Abstract
Methamphetamine has become a major public health issue in both the US and globally. Despite this, no effective pharmacotherapy for methamphetamine abuse has been developed to date. This 6 week, open-label pilot clinical trial examined the safety and tolerability of modafinil up to 400mg/day in eight methamphetamine dependent individuals. Subjects were inducted onto modafinil at 400 mg/day over three days and remained on 400 mg/day for 4.5 weeks. Participants received weekly blister packs and underwent weekly individual cognitive behavioral therapy. Adjunctive contingency management procedures were used to enhance retention. Vital signs and supervised urine samples were obtained thrice weekly and self-reported drug use and Hamilton Anxiety and Depression ratings were completed once weekly. Eight subjects (50% female, 100% Caucasian, aged 35-52 yrs) were enrolled. Four completed the 6-wk study, 3 completed a portion and one withdrew consent before completing intake. Results showed that systolic blood pressure (t=1.09, p=0.28), diastolic blood pressure, (t=1.18, p=0.24) and heart rate (t=1.55, p=0.13) did not change over time. Scores on the modafinil side effects checklist (t=−2.63, p=0.01), Hamilton Anxiety scale (t=−2.50, p=0.018) and Hamilton Depression scale (t=−3.25, p=0.003) all decreased over time. The proportion of urine positive for amphetamines did not change over time (t=−0.52, p=0.61), whereas self-reported methamphetamine use did (t=−2.86, p<0.005). These results suggest that modafinil at 400 mg/day is safe and tolerable for methamphetamine dependent individuals.
Methamphetamine has become a major public health issue both in the US and globally;1-3 however, no effective pharmacotherapy for methamphetamine abuse has been developed. Moreover, there is a paucity of studies examining pharmacotherapies for methamphetamine dependence. Thus, the purpose of this pilot study is to obtain preliminary safety data on a potential treatment agent for this disorder; that is, modafinil.
Amphetamines increase the release of newly synthesized norepinephrine and dopamine4 and enhanced release of dopamine is thought to be responsible for the reinforcing and psychomotor stimulant effects of amphetamine.5 Amphetamines release dopamine through the dopamine transporter (DAT) by means of reverse transport mechanisms, which contrasts with cocaine and it’s mechanism of inhibiting dopamine reuptake into the presynaptic nerve endings.6-8 Additionally, methamphetamine results in greater accumulation of dopamine in the extracellular space than cocaine when administered to rats during early withdrawal.9 The purpose of this study is to examine an agent with dopaminergic effects; that is, modafinil, as a potential treatment for methamphetamine dependence.
Modafinil is a wakefulness promoting agent with FDA-approved indications to improve wakefulness in patients with excessive sleepiness associated with narcolepsy, obstructive sleep apnea, and shift work sleep disorder.10 Its stimulant effects appear to be different from those of amphetamine based on sleep deprivation studies in cats, in that modafinil results in wakefulness without behavioral excitation and sleep rebound whereas amphetamine does result in sleep rebound and behavioral excitation.11 In addition, it has been found that modafinil and amphetamine differ regionally in the structures of the brain in which they act, suggesting that they differ in their mechanism to promote wakefulness.12 In cats and rats the primary target area of modafinil appears to be in the anterior hypothalamus and adjacent areas as opposed to the striatum and cortex for methamphetamine.13,14 Despite these differences, there are apparent similarities between the mechanism of action for modafinil and amphetamine. For instance, DAT-knockout mice show a similar lack of responsiveness to modafinil and methamphetamine,15 suggesting a dopaminergic mechanism of action for modafinil.
Modafinil-cocaine interactions have been examined in humans.16,17 Modafinil significantly reduced the euphoric effects of intravenously-administered cocaine in seven cocaine-abusing volunteers under controlled laboratory conditions.16 Moreover, in an outpatient clinical trial, modafinil significantly reduced cocaine use relative to placebo in cocaine-dependent participants.17 These data indicate that the stimulant effects of modafinil may block the stimulant effects of cocaine, reducing cocaine’s effects.
