, Hunter GR, Heini AF, Goran MI, Sell SM. The etiology of obesity: relative contribution of metabolic factors, diet and physical activity. American Journal of Medicine. 1998;105:145–150. doi: 10.1016/s0002-9343(98)00190-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


