Abstract
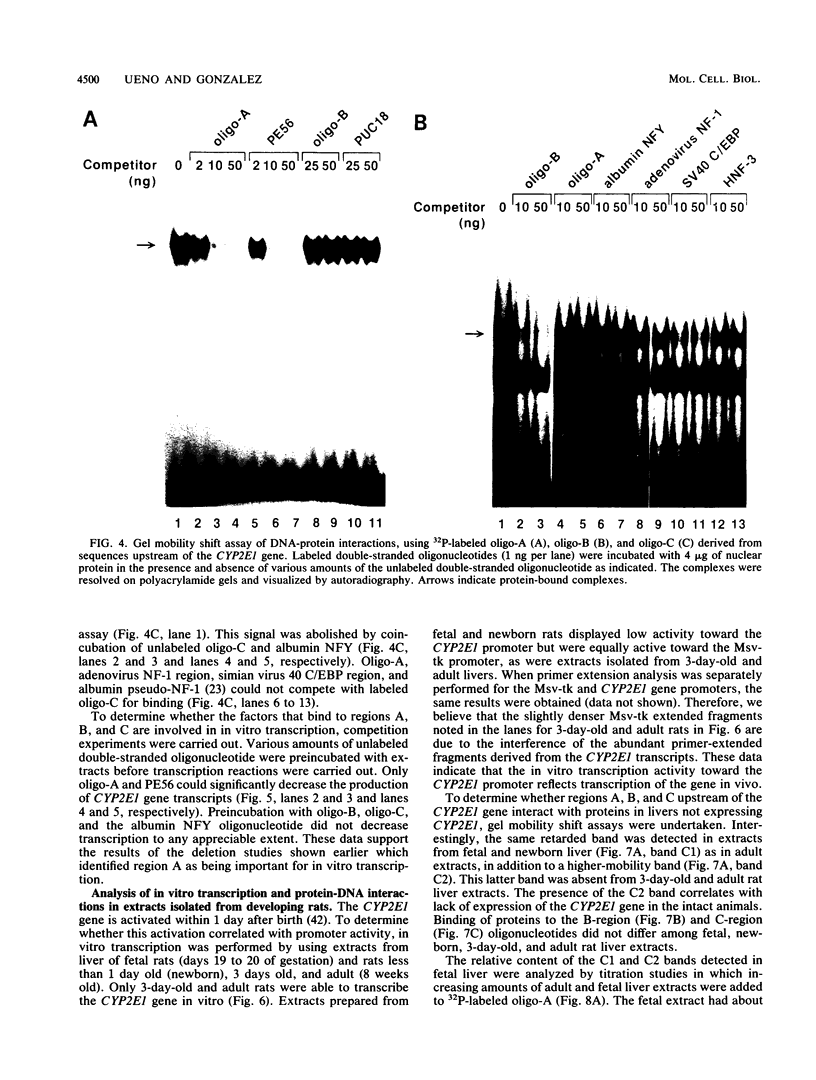
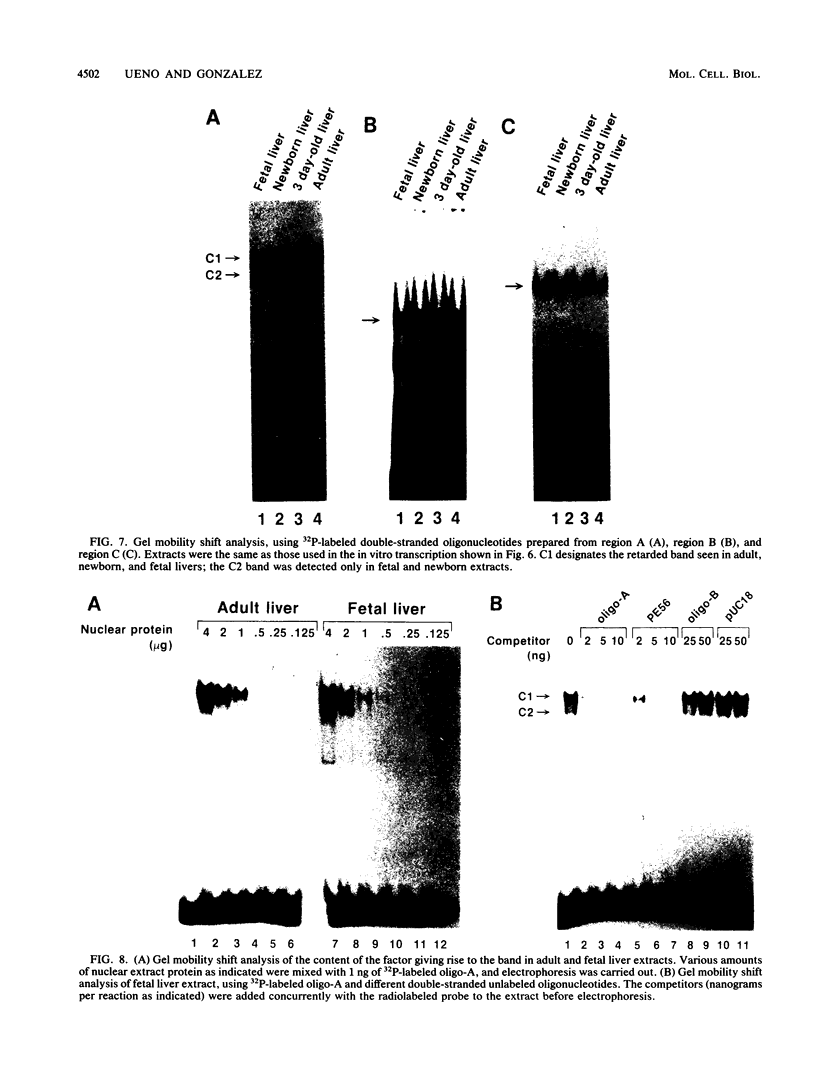
The rat hepatic CYP2E1 gene becomes transcriptionally activated within 1 day after birth. This activation can be mimicked by using the 5' end of the gene in a cell-free nuclear extract prepared from hepatocytes taken from rats at different developmental stages. Deletion analysis revealed that a positive element located between -127 and -89 was responsible for 90% of the in vitro transcription activity of adult liver extracts. Protein binding studies revealed that this region was operationally equivalent to the binding site for the factor HNF-1. Two other protein-binding regions were uncovered, one of which corresponded to the site for a CCAAT-binding factor NFY. The other site was a palindrome sequence unique to the CYP2E1 gene. These latter two factors did not significantly contribute to transcriptional activity in vitro and were not conserved between the rat and human CYP2E1 genes. Extracts prepared from fetal and newborn livers were transcriptionally inactive, whereas extracts from livers of 3-day-old rats were fully active toward the CYP2E1 gene. DNase I footprinting patterns indistinguishable between fetal and adult extracts were obtained for all three factors. However, gel mobility shift assays revealed a second, higher-mobility band produced by fetal and newborn liver extracts bound to the HNF-1 oligomer. UV-cross-linking studies showed that adult and fetal extracts had only a single 98-kilodalton protein that bound to this oligomer. In contrast, adult lung samples, also transcriptionally inactive toward the CYP2E1 gene, contained two proteins of slightly greater than 110 kilodaltons. These results suggest that the CYP2E1 gene is positively regulated in adult rats by HNF-1 or a protein similar in DNA-binding properties to HNF-1. The role of this factor or other protein-protein interactions in the lack of CYP2E1 transcription in fetal and newborn animals remains unclear.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumhueter S., Courtois G., Crabtree G. R. A variant nuclear protein in dedifferentiated hepatoma cells binds to the same functional sequences in the beta fibrinogen gene promoter as HNF-1. EMBO J. 1988 Aug;7(8):2485–2493. doi: 10.1002/j.1460-2075.1988.tb03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumhueter S., Mendel D. B., Conley P. B., Kuo C. J., Turk C., Graves M. K., Edwards C. A., Courtois G., Crabtree G. R. HNF-1 shares three sequence motifs with the POU domain proteins and is identical to LF-B1 and APF. Genes Dev. 1990 Mar;4(3):372–379. doi: 10.1101/gad.4.3.372. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Blumenfeld M., Yaniv M. A liver-specific factor essential for albumin transcription differs between differentiated and dedifferentiated rat hepatoma cells. Genes Dev. 1988 Aug;2(8):957–974. doi: 10.1101/gad.2.8.957. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Raymondjean M., Carranca A. G., Herbomel P., Yaniv M. Factors involved in control of tissue-specific expression of albumin gene. Cell. 1987 Aug 14;50(4):627–638. doi: 10.1016/0092-8674(87)90036-5. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Yaniv M., Cortese R. Hepatocyte dedifferentiation and extinction is accompanied by a block in the synthesis of mRNA coding for the transcription factor HNF1/LFB1. EMBO J. 1990 Jul;9(7):2257–2263. doi: 10.1002/j.1460-2075.1990.tb07396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Sharp P. A. A single polypeptide possesses the binding and transcription activities of the adenovirus major late transcription factor. Mol Cell Biol. 1986 Dec;6(12):4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- Frain M., Swart G., Monaci P., Nicosia A., Stämpfli S., Frank R., Cortese R. The liver-specific transcription factor LF-B1 contains a highly diverged homeobox DNA binding domain. Cell. 1989 Oct 6;59(1):145–157. doi: 10.1016/0092-8674(89)90877-5. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinari P., La Bella F., Heintz N. Characterization and purification of H1TF2, a novel CCAAT-binding protein that interacts with a histone H1 subtype-specific consensus element. Mol Cell Biol. 1989 Apr;9(4):1566–1575. doi: 10.1128/mcb.9.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Granner D., Pilkis S. The genes of hepatic glucose metabolism. J Biol Chem. 1990 Jun 25;265(18):10173–10176. [PubMed] [Google Scholar]
- Graves B. J., Eisenman R. N., McKnight S. L. Delineation of transcriptional control signals within the Moloney murine sarcoma virus long terminal repeat. Mol Cell Biol. 1985 Aug;5(8):1948–1958. doi: 10.1128/mcb.5.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Johnson P. F. Transcriptional activators in hepatocytes. Cell Growth Differ. 1990 Jan;1(1):47–52. [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Koop D. R., Casazza J. P. Identification of ethanol-inducible P-450 isozyme 3a as the acetone and acetol monooxygenase of rabbit microsomes. J Biol Chem. 1985 Nov 5;260(25):13607–13612. [PubMed] [Google Scholar]