A few studies have examined the efficacy of modafinil for methamphetamine dependence.18-20 For instance, a recent uncontrolled pilot study using modafinil (200 mg/day) plus cognitive behavioral therapy in HIV + men reported that six of ten study completers reduced their methamphetamine use by more than 50%, although two of the thirteen participants enrolled discontinued their participation due to side effects.18 Moreover, a double blind placebo-controlled trial of modafinil (200mg/day) supported the safety and tolerability of modafinil at this dose and modafinil produced a trend toward a decrease in the number of methamphetamine negative urines relative to placebo. 19 Meanwhile, an inpatient study examining the safety of modafinil and mirtazapine for methamphetamine withdrawal supported the safety of modafinil 400 mg daily in an abstinent population; however the safety of modafinil at 400 mg/day has not been established in patients who have the potential to use methamphetamine concurrently.20 Thus, the goal of this open label pilot study was to assess the safety and tolerability of modafinil at 400 mg/day in methamphetamine-dependent volunteers participating on an outpatient basis.
MATERIALS AND METHODS
Subjects
Individuals were recruited from the greater Little Rock area via newspaper and radio advertisements. Each gave written informed consent, as approved by the UAMS Institutional Review Board. Eligible subjects were between the ages of 18 and 65, not currently enrolled in a treatment program, met Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) criteria for amphetamine dependence, had a confirmed urine toxicology screen positive for amphetamines during the month prior to entry, and women of childbearing age had to have a negative pregnancy test to enroll. Exclusion criteria included a current diagnosis of physical dependence on alcohol, opiates or sedative/hypnotics, ill health, history of schizophrenia or bipolar I disorder, use of a medication that would have a major interaction with the study drug, current suicidality or psychosis, liver function tests three time normal, pregnancy or breastfeeding. While cocaine dependent individuals were not specifically excluded from this study, none of our participants were dependent on cocaine.
Design and Procedures
This study was a 6-wk, open label pilot study investigating the safety and tolerability of modafinil in methamphetamine dependent individuals. Subjects were started on modafinil dosed at 200mg daily for the first 3 days, then increased to 400mg daily. Procedures were in place to decrease the dose in 100mg increments to a minimum of 200mg daily if troublesome side effects emerged. They were maintained on the study drug for five weeks, and then observed for 5 days during week 6 after modafinil was discontinued. During the study each participant received weekly manual-driven cognitive behavior therapy (CBT) as well as contingency management in the form of monetary rewards in exchange for returning medication blister packs, submitting urine samples thrice-weekly for analysis and attending CBT sessions.
Measures
At intake, participants were interviewed using the Structured Clinical Interview for DSM-IV21 and the Addiction Severity Index.22 Primary measures of this study included thrice weekly vital signs, weekly Hamilton Depression and Anxiety scales23 and weekly Modafinil Side Effects Checklist, which consisted of 10 items rated on a scale from 0 (not at all) to 4 (very much), including headache, nausea, nervousness, rhinitis, diarrhea, back pain, anxiety, insomnia, dizziness, and dyspepsia.10 Secondary measures included drug use measures. Supervised urine samples were obtained thrice weekly and tested for the presence of amphetamines using a Hitachi 717 Automated Analyzer (Boehringer Mannheim Corp., Indianapolis, IN), with a cut-off concentration of 300 ng/ml. Self-reported methamphetamine use was obtained weekly using analog scales and instruments developed in previous studies.26,25 Participants were monitored at every visit for any adverse symptoms.
Data Analyses
Descriptive statistics are reported for demographic variables, retention and missed medication doses. Primary outcomes of interest included vital signs, self reported side effects and mood ratings to determine the safety and tolerability of modafinil. Secondary outcomes included amphetamine urine toxicology results and self reported use. Amphetamine urine results were classified into three categories: no positive urines during the week; some positive urines during the week; all positive urines during the week. Outcomes were analyzed using a random coefficient regression model, also known as hierarchical linear models (HLM) to determine whether results differentially changed over time.26,27 The HLM model was fit with time in weeks as a continuous coefficient. The time effect is used to determine whether measures increased or decreased over the course of the protocol. All outcomes except self-reported amphetamine use were fit using a normal error term. Self-reported use was non-normal count data skewed towards zero, so a log link function and Poisson error term was used. For the categorical outcome of qualitative urine data, estimates derived from the HLM analysis were expressed as logits much as with logistic regression. For the continuous outcomes, estimates derived from the HLM analysis were expressed in the same manner as a linear regression. T-value, degrees of freedom, and p-value are reported. These analyses were performed in SAS PROC GLIMMIX, an HLM modeling program within SAS with p<0.05 indicating statistical significance.
