Abstract
BACKGROUND
How Rh immune globulin (RhIG) prevents sensitization to D antigen is unclear. If RhIG Fc delivers a nonspecific immunosuppressive signal, then RhIG may inhibit sensitization to antigens other than D. HLA antibody prevalence was compared in previously pregnant RhD negative versus RhD positive women to investigate whether RhIG suppresses HLA sensitization.
STUDY DESIGN AND METHODS
In the Leukocyte Antibody Prevalence Study (LAPS)1, 7,920 volunteer blood donors were screened for anti-HLA antibodies and surveyed about prior pregnancies and transfusions. A secondary analysis of the LAPS database was performed.
RESULTS
RhD negative women ≤40 years old (presumed to have received antenatal ± postpartum RhIG in all pregnancies) had a significantly lower HLA sensitization rate than RhD positive women (RR 0.58, 95% CI 0.40–0.83). When stratified by deliveries (1, 2, 3, or ≥4), RhD negative women ≤40 were HLA sensitized less often than RhD positive women in every case. In contrast, a clear relationship between RhD type and HLA sensitization was not seen in older previously pregnant women whose childbearing years are presumed to have preceded the use of routine RhIG prophylaxis. In a multivariable logistic regression model, RhD negative women ≤40 years old remained significantly less likely to be HLA sensitized compared with RhD positive women after adjusting for parity, time from last pregnancy, lost pregnancies, and transfusions (OR 0.55, 95% CI 0.34–0.88).
CONCLUSION
Consistent with a nonspecific immunosuppressive effect of RhIG, younger previously pregnant RhD negative women were less likely than previously pregnant RhD positive women to be HLA sensitized.
Keywords: Rh immune globulin (RhIG), HLA sensitization
INTRODUCTION
During pregnancy, foreign antigens expressed by the fetus commonly sensitize the mother. The platelet surface antigen HPA-1a, implicated in most cases of neonatal alloimmune thrombocytopenia, provokes an antibody response in about 12% of HPA-1A-negative women.2 Before Rh immune globulin (RhIG) was introduced, ~17% of RhD negative women became sensitized to D antigen following a single pregnancy with an RhD positive fetus.3 But the fetal antigens that most often trigger maternal sensitization are HLA antigens. Over 24% of previously pregnant women have detectable circulating IgG antibodies against HLA.1 HLA antibodies are associated with transfusion-related acute lung injury (TRALI),4 organ rejection,5 and platelet refractoriness.6
Within the placenta, the fetal and maternal blood pools normally come into very close proximity without intermixing. As a result, immunogenic fetal antigens such as D and HLA are usually inaccessible to the mother’s immune system. Paternally-derived HLA is expressed on fetal leukocytes and other fetal cells, however no detectable HLA Class I or II is expressed on chorionic villi.7 Maternal sensitization can result when blood from the fetus and mother combine following disruption of the placental architecture. Most often this happens at delivery, although fetomaternal hemorrhage (FMH) followed by sensitization can occur earlier in gestation. In a prospective observational study of 256 pregnant women, Regan and colleagues demonstrated that anti-HLA antibodies rarely appear before week 28 of pregnancy. Most anti-HLA becomes detectable during the third trimester or post-delivery.8 Thus, the timing of HLA sensitization essentially tracks with the timing of detectable FMH.9 The frequency of both FMH and HLA antibody formation in pregnancy roughly correlates with the volume of the fetoplacental blood pool, which expands from about 25 mL at 20 weeks-gestation to 400 mL at term.7
Despite being used successfully for decades, the mechanism by which RhIG prevents sensitization to D antigen remains unclear. Four main hypotheses have been proposed: clearance of RhD positive RBCs before sensitization can take place10,11, antibody mediated immune suppression (AMIS),12 anti-idiotype networks,13 and steric masking.14 The evidence for and against these various mechanisms has been reviewed.15,16 To date, neither human studies nor animal models have provided much insight into how RhIG actually works. Recently, Ravetch and colleagues proposed a new model of how intravenous immunoglobulin (IVIG) might suppress inflammation in autoimmune disease, based on an elegant series of experiments in transgenic mice. Their data suggest that most of IVIG’s therapeutic effects may be attributable to a minor population of IgG molecules bearing Fc glycans that terminate in sialic acid residues. These sialylated IgGs uniquely bind via their Fc domains to the receptor DC-SIGN on macrophages or dendritic cells. This triggers local release of cytokines IL-33 and IL-4, and inhibitory signaling through FcγRIIB ultimately leads to an immunosuppressive state.17,18 We postulated that RhIG might act through a similar Fc-dependent inhibitory mechanism. If RhIG prevents anti-D formation solely by clearing D+ RBCs from the circulation, then we would predict that administering RhIG would essentially have no effect on HLA sensitization during pregnancy. Alternatively, if RhIG acts through a mechanism that is similar to sialylated IVIG, and RhIG Fc delivers a local nonspecific immunosuppressive signal, then we would predict that RhIG would suppress not only anti-D, but also antibody responses to other foreign antigens present at the same time as D, such as HLA. Thus, we hypothesized that previously pregnant RhD negative women (presumed to have received RhIG) would have a lower rate of HLA sensitization than previously pregnant RhD positive women (presumed not to have received RhIG.)