- Lamph W. W., Dwarki V. J., Ofir R., Montminy M., Verma I. M. Negative and positive regulation by transcription factor cAMP response element-binding protein is modulated by phosphorylation. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4320–4324. doi: 10.1073/pnas.87.11.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Schibler U. A glycosylated liver-specific transcription factor stimulates transcription of the albumin gene. Cell. 1989 Jun 30;57(7):1179–1187. doi: 10.1016/0092-8674(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Monaci P., Nicosia A., Cortese R. Two different liver-specific factors stimulate in vitro transcription from the human alpha 1-antitrypsin promoter. EMBO J. 1988 Jul;7(7):2075–2087. doi: 10.1002/j.1460-2075.1988.tb03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C. R., Maire P., Schibler U. DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell. 1990 Apr 20;61(2):279–291. doi: 10.1016/0092-8674(90)90808-r. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Numazaki M., Tsutsumi K., Tsutsumi R., Ishikawa K. Expression of aldolase isozyme mRNAs in fetal rat liver. Eur J Biochem. 1984 Jul 2;142(1):165–170. doi: 10.1111/j.1432-1033.1984.tb08265.x. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor contains a promoter-region-specific DNA-binding activity. Cell. 1984 Feb;36(2):357–369. doi: 10.1016/0092-8674(84)90229-0. [DOI] [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Identification of multiple protein binding domains in the promoter-regulatory region of the phosphoenolpyruvate carboxykinase (GTP) gene. J Biol Chem. 1989 Jun 5;264(16):9657–9664. [PubMed] [Google Scholar]
- Schorpp M., Döbbeling U., Wagner U., Ryffel G. U. 5'-flanking and 5'-proximal exon regions of the two Xenopus albumin genes. Deletion analysis of constitutive promoter function. J Mol Biol. 1988 Jan 5;199(1):83–93. doi: 10.1016/0022-2836(88)90380-4. [DOI] [PubMed] [Google Scholar]