RESULTS
Sample Characteristics
Seven Caucasian methamphetamine dependent individuals (3 males, 4 females), with a mean age of 45.3 (STD=7.2) years completed intake. All participants met DSM IV criteria for amphetamine dependence. All reported recent methamphetamine use with a mean use of 20.1 (STD=8.25) days out of the previous 30. Three subjects reported intravenous use and four subjects reported smoking methamphetamine. No participant met DSM IV criteria for alcohol dependence or current major depression. Participants reported a mean monthly income of $1,764 (STD=$2836) and a mean of 12.6 (STD=1.7) years of education.
Retention
Participants were retained for a mean of 5.4 (STD=2.4) weeks in the 7-week study (including one week of screening). Of the eight subjects who entered the study, 4 completed the entire study. One participant was enrolled but did not complete intake or begin the study medication. The other 3 subjects participated for less than 1, 4.1, and 5 weeks, respectively. Dropouts were associated with difficulty attending clinic due to transportation issues or employment requirements.
Adverse Events
A total of 5 adverse events occurred during the study since it began, of which 3 were at least probably related to study participation. These events included reports of tachycardia (n=1) and dry mouth (n=1). The third event occurred when a subject missed a dose on one day and took a double dose the following day. At that time the subject experienced tachycardia, dry mouth, nervousness, all of which resolved within one hour of modafinil ingestion. Study-related adverse events were mild to moderate overall. No participant required or requested a reduction in the dose of modafinil.
Treatment Outcomes
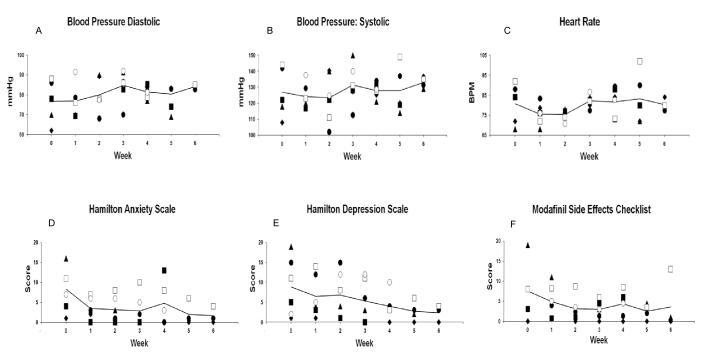
There was no significant change in vital signs over the course of the study (figure 1A-C). Systolic blood pressure essentially remained constant, with mean individual readings between 102 and 150 mmHg (t=1.09, df=85, p=0.28; figure 1A). Similarly, diastolic blood pressure did not change over time, ranging from 62 to 102 (t=1.18, df=85, p=0.24; figure 1B). In addition, heart rate remained relatively consistent over time with individual mean pulse generally remaining between 65 and 95 beats/min (t=1.55, df=84, p=0.13; figure 1C).
Figure 1.
Vital signs (A-C) and self-reported side effects and mood ratings (DEF) over the course of the 6-week trial. Each point represents mean data for one participant. The line represents the mean across participants.
