Abstract
Background
Autoimmune thyroid disease is an archetypal organ-specific autoimmune disorder that is characterized by the production of thyroid autoantibodies and lymphocytic infiltration into the thyroid. However, the underlying mechanisms by which specific thyroid antibodies are produced are largely unknown. Recent studies have shown that innate immune responses affect both the phenotype and the severity of autoimmune reactions. Moreover, it appears that even non-immune cells, including thyroid cells, have an ability to launch such responses. The aim of this study was to conduct a more detailed analysis of innate immune responses of the thyroid upon stimulation with various “non-self” and “self” factors that might contribute to the initiation of autoimmune reactions.
Methods
We used rat thyroid FRTL-5 cells, human thyroid cells, and mice to investigate the effects of various pathogen-associated molecular patterns (PAMPs), danger-associated molecular patterns (DAMPs), and iodide on gene expression and function that were related to innate immune responses.
Results
RT-PCR analysis showed that both rat and human thyroid cells expressed mRNAs for Toll-like receptors (TLRs) that sensed PAMPs. Stimulation of thyrocytes with TLR ligands resulted in activation of the interferon-beta (IFN-β) promoter and the nuclear factor kappa–light-chain-enhancer of activated B cells (NFκB)–dependent promoter. As a result, pro-inflammatory cytokines, chemokines, and type I interferons were produced. Similar activation was observed when thyroid cells were stimulated with double-stranded DNA, one of the typical DAMPs. In addition to these PAMPs and DAMPs, treatment of thyroid cells with high concentrations of iodide increased mRNA expression of various cytokines.
Conclusion
We show that thyroid cells express functional sensors for exogenous and endogenous dangers, and that they are capable of launching innate immune responses without the assistance of immune cells. Such responses may relate to the development of thyroiditis, which in turn may trigger autoimmune reactions.
Introduction
Autoimmune thyroid diseases (AITDs) are characterized by the production of pathogenic thyroid autoantibodies and lymphocyte infiltration (1,2). Autoantibodies against various thyroid antigens such as thyroid peroxidase (TPO), thyroglobulin (Tg), sodium iodide symporter (SLC5A5: NIS), and Pendred syndrome gene (SLC26A4: Pendrin) are present in the sera of patients with Hashimoto's thyroiditis (3,4). Furthermore, Graves' hyperthyroidism is caused by production of thyroid-stimulating antibodies (TSAbs) against the thyroid-stimulating hormone receptor (TSHR) (5). Although the production of autoantibodies requires disruption of self-tolerance and activation of an adaptive immune response, the underlying molecular mechanisms that trigger such changes are still unknown.
Host defense mechanisms consist of innate immunity, which mediates the initial phase of protection by inducing secretion of cytokines such as interferons (IFNs), followed by adaptive or acquired immunity, which develops slowly but is much more specific and effective. Infection or tissue injury triggers innate immune reactions that constitute the first line of defense against invading pathogens, leading to the initiation of inflammation, clearance of pathogens, and tissue repair (6–9).
Activation of innate immunity and the resultant secretion of pro-inflammatory cytokines stimulate B lymphocytes to differentiate into plasma cells and to produce antibodies (10). A primary challenge to the innate immune system is the recognition of a large number of pathogens by using a restricted number of receptors. This challenge has been met by the evolution of so-called pattern recognition receptors (PRRs). Toll-like receptors (TLRs) were the first such identified receptors that are responsible for sensing the presence of microorganisms and mounting innate immune responses (6,7,9). TLRs are named for their similarity to Toll, a Drosophila receptor essential for the protection against fungal infection by synthesizing antimicrobial peptides. After the discovery of TLRs, several classes of cytosolic PRRs, including Nod-like receptors (NLRs), retinoic acid–inducible gene (RIG)-I–like receptors (RLR), C-type lectin receptors (CLR), and cytosolic DNA sensors, were identified (6–9). Understanding of innate immune mechanisms greatly advanced with the discovery of PRRs. For example, it is now known that PRRs recognize conserved motifs on pathogens. They are now termed pathogen-associated molecular patterns (PAMPs). PAMPs include lipids, lipoproteins, proteins, and nucleic acids derived from a wide range of microbes such as bacteria, viruses, parasites, and fungi. PAMPs currently under analysis include lipopolysaccharides (LPS), peptidoglycans (PGN), and viral double-stranded RNAs (dsRNA) (11–15).
Increasing evidence indicates that PRRs are expressed not only by immunocompetent cells, but also by many other cells, including epithelial cells, endothelial cells, and fibroblasts (7,16). Moreover, in such cells, PRR-mediated activation of innate immune responses can lead to the development of various disorders, including autoimmunity (15,17,18). It was demonstrated that rat and human thyroid cells express functional TLR3, which recognizes dsRNA (19,20). Also, it was shown that TLR4, which mediates responses to LPS, is expressed in FRTL-5 cells (21,22). Moreover, the expression of TLR4 and accessory molecules with a basolateral localization has been described in normal rat thyroid cells (22). This evidence indicates that thyroid cells have the capacity to activate innate immune responses.
In addition to infection, sterile tissue damage can be caused by ischemia/reperfusion injury, trauma, or other noxious stimuli (23). Tissue damage releases intracellular molecules and/or cleaves extracellular matrix molecules that evoke innate immune responses aimed at repairing the damage. These molecules are now called danger (or damage)-associated molecular patterns (DAMPs). They include genomic DNA fragments, heat shock proteins, high-mobility group B1 protein, uric acid, and cleaved extracellular matrix molecules such as collagen and hyaluronic acid (24,25). DAMPs are recognized by a wide variety of cells including thyrocytes (25–28), and they can further activate innate immune responses, leading to acquired immune responses that partly overlap with those of PAMPs (15,23). Cellular recognition of PAMPs and DAMPs and the activation of innate immune responses establish the molecular basis of adjuvant effects that enhance adaptive immune responses (28,29). When genetic factors that enhance autoimmune susceptibility and environmental factors are combined, a persistent cycle of autoimmune reactivity may be initiated (30).