To investigate this hypothesis, we took advantage of a large dataset that had been assembled previously for the REDS-II Leukocyte Antibody Prevalence Study (LAPS).1 LAPS was a study of volunteer blood donors aimed at defining risk factors for HLA sensitization to help inform TRALI prevention strategies. 7,920 donors, including 3,983 previously pregnant women, were screened for HLA Class I and II antibodies by multiantigen bead flow. We conducted a secondary analysis of the LAPS database to examine whether the HLA sensitization risk in previously pregnant women varied by RhD type, as a surrogate for prior RhIG prophylaxis.
MATERIALS AND METHODS
LAPS was a cross-sectional multicenter study of volunteer blood donors conducted by the REDS-II group at six U.S. sites. In LAPS, pregnancy and transfusion histories were obtained by questionnaire, and HLA Class I and II antibody screening was performed on blood samples at a central laboratory using multiantigen bead flow as described previously.1 For this study, the LAPS database was reanalyzed to investigate whether a relationship existed between RhD type and HLA sensitization risk among previously pregnant LAPS subjects.
Currently in the U.S., virtually all RhD negative women receive prophylactic antenatal RhIG (300 mcg) at or around week 28 of pregnancy. After delivery, if the baby types as RhD positive, the mother receives an additional 300 mcg dose of RhIG (more for a large FMH). If the baby types as RhD negative, no postpartum RhIG is administered to the mother. The percentage of RhD negative women expected to deliver an RhD positive baby is calculated as: 35% DD + (½) 50% Dd + (0) 15% dd = 60%.11 That is, assuming a White population, the baby’s father will be homozygous for RhD 35% of the time and heterozygous for RhD 50% of the time, so the baby will be RhD positive (and therefore the mother will receive postpartum RhIG) in 35% + 25% = 60% of RhD negative pregnancies. Over 90% of LAPS subjects were White.1 RhIG was licensed in the U.S. in 1968. Before 1968, RhD negative mothers would not have received any RhIG prophylaxis. In the period from 1968 through 1983, only postpartum RhIG was administered to at-risk RhD negative women. Beginning in 1984, an additional antenatal (week 28) dose of RhIG was recommended by the American Congress of Obstetricians and Gynecologists (ACOG) for routine prophylaxis.19 In summary, whether a previously pregnant RhD negative LAPS subject received no RhIG, postpartum RhIG only, or antenatal RhIG plus postpartum RhIG depended largely on the year of pregnancy (Fig. 1). For each LAPS subject, the age at LAPS enrollment was known, as were the RhD type, total number of pregnancies and deliveries, and the date of the last pregnancy. Dates of earlier pregnancies and data on RhIG administration were not available. Therefore, our analysis was based on subject age at LAPS enrollment as described below.
Figure 1. Timeline of RhIG prophylaxis in the U.S.
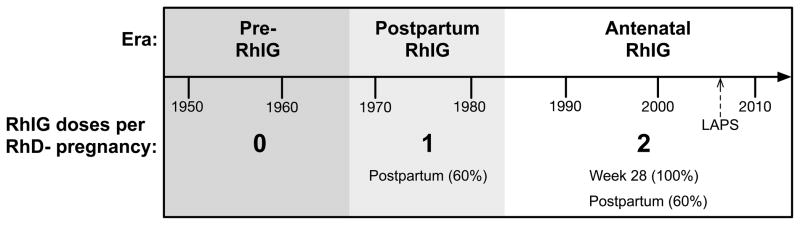
Postpartum RhIG to prevent D sensitization was first licensed in the U.S. in 1968. In 1984, antenatal (week 28) RhIG prophylaxis was first recommended in the U.S. for all RhD negative pregnancies. RhD negative women who were pregnant during the Pre-RhIG era (dark gray) are assumed not to have received RhIG prophylaxis. RhD negative women who were pregnant during the Postpartum RhIG era (light gray) are assumed to have received a postpartum dose of RhIG in the approximately 60% of pregnancies in which the baby would have tested RhD positive. RhD negative women who were pregnant during the Antenatal RhIG era (white) are assumed to have received antenatal RhIG in 100% of pregnancies, and an additional postpartum RhIG dose in the approximately 60% of pregnancies in which the baby would have tested RhD positive.
Previously pregnant female LAPS subjects were grouped into one of five age cohorts: ≤40, 41–50, 51–60, 61–70, and ≥71 years old. Women 41–50 years old at LAPS enrollment in 2006–7 would have been would have been ≤12 years old (i.e. likely nulliparous) in 1968 when postpartum RhIG was first licensed. The RhD negative women in this age cohort would be expected to have received postpartum RhIG in the ~60% of pregnancies in which the baby was RhD positive. Presumably, antenatal RhIG was administered in some but not all RhD negative pregnancies in this age cohort. Women ≤40 years old at LAPS enrollment would have been ≤18 years old (i.e. likely nulliparous) in 1984. The RhD negative women in this age cohort would be expected to have received antenatal RhIG in virtually all pregnancies, plus postpartum RhIG in 60% of pregnancies. In contrast, the childbearing years of many of the women in older age groups would have predated the postpartum RhIG era. For example, a woman who was 75 years old at LAPS enrollment would have been 53 years old in 1984 and 37 years old in 1968, so she would have been unlikely to receive RhIG prophylaxis in pregnancy.