Mean scores on the side effects checklist were highest prior to starting modafinil and decreased as the study progressed (t=−2.63, df=77, p=0.01; figure 1D). Scores on the Hamilton anxiety scale ranged from 1 to 16 at the beginning of the study and decreased as the study progressed (t=−2.50, df=29, p=0.018; figure 1E). Similarly, Hamilton depression scores initially varied across subjects, ranging from 1 to 19 prior to starting modafinil, and decreased over time as the study progressed (t=−3.25, df=29, p=0.003; figure 1F). Scores on the AWQ
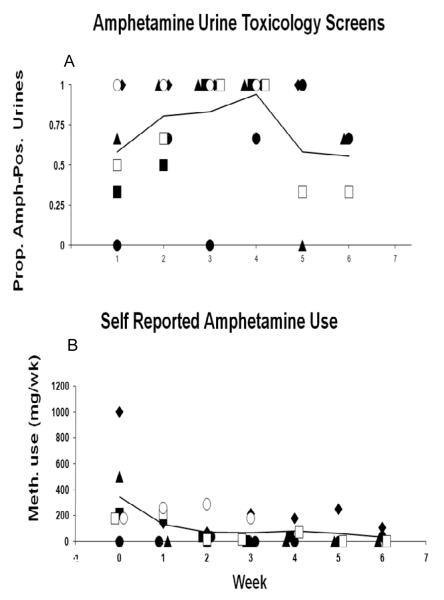
All subjects had urine samples that tested positive for amphetamines during the study with at least one-half of urine samples testing positive per week (figure 2A). There was no change in positive urine screens over time during the trial (t=−0.52, df=23, p=0.61), even though self-reported methamphetamine use showed a significant decrease over time (t=−2.86, df=259, p<0.005; figure 2B).
Figure 2.
Proportion of amphetamine-positive urine screens (A) and self-reported methamphetamine use (B) during the 6-week trial. Each point represents data for one participant. The line represents the mean across participants.
DISCUSSION
The results of this small, open label pilot study support modafinil as a safe and tolerable agent for use in methamphetamine dependent individuals. There was no consistent change in vital signs during the course of the study, suggesting that modafinil alone does not produce significant alterations in blood pressure, heart rate or temperature. This lack of clinically significant alteration in vitals was true even in the context of continued methamphetamine use. There were occasions in which participants did have elevated blood pressure in the context of methamphetamine use, however these were acute and not clinically relevant. These findings are consistent with prior reports in humans that modafinil does not enhance cocaine-induced increases in blood pressure and heart rate under controlled laboratory conditions.16,28
Self-reported modafinil side effects actually decreased as the study progressed. In addition, although anxiety is a potential side effect of modafinil, ratings on both the Hamilton anxiety and depression scales decreased over the course of the study. Adverse events were few, generally mild, did not result in participant dropout, and did not necessitate a decrease in dose. These events are similar to those reported in a modafinil trial with cocaine dependent participants.17
Whether modafinil has efficacy for treating methamphetamine dependence was not the primary aim of this study and, although self-reported methamphetamine use decreased over the course of the trial, amphetamine-positive urine screens did not change. This preliminary result does suggest, however, that modafinil may not exacerbate methamphetamine use. Interestingly, a recent case report relates that an amphetamine-dependent woman reported reduced craving for amphetamines, improved anxiety and depression, reduced preoccupation with acquiring amphetamines, and a different “high” with amphetamines while taking modafinil.29
Limitations of this study include the lack of blinding, the small sample size, and the lack of a placebo control. However, despite these limitations the findings of this study support modafinil at a dose of 400 mg daily as a safe and tolerable agent in methamphetamine dependent individuals. Further work is warranted to determine efficacy, if any, in this population.
ACKNOWLEDGMENTS
This work was supported by P50-DA12762 from the National Institute on Drug Abuse as well as pilot funds from the Department of Psychiatry and College of Medicine at the University of Arkansas for Medical Sciences. The authors thank Venus Smith for her assistance with the preparation of the manuscript.
Footnotes
This work was supported by P50-DA12762 from the National Institute on Drug Abuse as well as pilot funds from the Department of Psychiatry and College of Medicine at the University of Arkansas for Medical Sciences.
A preliminary report of this work was presented at the Annual Meeting of the College on Problems of Drug Dependence on June 18, 2008 in San Juan, P.R.
AUTHOR DISCLOSURE INFORMATION Authors have no disclosures.
REFERENCES
- 1.Baberg HT, Nelesen RA, Dimsdale JE. Amphetamine use: return of an old scourge in a consultation psychiatry setting. Am J Psychiatry. 1996;153:789–793. doi: 10.1176/ajp.153.6.789. [DOI] [PubMed] [Google Scholar]
- 2.Shaw KP. Human methamphetamine-related fatalities in Taiwan during 1991-1996. J Forensic Sci. 1999;44:27–31. [PubMed] [Google Scholar]
- 3.NACO The Meth Epidemic in America: Two Surveys of U.S. Counties. Jul 5, 2005.