Thus, innate immune activation plays essential roles in the killing or clearance of pathogens and dead cells, causing local inflammation and triggering acquired immune reactions. In some cases, those reactions may be directed against autoantigens in the thyroid. To understand the ability of thyroid cells to launch such innate immune responses, we have performed in-depth studies to evaluate the gene expression patterns related to innate immune activation against various PAMPs and DAMPs using primary cultures of human and mouse thyroid cells, as well as the rat thyroid cell line, FRTL-5.
Materials and Methods
Preparation of human thyroid follicles in suspension culture
This study was approved by the Ethics Committee of Tokyo Women's Medical University. Informed consent was obtained from all patients with Graves' disease before subtotal thyroidectomy. Human thyroid follicles were prepared and used as previously reported (20,31). In brief, thyroid tissue (15–30 g) obtained by subtotal thyroidectomy from patients with Graves' disease was minced with scissors into small pieces (3 mm×3 mm×3 mm). The dissected thyroid tissue was digested with 0.3 mg/mL collagenase (type IV; Worthington Biochemical Corp., Lakewood, NJ) and 5 mg/mL dispase (Godo Shusei Co., Tokyo, Japan) in Hanks' balanced salt solution at 32°C for 30 min. The digested material was filtered through nylon mesh (80 mesh), and the undigested tissue fragments were processed in the same manner once more. The second filtrate (containing thyroid follicles) was centrifuged at 70 g for 5 min, and the pellet was washed three times with F-12/RPMI-1640 (Sigma-Aldrich, St. Louis, MO) medium until the color of the pellet became white. We confirmed by microscopic observation and by the expression of lymphocyte-specific genes such as T-cell receptor and CD3 that there was no contamination of the immune cells. The washed thyroid follicles were resuspended in 15 mL of F-12/RPMI-1640 (1:1) medium supplemented with 0.5% fetal bovine serum and 10−8 M NaI (standard medium; about 1000–2000 follicles/mL). The follicles were added to 10 cm dishes, the bottoms of which had been coated with agarose.
Rat thyroid FRTL-5 cell culture
FRTL-5 rat thyroid cells were grown in Coon's modified Ham's F-12 medium containing 5% heat-treated, mycoplasma-free bovine serum (Invitrogen, Carlsbad, CA) and a mixture of six hormones, including bovine TSH (1 mU/mL), insulin (10 μg/mL), hydrocortisone (0.36 ng/mL), transferrin (5 μg/mL), glycyl-L-histidyl-L-lysine acetate (2 ng/mL), and somatostatin (10 ng/mL) as described (32). These reagents were purchased from Sigma-Aldrich.
Preparation and culture of mouse thyrocytes and myocytes
Female BALB/c mice were purchased from Japan SLC Inc. (Shizuoka, Japan) and housed in the animal facility of Yokohama City University Graduate School of Medicine. Animal experiments were conducted with the approval of the institutional laboratory animal care and use committee. Under anesthesia (subcutaneous injection of a 4:1 mixture of ketamine and xylazine), thyroid lobes were collected and mechanically disrupted using two 24-gauge needles in the same medium used for FRTL-5 cell culture. Tissue fragments were centrifuged for 1 min in a microcentrifuge. The fragments were dissolved in a 1.5 mL tube containing 1 mL digestion medium, which consisted of 0.9 mg/mL type I collagenase (Sigma-Aldrich) and 12 mg/mL dispase I (Godo Shusei Co.) in medium lacking serum. The suspension was incubated at 37°C for 45 min with shaking. After digestion, cells were centrifuged for 1 min in a microcentrifuge, and resuspended in 1 mL of medium and seeded in a poly-d-lysine coated 24-well plate (Greiner Bio One, Kremsmünster, Austria). Virtually all follicles and individual cells attached to the bottom of the dish after 24 h.
Myocytes were prepared by treating fascia and surrounding muscle tissues with 1 mL of digestion medium, which consisted of 0.9 mg/mL type I collagenase (Sigma-Aldrich), 2 mg/mL trypsin (Invitrogen), and 0.2% EDTA (Invitrogen) in growth medium (Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin; Sigma-Aldrich). After digestion, cells were centrifuged for 1 min in a microcentrifuge, and resuspended in 1 mL of growth medium. Cells were seeded in a poly-d-lysine coated 24-well plate (Greiner Bio One), and maintained in growth medium in a 37°C CO2 incubator.
Transfection of ds nucleic acids
One microgram of synthetic polynucleotides, poly(dA:dT) and poly(I:C) (GE Healthcare, Little Chalfont, United Kingdom) was mixed with 3 μL of Fugene6 transfection reagent (Roche Diagnostics, Basel, Switzerland) and 100 μL of serum-free medium and then incubated for 45 min at room temperature. The solution was added to cells and incubated for 6 h at 37°C in a CO2 incubator after which the medium was replaced with normal serum-containing culture medium.
Reverse transcription polymerase chain reaction and real-time polymerase chain reaction
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and reverse transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), as previously described (26). Touchdown PCR was performed using TaKaRa Ex Taq Hot Start Version (Takara Bio, Otsu, Japan) under the following conditions: 5 min at 94°C, 20 cycles of 30 sec at 94°C, 30 sec at 65–55°C (decreased in steps of 0.5°C/cycle until a temperature of 55°C was reached), 30 sec at 72°C, and then an additional 10–20 cycles (depending on the primer) of 30 sec at 94°C, 30 sec at 55°C, 30 sec at 72°C, and 7 min at 72°C. The polymerase chain reaction (PCR) products were separated by agarose gel electrophoresis and the DNA bands were visualized under ultraviolet light.
For real time PCR analysis, cDNAs were analyzed using either the TaqMan Gene Expression Assay or the SYBR green PCR Master Mix in accordance with the manufacturer's instructions using an ABI PRISM 7700 Sequence Detector (Applied Biosystems). The PCR thermal cycle conditions were set at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. Data were analyzed using ABI PRISM 7000 SDS Software Version 1.1 (Applied Biosystems). All samples were amplified in triplicate from the same RNA preparation, and the experiment was repeated three times. All the primers used in this study are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/thy).