Statistical Analysis
Descriptive statistics of donor demographics were stratified by RhD type. To examine the relationship between RhD type and HLA sensitization across age groups, relative risks (RRs) and 95% confidence intervals were calculated. Multivariable logistic regression models were constructed to evaluate the effect of RhD type and subject age on the prevalence of HLA antibody (Any HLA, Class I and Class II). The classification of RhD type and subject age was; RhD+ women, RhD− women with 0 deliveries, and RhD− women with >0 deliveries further sub-classified as RhD− women age ≤40 years old, RhD− women age 41–50 years old, RhD− women age 51–60 years old, RhD− women age 61–70 years old, and RhD− women age ≥71 years old. Models adjusted for other donor characteristics including the number of deliveries, elapsed time from last pregnancy, number of lost pregnancies and transfusion history. All analyses were done using SAS 9.2 (2008; SAS Institute Inc., Cary, NC).
RESULTS
Demographic characteristics of all female LAPS subjects are reported in Table 1. Of 5,819 female LAPS subjects, 1,069 (18.4%) were RhD negative and 4,750 were RhD positive (81.6%). The age distribution of RhD negative and RhD positive individuals was similar. The RhD negative group was slightly enriched for individuals who were White (94.9% v. 88.9%), while fewer RhD negative individuals were Asian. RhD negative and RhD positive women were similar with respect to parity and lost pregnancies, as well as previous transfusions.
Table 1.
Demographic characteristics of all female LAPS subjects
| Total n (%) | RhD Negative n (%) | RhD Positive n (%) | |
|---|---|---|---|
| All Women * | 5,819 | 1,069 | 4,750 |
| Age (in years) | |||
| ≤40 | 1,904 (32.7) | 315 (29.5) | 1,589 (33.5) |
| 41–50 | 1,527 (26.2) | 297 (27.8) | 1,230 (25.9) |
| 51–60 | 1,534 (26.4) | 296 (27.7) | 1,238 (26.1) |
| 61–70 | 669 (11.5) | 126 (11.8) | 543 (11.4) |
| ≥71 | 185 (3.2) | 35 (3.4) | 150 (3.2) |
| Missing | 1 (0.0) | 0 (0.0) | 1 (0.0) |
| Race/Ethnicity | |||
| White | 5,238 (90.0) | 1,014 (94.9) | 4,224 (88.9) |
| Black | 177 (3.0) | 15 (1.4) | 162 (3.4) |
| Hispanic | 186 (3.2) | 25 (2.3) | 161 (3.4) |
| Asian | 134 (2.3) | 2 (0.2) | 132 (2.8) |
| Other | 74 (1.3) | 10 (0.9) | 64 (1.3) |
| Missing | 10 (0.2) | 3 (0.2) | 7 (0.1) |
| Parity | |||
| 0 | 2,051 (35.2) | 341 (31.9) | 1,710 (36.0) |
| 1 | 684 (11.8) | 122 (11.4) | 562 (11.8) |
| 2 | 1,655 (28.4) | 339 (31.7) | 1,316 (27.7) |
| 3 | 906 (15.6) | 167 (15.6) | 739 (15.6) |
| ≥4 | 463 (8.0) | 94 (8.8) | 369 (7.8) |
| Missing | 60 (1.0) | 6 (0.6) | 54 (1.1) |
| Lost Pregnancies | |||
| 0 | 4,071 (70.0) | 723 (67.6) | 3,348 (70.5) |
| 1 | 1,009 (17.3) | 207 (19.4) | 802 (16.9) |
| ≥2 | 595 (10.2) | 120 (11.2) | 475 (10.0) |
| Missing | 144 (2.5) | 19 (1.8) | 125 (2.6) |
| Previously Transfused | |||
| Yes | 348 (6.0) | 62 (5.8) | 286 (6.0) |
| No | 5,337 (91.7) | 983 (92.0) | 4,354 (91.7) |
| Missing | 134 (2.3) | 24 (2.2) | 110 (2.3) |
5 women were missing Rh information
Relative risks of HLA sensitization in RhD negative v. RhD positive women
2×2 analyses of previously pregnant female LAPS subjects analyzed by age group are reported in Table 2. Among the 625 previously pregnant women who were ≤40 years old at LAPS enrollment, the risk of HLA sensitization (any HLA class) was significantly lower for RhD negative individuals than RhD positive individuals (RR 0.58, 95% CI 0.40–0.83). As described above, RhD negative women in this age cohort would be expected to have received antenatal RhIG in 100% of pregnancies at week 28, and postpartum RhIG in an additional 60% of pregnancies. Statistically significant differences in HLA sensitization based on RhD type were not observed for any other age cohort. RhD negative individuals age 41–50 should have received postpartum RhIG in 60% of their pregnancies; some but not all would have received antenatal RhIG depending on when they were pregnant. These women were only slightly less likely than RhD positive individuals to be HLA sensitized. Many of the older LAPS subjects would be expected to have been pregnant before postpartum and/or antenatal RhIG prophylaxis was used routinely. RhD negative individuals age 51–60 were slightly more likely to be HLA sensitized than their RhD positive counterparts, as were RhD negative individuals ≥71 years old. In contrast, RhD negative women 61–70 were less likely than RhD positive individuals to be HLA sensitized. A statistically significant relationship between RhD type and HLA sensitization was not observed among 1801 never-pregnant women (0.56, 0.17–1.83) or among 893 previously transfused men (2.47, 0.85–7.27).