- 4.Ellinwood EH, Jr, Kilbey MM. Fundamental mechanisms underlying altered behavior following chronic administration of psychomotor stimulants. Biol Psychiatry. 1980;15:749–757. [PubMed] [Google Scholar]
- 5.Abi-Dargham A, Kegeles LS, Martinez D, Innis RB, Laruelle M. Dopamine mediation of positive reinforcing effects of amphetamine in stimulant naive healthy volunteers: results from a large cohort. Eur Neuropsychopharmacol. 2003;13:459–468. doi: 10.1016/j.euroneuro.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Marek GJ, Vosmer G, Seiden LS. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res. 1990;513:274–279. doi: 10.1016/0006-8993(90)90467-p. [DOI] [PubMed] [Google Scholar]
- 7.Kitayama S, Dohi T. Cellular and molecular aspects of monoamine neurotransmitter transporters. Jpn J Pharmacol. 1996;72:195–208. doi: 10.1254/jjp.72.195. [DOI] [PubMed] [Google Scholar]
- 8.Fleckenstein AE, Metzger RR, Wilkins DG, et al. Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- 9.Zhang Y, Loonam TM, Noailles PA, et al. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 10.Physicians Desk Reference. Medical Economics Company, Inc; Montvale, NJ: 2005. [Google Scholar]
- 11.Hou YP, Lin JS. Effects of modafinil and amphetamine on sleep-wake cycle after sleep deprivation in cats. Zhongguo Yao Li Xue Bao/Acta Pharmacologica Sinica. 1999;20:813–818. [PubMed] [Google Scholar]
- 12.Engber TM, Dennis SA, Jones BE, et al. Brain regional substrates for the actions of the novel wake-promoting agent modafinil in the rat: comparison with amphetamine. Neuroscience. 1998;87:905–911. doi: 10.1016/s0306-4522(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 13.De Sereville JE, Boer C, Rambert FA, et al. Lack of pre-synaptic dopaminergic involvement in modafinil activity in anaesthetized mice: in vivo voltammetry studies. Neuropharmacology. 1994;33:755–61. doi: 10.1016/0028-3908(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 14.Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Nat Acad Sci USA. 1996;93:14128–33. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisor JP, Nishino S, Sora I, et al. Dopaminergic role in stimulant-induced wakefulness. J Neuroscience. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dackis DA, Lynch LG, Yu E, et al. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 17.Dackis CA, Kampman KM, Lynch KG, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacol. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 18.McElhiney MC, Rabkin JG, Rabkin R, Nunes EV. Provigil (modafinil) plus cognitive behavioral therapy for methamphetamine use in HIV+ gay men: a pilot study. Am J Drug Alcohol Abuse. 2009;35:34–7. doi: 10.1080/00952990802342907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shearer J, Darke S, Rodgers C, Slate T, Van Beek I, Lewis J, Brady D, McKetin R, Mattick RP, Wodak A. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009 Feb;104:224–33. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 20.McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. J Subst Abuse Treat. 2008 Oct;35:334–42. doi: 10.1016/j.jsat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV. patient ed American Psychiatric Press; Washington, D.C.: 1995. [Google Scholar]
- 22.McLellan AT, Luborsky L, Woody GE, et al. An improved diagnostic instrument for substance abuse subjects: The Addiction Severity Index. J Nerv Mental Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosten TR, Oliveto A, Feingold A, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 25.Oliveto A, Poling J, Sevarino K, et al. Efficacy of dose and contingency management procedures in LAAM-maintained cocaine-dependent patients. Drug Alcohol Depend. 2005;79:157–166. doi: 10.1016/j.drugalcdep.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bull. 1987;101:147–158. [Google Scholar]
- 27.Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analyses of longitudinal psychiatric data. Arch Gen Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 28.Hart CL, Haney M, Vosburg SK, et al. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33(4):761–8. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- 29.Camacho A, Stein MB. Modafinil for social phobia and amphetamine dependence. Am J Psychiatry. 2002;159:1947–1948. doi: 10.1176/appi.ajp.159.11.1947-a. [DOI] [PubMed] [Google Scholar]