Immunocytochemistry and acid-fast staining
FRTL-5 cells were grown on glass coverslips in 24-well plates for 24 h before the culture medium was exchanged with 6H5 containing Mycobacterium leprae and incubated for 24 h. M. leprae was prepared from the footpads of nude mice as described previously (33). Cells were fixed in 10% neutral buffered formalin for 5 min. They were then washed with Dulbecco's phosphate-buffered saline (DPBS) containing 0.4% Triton-X 100 (DPBST), incubated with either anti-TLR2 or TLR4 antibody (clones sc-10739 or sc-12511 respectively; Santa Cruz Biotechnology, Santa Cruz, CA) diluted to 1:100 for 24 h at 4°C, and washed again with DPBST. The signal was detected using peroxidase-labeled streptavidin-biotin (LSAB2 Kit; Dako, Carpenteria, CA) and 3,3-diaminobenzidine tetrahydrochloride (DAB) (33). Cells were then stained with carbol fuchsin and counterstained with hematoxylin to visualize acid-fast mycobacteria.
DNA microarray analysis
After the RNA had been extracted, oligo-DNA microarray was performed as described previously (20). Total RNA was isolated using an RNeasy Mini Kit (Qiagen) and reverse transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). cDNA obtained from thyroid follicles cultured in control or treated media was labeled with Cy3 or Cy5 respectively, and the expression levels of 41,000 gene spots were analyzed using cDNA microarray (Whole Human Genome Oligo Microarray Kit, Product No. G4112A; Agilent Technologies, Palo Alto, CA). Laser detection of the Cy3 and Cy5 signals on the microarray was performed with a nonconfocal laser leader, Gene PX 4000A (Axon Instruments, Union, CA). Fluorescence signal intensities and the Cy5/Cy3 ratios for each of the 41,000 oligo-DNAs were analyzed using the Gene PixPro 30 software package (Axon Instruments).
Transient transfections of plasmids and reporter gene assays
FRTL-5 cells (1×104) plated on poly-d-lysine coated 24-well plates (Greiner Bio One) were transfected with 0.3 μg of either a rat IFN-β promoter luciferase reporter plasmid (pGL3 IFN-β luc) or a 5×nuclear factor kappa–light-chain-enhancer of activated B cells (NFκB)–dependent luciferase reporter plasmid (pNFκB luc) using Fugene6 (Roche Diagnostics), as previously described (26). Cells were then stimulated with various ligands and reporter gene assays were performed with Bright-GloTM Luciferase assay systems (Promega) at the indicated times after stimulation. Luciferase activities were measured by FLUOstar galaxy (BMG Labtech, Offenburg, Germany), and the data were normalized to corresponding protein concentrations, which were determined using Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA).
Other reagents
Human spleen and human thyroid total RNAs were purchased by Clontech Laboratories (Mountain View, CA). Mouse IFN-γ was purchased from Roche. Peptidoglycan (PGN) from Staphylococcus aureus and lipopolysaccharides (LPS) from Escherichia coli 0111:B4 were purchased from Sigma-Aldrich. The nucleotide sequence of unmethylated CpG oligodeoxynucleotide (ODN) A3 was 5′-ATC GAC TCT CGA GCG TTC TC-3′ (11).
Results
Thyroid cells express functional TLRs
To study the expression pattern of TLRs in thyroid cells, we first examined mRNA from human thyroid tissue and rat thyroid cells, FRTL-5. Total RNA from human spleen cells, which express most members of the TLR family, was used as a control. As shown in Figure 1, both human and rat thyroid expressed variable levels of TLRs. Although mRNA from human thyroid tissue could contain mRNAs from other types of cells (fibroblasts, endothelial cells, and infiltrated leukocytes), FRTL-5 cells are pure thyroid cells without contamination by other cell types. Therefore, the results indicate that thyroid cells actually express TLRs as previously demonstrated for TLR3 and TLR4 expression in human and rat thyroid cells (19–22).
FIG. 1.
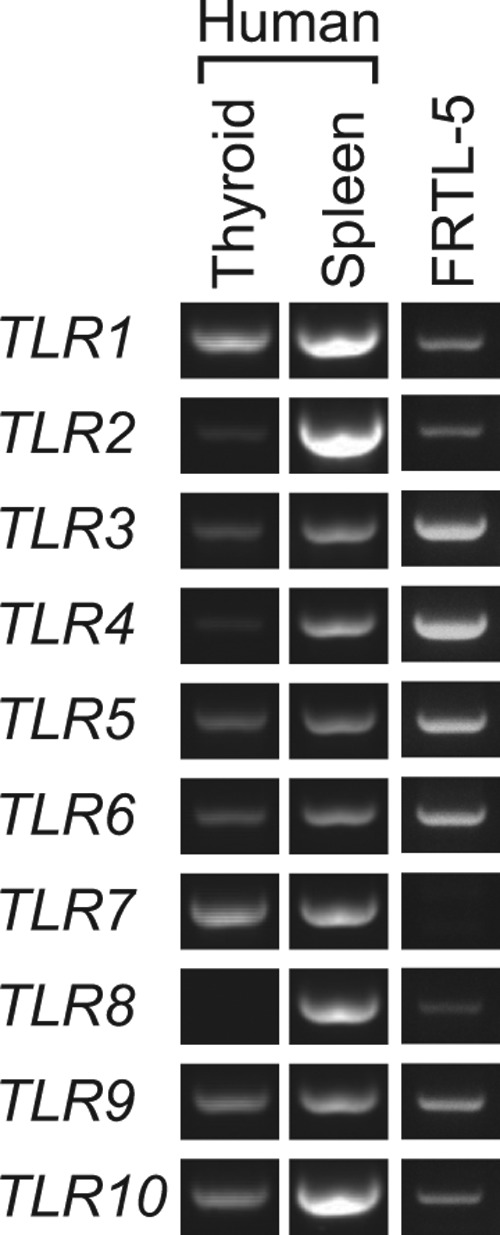
Toll-like receptor (TLR) mRNA was expressed in human thyroid tissues and rat thyroid FRTL-5 cells. Total RNA from human thyroid and spleen, as well as rat thyroid FRTL-5 cells, were reverse transcribed, and polymerase chain reaction (PCR) was performed targeting human and rat TLR1 through TLR10. PCRproducts were separated on 2.0% agarose gels, and DNA fragments of expected length were visualized under ultraviolet light after ethidium bromide staining. Human spleen cells served as controls for TLRs expression. Typical results from three different experiments are shown.