Table 2.
Relative risk (RR) of HLA sensitization in RhD negative women with one or more deliveries, stratified by age.
| Women with ≥ 1 delivery | HLA sensitization (any class) | |
|---|---|---|
| Age Group (in years) | n | RR (95% CI) RhD neg v. RhD pos |
| ≤40 | 625 | 0.58 (0.40–0.83) |
| 41–50 | 1,176 | 0.92 (0.73–1.17) |
| 51–60 | 1,202 | 1.28 (1.01–1.64) |
| 61–70 | 548 | 0.65 (0.39–1.06) |
| ≥71 | 157 | 1.16 (0.58–2.30) |
HLA sensitization rates stratified by delivery number
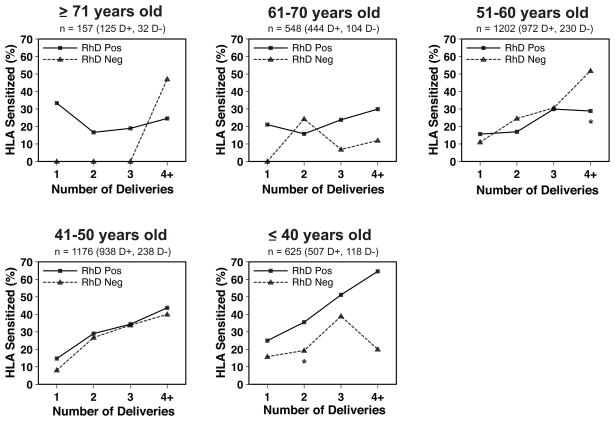
By far the most significant factor affecting HLA sensitization identified in the original LAPS study1 was the number of pregnancies or deliveries. Female LAPS subjects with no prior deliveries had an HLA sensitization rate of only 2.0%. In contrast, women with 1, 2, 3, or 4 more deliveries had HLA sensitization rates of 17.0%, 24.2%, 34.2%, or 35.0%, respectively.1 Therefore, we examined HLA sensitization in RhD negative versus RhD positive women after stratifying by number of deliveries. As shown in Fig. 2, no clear relationship was seen between RhD type and HLA sensitization among women who were ≥71, 61–70, or 51–60 years old at LAPS enrollment. Among the 51–60 year-old women, RhD negative individuals with 4 or more deliveries had a significantly higher rate of HLA sensitization than RhD positive individuals; sensitization rates were similar in individuals with 1, 2, or 3 deliveries. Among women 41–50 years old, RhD negative individuals had slightly lower rates of HLA sensitization than RhD positive individuals for all delivery strata, although the trend lines were very similar. Among women ≤40 years old, RhD negative individuals were less likely to be HLA sensitized than RhD positive individuals for all delivery strata. HLA sensitization was significantly lower for these RhD negative women overall, and for the subset with two deliveries.
Figure 2. HLA sensitization rates in previously pregnant LAPS subjects, stratified by delivery number.
The percentage of previously pregnant women with detectable anti-HLA antibody (any class) is shown for RhD positive women (squares, solid lines) and RhD negative women (triangles, dashed lines) with 1, 2, 3, or 4 or more deliveries. Percent HLA sensitization is plotted against deliveries for women who were: ≥71; 61–70; 51–60; 41–50; or ≤40 years old at LAPS enrollment in 2006–07. *Significant at the p≤0.05 level.
Logistic regression analysis
The results of multivariable logistic regression analysis are reported in Table 3. RhD negative women ≤40 years old were significantly less likely than RhD positive women to be sensitized to any HLA class (adjusted OR 0.55, 95% CI 0.34–0.88) or to HLA Class I (0.50, 0.29–0.87). There was a non-significant trend for lower HLA Class II sensitization among RhD negative women ≤40 as well (0.66, 0.38–1.12). Statistically significant relationships between RhD type and HLA sensitization were not seen in any other age cohort. As in the original LAPS report1, the amount of time elapsed from the last pregnancy was inversely correlated with the detection of anti-HLA antibodies. The number of deliveries was again observed to be the strongest predictor of HLA sensitization. Lost pregnancies and transfusions both appeared to weakly increase HLA sensitization rates.
Table 3.