- Schuetz E. G., Li D., Omiecinski C. J., Muller-Eberhard U., Kleinman H. K., Elswick B., Guzelian P. S. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988 Mar;134(3):309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- Short J. M., Wynshaw-Boris A., Short H. P., Hanson R. W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. II. Identification of cAMP and glucocorticoid regulatory domains. J Biol Chem. 1986 Jul 25;261(21):9721–9726. [PubMed] [Google Scholar]
- Song B. J., Matsunaga T., Hardwick J. P., Park S. S., Veech R. L., Yang C. S., Gelboin H. V., Gonzalez F. J. Stabilization of cytochrome P450j messenger ribonucleic acid in the diabetic rat. Mol Endocrinol. 1987 Aug;1(8):542–547. doi: 10.1210/mend-1-8-542. [DOI] [PubMed] [Google Scholar]
- Song B. J., Veech R. L., Park S. S., Gelboin H. V., Gonzalez F. J. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989 Feb 25;264(6):3568–3572. [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Bandiera S., Maines S. L., Ryan D. E., Levin W. Regulation of cytochrome P-450j, a high-affinity N-nitrosodimethylamine demethylase, in rat hepatic microsomes. Biochemistry. 1987 Apr 21;26(8):2280–2289. doi: 10.1021/bi00382a031. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Ito K., Ishikawa K. Developmental appearance of transcription factors that regulate liver-specific expression of the aldolase B gene. Mol Cell Biol. 1989 Nov;9(11):4923–4931. doi: 10.1128/mcb.9.11.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeno M., McBride O. W., Yang C. S., Gelboin H. V., Gonzalez F. J. Human ethanol-inducible P450IIE1: complete gene sequence, promoter characterization, chromosome mapping, and cDNA-directed expression. Biochemistry. 1988 Dec 13;27(25):9006–9013. doi: 10.1021/bi00425a019. [DOI] [PubMed] [Google Scholar]
- Umeno M., Song B. J., Kozak C., Gelboin H. V., Gonzalez F. J. The rat P450IIE1 gene: complete intron and exon sequence, chromosome mapping, and correlation of developmental expression with specific 5' cytosine demethylation. J Biol Chem. 1988 Apr 5;263(10):4956–4962. [PubMed] [Google Scholar]
- Vaulont S., Puzenat N., Kahn A., Raymondjean M. Analysis by cell-free transcription of the liver-specific pyruvate kinase gene promoter. Mol Cell Biol. 1989 Oct;9(10):4409–4415. doi: 10.1128/mcb.9.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Morrissey J. J., LeBlanc G. A. Female-predominant rat hepatic P-450 forms j (IIE1) and 3 (IIA1) are under hormonal regulatory controls distinct from those of the sex-specific P-450 forms. Endocrinology. 1989 Jun;124(6):2954–2966. doi: 10.1210/endo-124-6-2954. [DOI] [PubMed] [Google Scholar]