We next investigated whether these TLRs were functionally active and able to detect PAMPs and transduce intracellular signals. To do this, FRTL-5 cells were stimulated with various TLR ligands, such as dsRNA, LPS, CpG ODN, and PGN. Luciferase reporter gene activities for the IFN-β promoter and the NFκB-dependent promoter were evaluated (26,33,34). In addition to these PAMPs, the role of dsDNA as one of the DAMPs was also examined. We found that dsRNA (a ligand for TLR3), LPS (a ligand for TLR4), CpG ODN (a ligand for TLR9), and PGN (a ligand for TLR2) increased IFN-β promoter activity (Fig. 2A). dsRNA and LPS increased NFκB activity (Fig. 2B), which is consistent with the previous reports showing the functional expression of TLR3 and TLR4 in FRTL-5 cells (19–22). dsDNA also stimulated these two reporter genes as previously described (26).
FIG. 2.
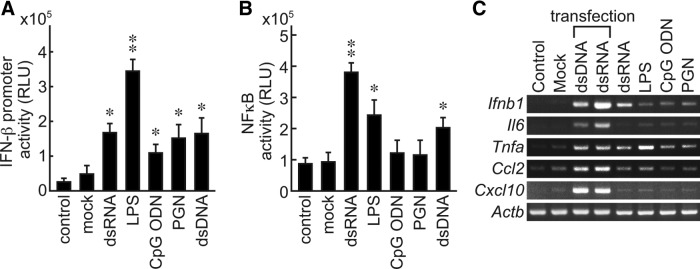
FRTL-5 thyroid cells responded to PAMPsand DAMPs to induce innate immune responses. FRTL-5 cells were transfected with a pGL3 IFN-β luc (A) or a pNFκB luc (B) and incubated for 24 h. Cells were stimulated with TLR ligands (dsRNA: 20 μg/mL; LPS: 0.3 μg/mL; CpG DNA: 5 μg/mL; PGN: 10 μg/mL) and incubated for another 6 h (A) or 12 h (B). Luciferase activities were measured and expressed as means±SD of relative light units (RLU) from four samples. *p≤1×10−2, **p≤1×10−5, compared with the control. (C) FRTL-5 cells were stimulated with TLR ligands (dsRNA: 20 μg/mL; LPS: 0.3 μg/mL; CpG DNA: 5 μg/mL PGN: 10 μg/mL). Cells transfected (tfx) with synthetic dsDNA (1.0 μg/well) or dsRNA (1.0 μg/well) served as controls. Total RNA was isolated at 6 h, and reverse transcription–PCR was performed. Typical results from at least four different experiments on different batches of cells are shown. PAMP, pathogen-associated molecular pattern; DAMP, danger-associated molecular pattern; IFN, interferon; pGL3 IFN-β luc, rat IFN-β promoter luciferase reporter plasmid; NFκB, nuclear factor kappa–light-chain-enhancer of activated B cells; pNFκB luc, 5×NFκB-dependent luciferase reporter plasmid; dsRNA, viral double-stranded RNAs; LPS, lipopolysaccharides; PGN, peptidoglycans.
To evaluate whether stimulation of TLRs actually induced innate immune responses in the thyroid cells, we examined the induction of mRNAs for several cytokines. Stimulation of the cells with synthetic dsDNA or dsRNA with a transfection reagent, which was shown to activate innate immune responses in FRTL-5 cells, served as positive controls (26,32). RT-PCR analysis revealed that dsRNA, LPS, CpG ODN, and PGN, ligands for TLR3, TLR4, TLR9, and TLR2 respectively, induced mRNA expression for Ifnb1 (IFN-β), Il6, tumor necrosis factor-α (Tnfa), Ccl2, and Cxcl10 (IP10) following stimulation (Fig. 2C). These responses were supported by observations that FRTL-5 cells rapidly engulfed bacteria and transported them from the plasma membrane to phagosomes (Fig. 3A) where TLR2 and TLR4 are colocalized (Fig. 3B and 3C respectively).
FIG. 3.