Effects of age, RhD type and other variables on HLA sensitization
| Variable | Any HLA Adjusted OR (95% CI) |
Class I Adjusted OR (95% CI) |
Class II Adjusted OR (95% CI) |
|---|---|---|---|
|
| |||
| RhD Status and age * | |||
| All RhD+ women | 1.0 | 1.0 | 1.0 |
| RhD− women, ≤40 years old | 0.55 (0.34–0.88) | 0.50 (0.29–0.87) | 0.66 (0.38–1.12) |
| RhD− women, 41–50 years old | 0.89 (0.65–1.21) | 0.94 (0.65–1.34) | 0.84 (0.59–1.20) |
| RhD− women, 51–60 years old | 1.20 (0.88–1.65) | 1.12 (0.75–1.66) | 1.39 (0.99–1.96) |
| RhD− women, 61–70 years old | 0.63 (0.36–1.12) | 0.89 (0.43–1.83) | 0.66 (0.34–1.26) |
| RhD− women, ≥71 years old | 1.02 (0.44–2.35) | 0.84 (0.24–2.87) | 1.04 (0.41–2.63) |
|
| |||
| Time since last pregnancies (in years) | |||
| <10 | 1.0 | 1.0 | 1.0 |
| 10–20 | 0.62 (0.50–0.76) | 0.47 (0.37–0.60) | 0.78 (0.62–0.98) |
| 21–30 | 0.47 (0.37–0.59) | 0.34 (0.26–0.45) | 0.58 (0.44–0.75) |
| >30 | 0.33 (0.26–0.43) | 0.19 (0.14–0.26) | 0.46 (0.35–0.61) |
|
| |||
| Deliveries (live and still births combined) | |||
| 0 | 1.0 | 1.0 | 1.0 |
| 1 | 14.75 (9.84–22.10) | 15.96 (9.77–26.07) | 19.20 (10.72–34.37) |
| 2 | 24.63 (16.91–35.87) | 24.88 (15.79–39.19) | 31.37 (18.06–54.49) |
| 3 | 36.42 (24.65–53.81) | 35.79 (22.36–57.30) | 46.81 (26.66–82.18) |
| ≥4 | 46.49 (30.56–70.72) | 44.19 (26.68–73.19) | 55.51 (30.85–99.87) |
|
| |||
| Lost pregnancies (pregnancies medically or spontaneously terminated) | |||
| 0 | 1.0 | 1.0 | 1.0 |
| 1 | 1.10 (0.92–1.32) | 1.16 (0.94–1.44) | 1.04 (0.85–1.28) |
| ≥2 | 1.18 (0.94–1.47) | 1.21 (0.93–1.57) | 1.02 (0.79–1.31) |
|
| |||
| Transfused | |||
| No | 1.0 | 1.0 | 1.0 |
| Yes | 1.53 (1.17–1.99) | 1.64 (1.20–2.26) | 1.70 (1.27–2.27) |
RhD− women with no pregnancies are all assumed to have same HLA sensitization regardless of age
DISCUSSION
In the clinical setting, fetal RBC clearance is the only early indicator that can be used to assess the adequacy of RhIG dosage.20 However, previous human studies have demonstrated that complete RBC clearance is not actually required for RhIG to suppress anti-D formation. In 1971, Pollack and colleagues21 reported a trial of large-volume D+ RBC transfusions in RhD negative men randomized to receive or not receive RhIG. Each subject was transfused with a 500 mL whole blood unit from an ABO compatible, RhD positive donor. Subjects in the treatment arm were administered RhIG at a dose of approximately 20 mcg/mL D+ RBCs, calculated to be sufficient to provide D suppression. After one month, 8/20 (36%) of subjects in the treatment arm and 22/22 (100%) of control subjects still had detectable circulating D+ RBCs. Yet after five months of observation, 0/20 subjects in the treatment arm had formed anti-D, compared with 18/22 (81.8%) sensitization in the control arm. Similar findings were reported in a 1999 case series of six postpartum women who were treated with RhIG after Kleihauer-Betke testing revealed the presence of large-volume FMH (range: 11.7–153.4 mL). As many as 36% of the D+ fetal RBCs remained detectable in the circulation on day 5–6 post-RhIG administration, yet months later none of the women had become sensitized to D.22 It is perhaps not surprising that D+ RBCs can sometimes be detected in the circulation several days after RhIG administration. Based on the law of mass action, it has been calculated that in a bleed of 5 mL D+ RBCs treated with 100 mcg RhIG, only 8.7% of D antigen sites would be bound by anti-D.23
The phenomenon of AMIS, in which the passive infusion of antibody directed against a specific antigen can suppress the endogenous immune response to that antigen, was first described over a century ago.24 RhIG has long been hypothesized to prevent anti-D formation via an AMIS mechanism.15 Proof of this idea has remained elusive, however. Studies of AMIS in animal models have yielded conflicting results. Some animal experiments suggest that IgG Fc is required for AMIS,25 while other experiments suggest that Fc is dispensable.14,26 Even the question of whether AMIS is truly antigen-specific remains controversial. In 1968, Pollack and colleagues27 reported the results of several AMIS experiments in a rabbit model. Unlike mice, and like humans, rabbits have blood group antigens. In one experiment, HgA−,F− rabbits were injected with HgA+,F+ RBCs. Some of the rabbits were also injected with IgG anti-HgA. The anti-HgA was observed to suppress anti-HgA formation, but not anti-HgF formation. Thus, the AMIS effect exerted by anti-HgA appeared to be specific for the cognate HgA antigen. The opposite result was reported in an analogous human study published by Woodrow and colleagues in 1975.28 62 D−,K− male volunteers were injected with 1 mL D+,K+ RBCs. Half of the subjects (treatment arm) were then administered a single dose of IgG anti-K. 11/31 (36%) of control subjects formed anti-D, compared with only 1/31 anti-K-treated subjects (3%, p=0.001). So in contrast to the rabbit experiment, antibody suppression did not appear to be antigen-specific in the human study. One published case report additionally suggests a possible non-D-specific suppressive effect of RhIG. Branch et al reported a case of a pregnant woman with a high-titer anti-Fya antibody. Her anti-Fya titer was observed to decrease dramatically following the administration of multiple doses of prophylactic antenatal RhIG.29