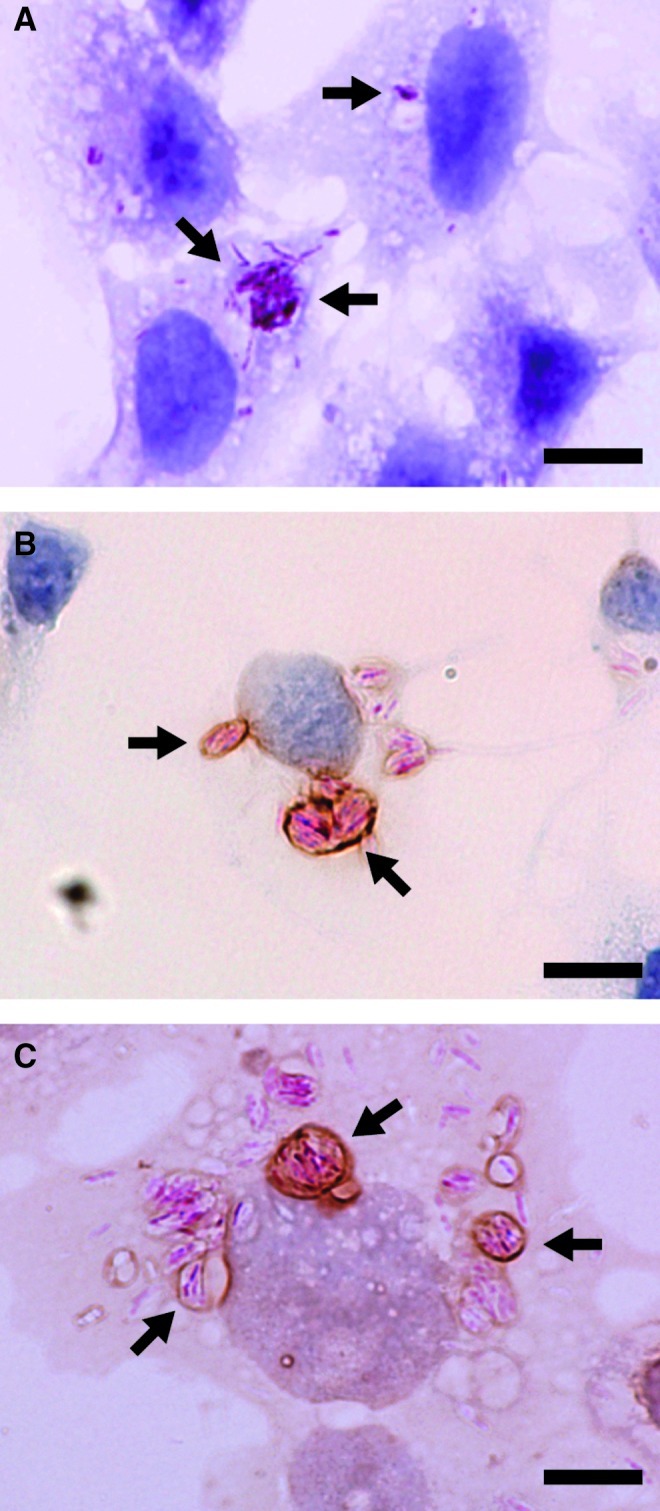
Engulfment of bacteria by FRTL-5 cells. FRTL-5 cells were cultured in a six-well plate and infected with Mycobacterium leprae (33). (A) Bacteria were visualized by acid-fast staining (pink-red coloration) and cells were counterstained with hematoxylin (purple). (B, C) FRTL-5 cells were immunostained for TLR2 (B) or TLR4 (C) followed by acid-fast staining and light hematoxylin counter-staining. Arrows indicate bacteria phagocytized in phagosomes. Original magnification:×1000 (scale bar=10 μm).
DNA microarray analysis of genes affected by dsRNA in human thyroid follicles
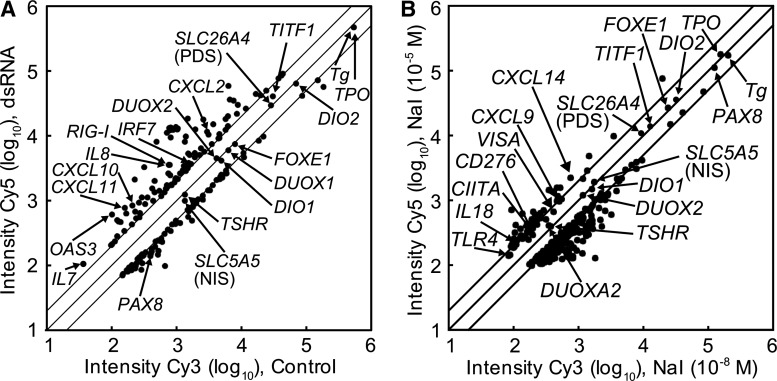
Among the TLR ligands tested, dsRNA showed the strongest ability to induce cytokines in FRTL-5 cells (Fig. 2C). We previously examined the effect of dsRNA on human thyroid after 6 h (20). However, to analyze thyrocyte responses better against PAMPs, we treated primary human thyroid follicles with dsRNA for 72 h and comprehensively evaluated affected genes using DNA microarray analysis. After 72 h of stimulation, dsRNA significantly increased the expression of various IFN-responsive genes, cytokines, and chemokines (Fig. 4A and Table 1). In addition to the cytokines, the expression level of retinoic acid-inducible gene (RIG-I), one of the receptors of dsRNA, was also upregulated 4.6-fold. Although expression of genes involved in the thyroid function was slightly decreased, PAX8 expression was suppressed to less than half of the original levels by dsRNA after 72 h (Supplementary Table S2).
FIG. 4.
DNA microarray analysis of genes expressed in cultured human thyroid follicles. Human thyroid follicles were pre-cultured in the absence of TSH for 4 days and in the presence of TSH (30 μU/mL) for 1 day. (A) dsRNA was added to a final concentration of 25 μg/mL. After an additional 2 days of culture, total RNA was extracted. (B) Cells were treated with low (10−8 M) or high (10−5 M) concentrations of NaI. After an additional 6 h of culture, total RNA was extracted. The mRNA expression levels were analyzed by DNA microarray as described in the Materials and Methods section. Dots in the scatter plot correspond to the fluorescence intensity of each gene on the microarray. Twofold and half-fold changes in expression are indicated as parallel lines.
Table 1.
Representative Genes Whose Expression Levels Were Significantly Increased After 72 h of dsRNA Stimulation in Human Thyroid
| Gene name | Description | Fold change |
|---|---|---|
| IFIT1 | Homo sapiens interferon-induced protein with tetratricopeptide repeats 1 (IFIT1), mRNA [NM_001548] | 12.20 |
| IFI27 | Homo sapiens interferon, alpha-inducible protein 27 (IFI27), transcript variant a, mRNA [NM_005532] | 11.40 |
| NM_080657 | Homo sapiens viperin (cig5), mRNA [NM_080657] | 9.10 |
| IFI44 | Homo sapiens interferon-induced protein 44 (IFI44), mRNA [NM_006417] | 6.95 |
| IFIT2 | Homo sapiens interferon-induced protein with tetratricopeptide repeats 2 (IFIT2), mRNA [NM_001547] | 6.77 |
| IFITM1 | Homo sapiens interferon induced transmembrane protein 1 (9–27) (IFITM1), mRNA [NM_003641] | 6.28 |
| OAS3 | Homo sapiens 2′-5′-oligoadenylate synthetase 3, 100kDa (OAS3), mRNA [NM_006187] | 5.96 |
| AF038963 | Homo sapiens RNA helicase (RIG-I) mRNA, complete cds [AF038963] | 4.55 |
| MX1 | Homo sapiens myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 (mouse) (MX1), mRNA [NM_002462] | 4.49 |
| IL8 | Homo sapiens interleukin 8 (IL8), mRNA [NM_000584] | 4.47 |
| BIRC3 | Homo sapiens baculoviral IAP repeat-containing 3 (BIRC3), transcript variant 1, mRNA [NM_001165] | 4.30 |
| OASL | Homo sapiens 2′-5′-oligoadenylate synthetase-like (OASL), transcript variant 1, mRNA [NM_003733] | 4.01 |
| CXCL11 | Homo sapiens chemokine (C-X-C motif) ligand 11 (CXCL11), mRNA [NM_005409] | 3.96 |
| CXCL2 | Homo sapiens chemokine (C-X-C motif) ligand 2 (CXCL2), mRNA [NM_002089] | 3.89 |
| EPSTI1 | Homo sapiens epithelial stromal interaction 1 (breast) (EPSTI1), mRNA [NM_033255] | 3.70 |
| IFIT4 | Homo sapiens interferon-induced protein with tetratricopeptide repeats 4 (IFIT4), mRNA [NM_001549] | 3.60 |
| CXCL10 | Homo sapiens chemokine (C-X-C motif) ligand 10 (CXCL10), mRNA [NM_001565] | 3.48 |
| NM_022147 | Homo sapiens 28kD interferon responsive protein (IFRG28), mRNA [NM_022147] | 3.44 |
| TRIM14 | Homo sapiens tripartite motif-containing 14 (TRIM14), transcript variant 2, mRNA [NM_033219] | 3.34 |
| SOD2 | Homo sapiens superoxide dismutase 2, mitochondrial (SOD2), mRNA [NM_000636] | 3.34 |
| CTSZ | Homo sapiens cathepsin Z (CTSZ), mRNA [NM_001336] | 3.33 |
| IL7 | Homo sapiens interleukin 7 (IL7), mRNA [NM_000880] | 3.07 |
| NFKBIA | Homo sapiens nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha (NFKBIA), mRNA [NM_020529] | 2.65 |
| IRF7 | Homo sapiens interferon regulatory factor 7 (IRF7), transcript variant d, mRNA [NM_004031] | 2.50 |
| CLDN1 | Homo sapiens claudin 1 (CLDN1), mRNA [NM_021101] | 2.32 |
| CXCL1 | Homo sapiens chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) (CXCL1), mRNA [NM_001511] | 2.31 |
| HLA-F | Homo sapiens major histocompatibility complex, class I, F (HLA-F), mRNA [NM_018950] | 2.14 |
| CXCL2 | Homo sapiens chemokine (C-X-C motif) ligand 2 (CXCL2), mRNA [NM_002089] | 2.02 |
Innate immune activation of the thyroid is induced by iodide
Excess iodide is related to various thyroid disorders, and iodide is known to induce chemokine secretion in human thyroid follicles in addition to the PAMPs and DAMPs (31). However, this study was performed 48–72 h after addition of iodide. Under those conditions, high concentrations of iodide may induce thyroid cell injury by modulating the redox state and generating reactive oxygen species (ROS) (35–37) and inducing necrosis and apoptosis in thyroid cells (38). Cell injury results in the release of DAMPs, for example genomic DNA fragments (26,32) that induce chemokine gene expression (26). We therefore re-examined the changes of gene expression of human thyroid follicles 6 h after addition of NaI in order to avoid possible effects of iodide-induced cell injury.
Primary human thyroid follicles were treated with a high concentration (10−5 M) of NaI for 6 h, and the pattern of gene expression was comprehensively evaluated using DNA microarray analysis and compared with a low concentration (10−8 M; Fig. 4B). Iodide increased mRNA levels of various chemokines after 6 h (Table 2), data that are in concordance with the previous results obtained after 72 h (31). Expression of thyroid-specific genes did not show significant changes after 6 h of iodide stimulation (Supplementary Table S3). In addition to the chemokines, mRNA levels of other immune-related genes (other than the chemokines listed in Table 2) were moderately increased, for example TLR4 (1.7-fold), mitochondrial antiviral-signaling protein (MAVS; 1.7-fold), and class II transactivator (CIITA; 1.7-fold). These results suggest that the changes in chemokine expression by iodide were direct effects rather than secondary to iodide-induced cell injury, although the underlying mechanism needs to be explored.
Table 2.