We speculated that RhIG might provide a local immunosuppressive signal through its Fc domain, as has been proposed for IVIG.17,18 As Fc itself is not antigen-specific, we hypothesized that the immune response to HLA antigens—present at the same time as D—would be inhibited. Therefore, we predicted that younger RhD negative women, whose childbearing years would have coincided with the routine use of RhIG prophylaxis, would have a lower HLA sensitization rate than RhD positive women. To test this prediction, we examined the HLA sensitization rates in RhD negative versus RhD positive individuals in different age groups. The rationale for this approach was that antenatal ± postpartum RhIG has been standard practice in the U.S. for many years, so without having to be concerned about the (largely unknown) details of pregnancy histories of the LAPS subjects, it was reasonable to assume that all younger--but not older--RhD negative women would have received RhIG in essentially all pregnancies. We found that RhD negative women ≤40 years old (≤18 years old when antenatal RhIG prophylaxis was first recommended) had a significantly lower HLA sensitization rate than their RhD positive counterparts. This difference persisted after stratifying for delivery number. In contrast, a clear relationship between RhD type and HLA sensitization was not seen in older previously pregnant women, men, or nulliparous women. In a multivariable logistic regression analysis, RhD negative women ≤40 years old remained significantly less likely to be HLA sensitized compared with RhD positive women after adjusting for independent risk factors identified in the original LAPS study1: parity, time from last pregnancy, lost pregnancies, and transfusions. Overall, our data are consistent with the hypothesis that RhIG may suppress HLA sensitization in pregnancy. If real, however, the effect of RhIG on HLA sensitization appears modest, and much smaller than the effect exerted by pregnancy or delivery number. Alternatively, the observation of lower HLA sensitization among previously pregnant younger RhD negative individuals may be due to chance or other, unknown factors.
This study had several important limitations. As in all retrospective analyses, the data were potentially subject to confounding and systematic bias. No information was available on RhIG administration during pregnancy. Consequently, RhIG use had to be inferred based on the estimated range of childbearing years. This approach should have been reasonably accurate for the youngest (≤40) and oldest (≥71) female LAPS subjects, but less accurate for subjects in the middle of the age spectrum. Postpartum RhIG was first licensed in the U.S. in 1968. CDC surveillance data shows that by 1970, postpartum RhIG was being used in 79% of indicated pregnancies. Postpartum RhIG utilization increased to over 90% in many states over the next few years.30 Antenatal RhIG was first recommended by ACOG in 1984.19,31 Unfortunately, utilization data for the years after 1984 are unavailable,32 so it is unclear when antenatal RhIG prophylaxis became the standard of care in the U.S. In addition to having no direct information about RhIG use in LAPS subjects, no data were available on when HLA antibodies first became detectable in sensitized LAPS subjects. Therefore, it was not possible in this study to directly investigate a causal relationship between RhIG administration and HLA antibody suppression.
There were other, less critical limitations of this study. The RR of 0.56 observed in RhD negative versus RhD positive nulliparous women is difficult to explain, although the HLA sensitization rate in this group was low, so the 95% CI associated with this point estimate was very wide and failed to exclude 1 (0.17–1.83). It is highly unlikely that younger RhD negative woman were less likely than RhD positive women to become HLA sensitized due to a genetic factor. This is simply because all older women were once younger women. If a genetic factor suppressed HLA antibody formation specifically in young RhD negative women, then as cohorts of young women aged, we would have expected to observe a lower prevalence of HLA antibodies in RhD negative women of all ages. This was not seen our study, however. In both the original LAPS analysis1 and in this study, it was found that the more time that has elapsed since the last pregnancy, the less likely that anti-HLA antibodies will be detectable.1 We assume that the probability of an HLA antibody becoming undetectable over time is equivalent for RhD negative and RhD positive women, but this is unproven. In a 1974 study of previously pregnant White South African women, Brain and colleagues reported that nonresponders to D tended not to form anti-HLA antibodies, and women sensitized to D tended to be HLA sensitized as well.33 All LAPS subjects were volunteer blood donors. Typically, individuals who test positive for non-ABO anti-RBC antibodies are excluded from donating blood. It is plausible that among older, RhD negative LAPS subjects, some “responders” likely to form anti-HLA could have been excluded from LAPS on the basis of prior D sensitization in pregnancy, potentially skewing the older RhD negative group towards a lower rate of HLA sensitization. If this skewing effect occurred, it would have been modest, and would only have affected those RhD negative LAPS subjects who became pregnant prior to the routine use of RhIG prophylaxis. Finally, while an exploration of the potential mechanism(s) of HLA antibody suppression by RhIG was beyond the scope of this study, one notable caveat is that commercial RhIG preparations do contain antibody specificities other than anti-D e.g. anti-HLA (data not shown).