Representative Genes Whose Expression Levels Were Increased After 6 h of Iodide Stimulation of Human Thyroid Follicles
| Gene name | Description | Fold change |
|---|---|---|
| MED18 | Homo sapiens mediator of RNA polymerase II transcription, subunit 18 homolog (S. cerevisiae) (MED18), mRNA [NM_017638] | 10.89 |
| BPNT1 | Homo sapiens 3'(2'), 5'-bisphosphate nucleotidase 1 (BPNT1), mRNA [NM_006085] | 4.05 |
| ICAM4 | Homo sapiens intercellular adhesion molecule 4 (Landsteiner-Wiener blood group) (ICAM4), transcript variant 1, mRNA [NM_001544] | 3.63 |
| CYCS | Homo sapiens cytochrome c, somatic (CYCS), nuclear gene encoding mitochondrial protein, mRNA [NM_018947] | 3.61 |
| GNL3L | Homo sapiens guanine nucleotide binding protein-like 3 (nucleolar)-like (GNL3L), mRNA [NM_019067] | 3.59 |
| METTL7A | Homo sapiens methyltransferase like 7A (METTL7A), mRNA [NM_014033] | 3.53 |
| APOL6 | Homo sapiens apolipoprotein L, 6 (APOL6), mRNA [NM_030641] | 3.26 |
| GIPR | Homo sapiens gastric inhibitory polypeptide receptor (GIPR), mRNA [NM_000164] | 3.16 |
| ARHGAP26 | Homo sapiens Rho GTPase activating protein 26 (ARHGAP26), mRNA [NM_015071] | 3.08 |
| HFE | Homo sapiens hemochromatosis (HFE), transcript variant 6, mRNA [NM_139006] | 2.90 |
| CTSS | Homo sapiens cathepsin S (CTSS), mRNA [NM_004079] | 2.56 |
| FOXP4 | Homo sapiens forkhead box P4 (FOXP4), transcript variant 1, mRNA [NM_001012426] | 2.39 |
| CXCL14 | Homo sapiens chemokine (C-X-C motif) ligand 14 (CXCL14), mRNA [NM_004887] | 2.08 |
| CIITA | Homo sapiens class II, major histocompatibility complex, transactivator (CIITA), mRNA [NM_000246] | 1.74 |
| MAVS | Mitochondrial antiviral-signaling protein (MAVS), mRNA [NM_020746] | 1.74 |
| TLR4 | Homo sapiens toll-like receptor 4 (TLR4), mRNA [NM_138554] | 1.72 |
| CXCL9 | Homo sapiens chemokine (C-X-C motif) ligand 9 (CXCL9), mRNA [NM_002416] | 1.67 |
| IL18 | Homo sapiens interleukin 18 (interferon-gamma-inducing factor) (IL18), mRNA [NM_001562] | 1.51 |
| CD276 | Homo sapiens CD276 molecule (CD276), transcript variant 2, mRNA [NM_025240] | 1.51 |
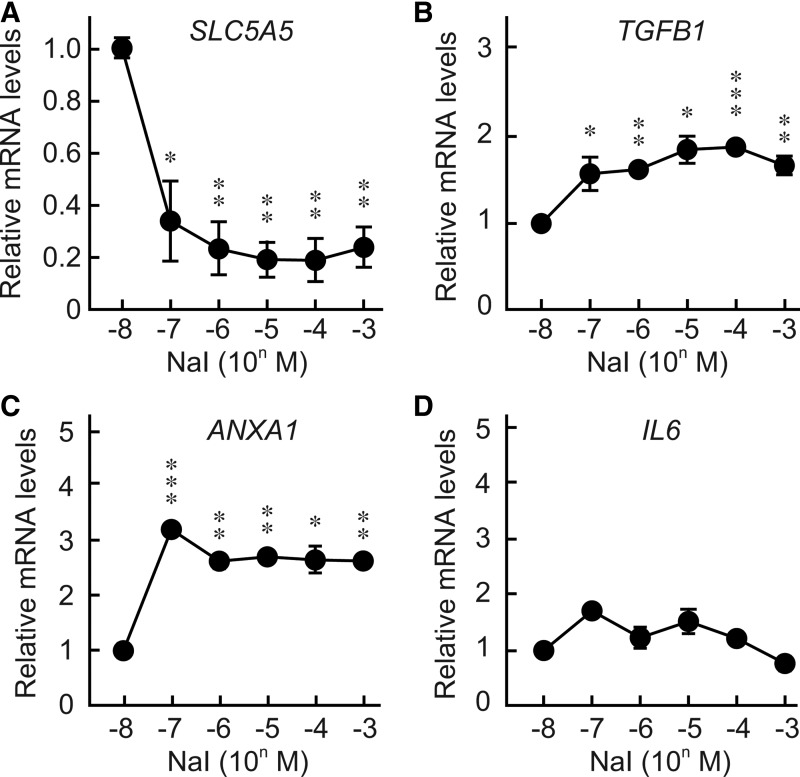
To clarify further the effect of iodide concentration on gene expression, quantitative real time PCR analysis was performed using mRNA from human thyroid follicles cultured in medium containing 10−8 to 10−3 M NaI. As previously reported, SLC5A5 expression was significantly suppressed by increasing concentrations of iodide (Fig. 5A) (27,31,39). The ability of iodide to decrease Slc5a5 expression was inhibited by perchlorate as seen in FRTL-5 cells (Supplementary Fig. S1). Realtime PCR analyses revealed that iodide increased the mRNA levels of TGFB1 (Fig. 5B), which regulates immune responses and inhibits thyroid growth and function by downregulating thyroid-specific genes, such as SLC5A5, TG, and TPO (40–43). Iodide also increased annexin-A1 (ANXA1), which is cleaved and released from apoptotic cells and inhibits the activation of monocytes and neutrophils (Fig. 5C) (44). Although expression of IL6 was significantly induced by DAMPs (Fig. 2C) (26) and PAMPs (20), it was not affected by iodide (Fig. 5A). This evidence again confirms that the observed effect was specific to iodide, but not secondary to effects of DAMPs.
FIG. 5.
Effects of various doses of iodide on the expression of mRNAs in cultured human thyroid follicles. Human thyroid follicles were pre-cultured for 5 days and then cultured in medium containing bovine TSH (30 μU/mL) and 10−8 to 10−3 M of NaI. After an additional 72 h of culture, total RNA was isolated, and the mRNA expression levels of SLC5A5 (NIS) (A), TGFB1 (B), ANAXA1 (C), and IL6 (D) were analyzed by real-time PCR. Results are expressed relative to mRNA levels found in human thyroid follicles treated with 10−8 M NaI. Data are means±SD of three samples. *p≤0.05, **p≤0.01, ***p≤0.001, compared with the control.