Given the limitations of this preliminary analysis, prospective studies are needed to determine definitively whether RhIG can affect HLA sensitization in pregnancy. If RhIG does suppress anti-HLA antibody formation, we predict that the effect will be somewhat stronger than what was observed in this study, as the RhD negative women in LAPS would not have received postpartum RhIG in about 40% of pregnancies. Immunoglobulin therapies have successfully been used to prevent the serious clinical consequences of HPA-1A and RhD sensitization in pregnancy. In contrast, nothing is done currently to try to avoid pregnancy-related HLA sensitization, the most common form of alloimmunization in pregnancy, and one that is associated with a host of potential adverse consequences downstream: TRALI, platelet refractoriness, and stem cell and solid organ transplant rejection. Based on the preliminary results from this study, we believe that further investigation of the potential for RhIG to suppress HLA sensitization is warranted.
Acknowledgments
This work was supported by NHLBI contracts N01-HB-47168, -47169, -47170, -47171, -47172, -47174, -47175, and -57181
Appendix
The authors thank the staff at all six participating blood centers. Without their help, this study would not have been possible. Also, special thanks to Yu Sun from Westat for her work on the analyses.
The Retrovirus Epidemiology Donor Study-II was the responsibility of the following persons:
Blood Centers
American Red Cross Blood Services, New England Region
R. Cable, J. Rios and R. Benjamin
American Red Cross Blood Services, Southern Region/Department of Pathology and Laboratory Medicine, Emory University School of Medicine
J.D. Roback
Hoxworth Blood Center, University of Cincinnati Academic Health Center
R.A. Sacher, S.L. Wilkinson and P.M. Carey
Blood Centers of the Pacific, University of California San Francisco, Blood Systems Research Institute
E.L. Murphy, B. Custer and N. Hirschler
The Institute for Transfusion Medicine
D. Triulzi, R. Kakaiya and J. Kiss
Blood Center of Wisconsin
J.L. Gottschall and A.E. Mast
Coordinating Center: Westat, Inc
J. Schulman and M. King
National Heart, Lung, and Blood Institute, NIH
G. Nemo and S.A Glynn
Central Laboratory: Blood Systems Research Institute
M.P. Busch and P. Norris
Footnotes
Reprints will not be available from the author.
Conflicts of interest: none.
References
- 1.Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, Hillyer CD, Carey P, Gottschall JL, Murphy EL, Rios JA, Ness PM, Wright DJ, Carrick D, Schreiber GB. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49:1825–35. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson LM, Hackett G, Rennie J, Palmer CR, Maciver C, Hadfield R, Hughes D, Jobson S, Ouwehand WH. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–7. [PubMed] [Google Scholar]
- 3.Woodrow JC. Rh Immunization and its Prevention. Copenhagen: Munksgaard; 1970. [Google Scholar]
- 4.Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, Lowell CA, Norris PJ, Murphy EL, Weiskopf RB, Wilson G, Koenigsberg M, Lee D, Schuller R, Wu P, Grimes B, Gandhi MJ, Winters JL, Mair D, Hirschler N, Sanchez Rosen R, Matthay MA. Transfusion related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–67. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, Warren DS, Simpkins CE, Dagher NN, Singer AL, Zachary AA, Segev DL. Desensitization in HLA-incompatible kidney recipients and survival. New England Journal of Medicine. 2011;365:318–26. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 6.Slichter SJ. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. New England Journal of Medicine. 1997;337:1861–9. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 7.Kumpel BM, Manoussaka MS. Placental immunology and maternal alloimmune responses. Vox Sanguinis. 2011;102:2–12. doi: 10.1111/j.1423-0410.2011.01533.x. [DOI] [PubMed] [Google Scholar]
- 8.Regan L, Braude PR, Hill DP. A prospective study of the incidence, time of appearance and significance of anti-paternal lymphocytotoxic antibodies in human pregnancy. Human Reproduction (Oxford, England) 1991;6:294–8. doi: 10.1093/oxfordjournals.humrep.a137325. [DOI] [PubMed] [Google Scholar]
- 9.Bowman JM, Pollock JM, Penston LE. Fetomaternal transplacental hemorrhage during pregnancy and after delivery. Vox Sanguinis. 1986;51:117–21. doi: 10.1111/j.1423-0410.1986.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumpel BM. On the immunologic basis of Rh immune globulin (anti-D) prophylaxis. Transfusion. 2006;46:1652–6. doi: 10.1111/j.1537-2995.2006.00924_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Klein HG, editor. Mollison’s Blood Transfusion in Clinical Medicine. 11. Oxford: Blackwell Publishing; 2005. [Google Scholar]
- 12.Brinc D, Lazarus AH. Mechanisms of anti-D action in the prevention of hemolytic disease of the fetus and newborn. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2009:185–91. doi: 10.1182/asheducation-2009.1.185. [DOI] [PubMed] [Google Scholar]
- 13.Semple JW, Kim M, Lazarus AH, Freedman J. Gamma-globulins prepared from sera of multiparous women bind anti-HLA antibodies and inhibit an established in vivo human alloimmune response. Blood. 2002;100:1055–9. doi: 10.1182/blood.v100.3.1055. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson MC, Wernersson S, Diaz de Ståhl T, Gustavsson S, Heyman B. Efficient IgG-mediated suppression of primary antibody responses in Fcgamma receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2244–9. doi: 10.1073/pnas.96.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumpel BM, Elson CJ. Mechanism of anti-D-mediated immune suppression--a paradox awaiting resolution? Trends in Immunology. 2001;22:26–31. doi: 10.1016/s1471-4906(00)01801-9. [DOI] [PubMed] [Google Scholar]
- 16.Kumpel BM. On the mechanism of tolerance to the Rh D antigen mediated by passive anti-D (Rh D prophylaxis) Immunology Letters. 2002;82:67–73. doi: 10.1016/s0165-2478(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 17.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature. 2011;475:110–3. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Annals of the New York Academy of Sciences. 2012;1253:170–80. doi: 10.1111/j.1749-6632.2011.06305.x. [DOI] [PubMed] [Google Scholar]
- 19.Goplerud CPGM, Pollack W, Scott JR. ACOG Technical Bulletin Update. 1984. The Selective Use of Rho(D) Immune Globulin (RhIG) pp. 1–5. [Google Scholar]
- 20.Kumpel BM. Efficacy of RhD monoclonal antibodies in clinical trials as replacement therapy for prophylactic anti-D immunoglobulin: more questions than answers. Vox Sanguinis. 2007;93:99–111. doi: 10.1111/j.1423-0410.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 21.Pollack W, Ascari W, Crispen J, O’Connor R, Ho T. Studies on Rh prophylaxis. II. Rh immune prophylaxis after transfusion with Rh-positive blood. Transfusion. 1971;11:340. doi: 10.1111/j.1537-2995.1971.tb04425.x. [DOI] [PubMed] [Google Scholar]
- 22.Lubenko A, Williams M, Johnson A, Pluck J, Armstrong D, MacLennan S. Monitoring the clearance of fetal RhD-positive red cells in FMH following RhD immunoglobulin administration. Transfusion Medicine. 1999;9:331–5. doi: 10.1046/j.1365-3148.1999.00217.x. [DOI] [PubMed] [Google Scholar]
- 23.Garraty G, editor. Hemolytic Disease of the Newborn. Arlington: American Association of Blood Banks; 1984. [Google Scholar]
- 24.Von Dungern F. Beiträge zur immunitätslehre. Munch Med Wochenschr. 1900;47:677–80. [Google Scholar]
- 25.Coopamah MD. Anti-D initially stimulates an Fc-dependent leukocyte oxidative burst and subsequently suppresses erythrophagocytosis via interleukin-1 receptor antagonist. Blood. 2003;102:2862–7. doi: 10.1182/blood-2003-04-1029. [DOI] [PubMed] [Google Scholar]
- 26.Crow AR, Freedman J, Hannach B, Lazarus AH. Monoclonal antibody-mediated inhibition of the human HLA alloimmune response to platelet transfusion is antigen specific and independent of Fcgamma receptor-mediated immune suppression. British Journal of Haematology. 2000;110:481–7. doi: 10.1046/j.1365-2141.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 27.Pollack W, Gorman JG, Hager HJ, Freda VJ, Tripodi D. Antibody-mediated immune suppression to the Rh factor: animal models suggesting mechanism of action. Transfusion. 1968;8:134–45. doi: 10.1111/j.1537-2995.1968.tb04891.x. [DOI] [PubMed] [Google Scholar]
- 28.Woodrow JC, Clarke CA, Donohow WT, Finn R, McConnell RB, Sheppard PM, Lehane D, Roberts FM, Gimlette TM. Mechanism of Rh prophylaxis: an experimental study on specificity of immunosuppression. British Medical Journal. 1975;2:57–9. doi: 10.1136/bmj.2.5962.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Branch DR, Scofield TL, Moulds JJ, Swanson JL. Unexpected suppression of anti-Fya and prevention of hemolytic disease of the fetus and newborn after administration of Rh immune globulin. Transfusion. 2010;51:816–9. doi: 10.1111/j.1537-2995.2010.02905.x. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control. Rh hemolytic disease surveillance. Lexington: University of Michigan Library; 2010. [Google Scholar]
- 31.Bowman JM. The Prevention of Rh Immunization. Transfusion Medicine Reviews. 1988;2:129–50. doi: 10.1016/s0887-7963(88)70039-5. [DOI] [PubMed] [Google Scholar]
- 32.Chávez GF, Mulinare J, Edmonds LD. Epidemiology of Rh hemolytic disease of the newborn in the United States. Journal of the American Medical Association. 1991;265:3270–4. doi: 10.1001/jama.1991.03460240066029. [DOI] [PubMed] [Google Scholar]
- 33.Brain P, Hammond MG. Association between histocompatibility type and the ability to make anti-Rh antibodies. European Journal of Immunology. 1974;4:223–5. doi: 10.1002/eji.1830040313. [DOI] [PubMed] [Google Scholar]