Immune activation in mouse primary thyrocytes and surrounding myocytes
In the case of infection and/or tissue injury, not only thyroid cells but also other surrounding cells and tissues could become involved, events that could affect the innate immune activation of the thyroid. We previously showed that both primary and cultured mouse fibroblasts respond to PAMPs (virus infection) and DAMPs (genomic DNA fragments) to activate innate immunity (29,45,46). The thyroid gland is covered with a thin layer of fascia and is surrounded by muscular tissues such as sternothyroid and sternocleidomastoid muscles. To elucidate further the immune activation of the thyroid and surrounding tissues, we simultaneously prepared primary mouse thyrocytes and surrounding myocytes in culture. They were stimulated by dsDNA and IFN-γ, and we compared the induction of gene expression in the cells with regard to innate and adaptive immunity by real time PCR analysis.
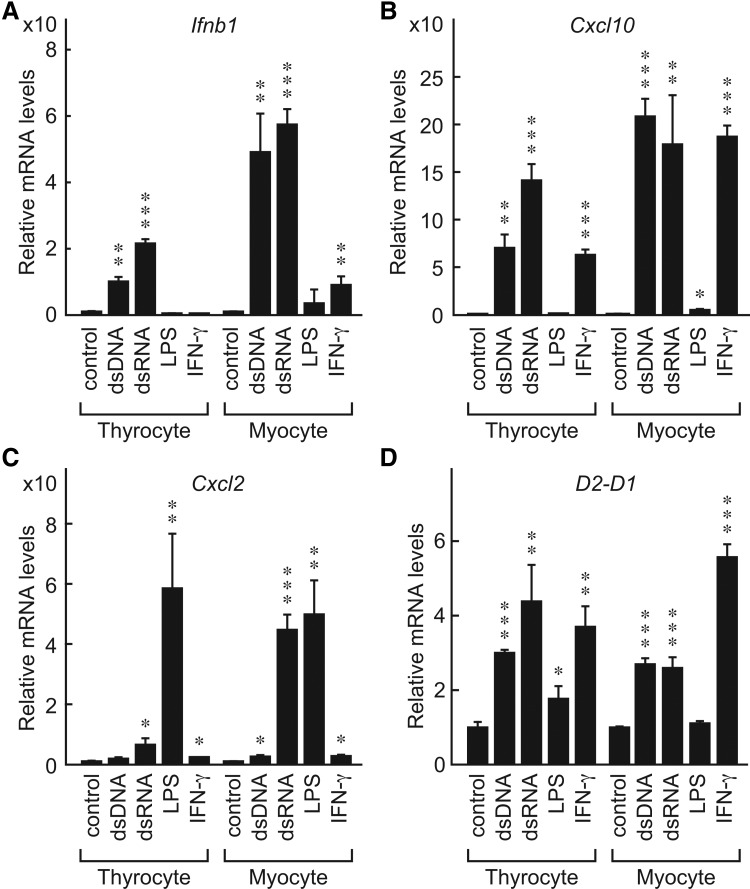
Most genes in primary thyrocytes and surrounding myocytes responded similarly (Fig. 6). In thyrocytes and myocytes, the expression of Ifnb1 (Fig. 6A) and Cxcl10 (Fig. 6B) was significantly induced by dsDNA and dsRNA. However, there were some differences in gene activation between the two cell types in responses to particular ligands. Thus, although IFN-γ increased Cxcl10 (Fig. 6B) and H2-D1 (Fig. 6D) in thyrocytes and myocytes, the effects on the expression of Ifnb1 and Cxcl2 were much weaker (Fig. 6A and C respectively). Expression of Cxcl2, but not other genes, was significantly induced by LPS in both cell types (Fig. 6C). These results suggest that in cases of infection or tissue injury, both thyrocytes and surrounding myocytes coordinately respond to the stimuli and activate both innate and adaptive immune responses that may induce local inflammation.
FIG. 6.
Primary mouse thyrocytes and surrounding myocytes coordinately responded to PAMPs. Primary mouse thyrocytes and surrounding myocytes were prepared from BALB/c mice as described in the Materials and Methods and stimulated with dsDNA (0.3 μg/well), dsRNA (0.3 μg/well), LPS (0.1 μg/mL), or IFN-γ (100 U/mL). Total RNA was isolated and the mRNA expression levels of Ifnb1 (A), Cxcl10 (B), Cxcl2 (C), and MHC class I (H2-D1) (D) were analyzed by real-time PCR. Results are expressed relative to control mRNA levels. Data are means±SD of three samples. *p≤0.05, **p≤0.01, ***p≤0.001, compared with the control.
Discussion
In this report, we utilized suspension cultures of primary human thyroid follicles, primary mouse thyroid cells, and cultured rat thyroid FRTL-5 cells, and demonstrated that thyroid cells have the ability to respond to various PAMPs, DAMPs, and high doses of iodide, thereby inducing immune activation in the absence of immune competent cells.
We have demonstrated in this study that FRTL-5 cells express functional PRRs such as TLR3, TLR4, TLR9, and TLR2, and produce proinflammatory cytokines and chemokines. We have also shown that TLR ligands such as LPS, dsRNA, CpG ODN, and PGN functionally activated the IFN-β promoter and/or NFκB activity in FRTL-5 cells. It was previously demonstrated that dsRNA is recognized by functional TLR3 in the human thyroid (19,20), that LPS is recognized by functional TLR4 in FRTL-5 cells (22), and that genomic dsDNA fragments are recognized by a cytosolic nucleic acid sensor, histone H2B, in FRTL-5 (26). Furthermore, it was clearly demonstrated that recognition of LPS by TLR4 results in activation of NFκB, thereby inducing the expression of the sodium iodide symporter (NIS; Slc5a5) in FRTL-5 cells (21). Although we assume dsRNA is mainly recognized by TLR3 (20), it is possible that endocytosed dsRNA is recognized by other molecules such as melanoma-differentiation-associated gene 5 (MDA5) and retinoic acid–inducible protein I (RIG-I) (47–49). Since FRTL-5 cells are an established thyroid cell line, the present results confirm that thyrocytes are capable of responding to various PAMPs to launch innate immune reactions, even in the absence of infiltrating immune cells. Although the recognition of PAMPs requires attachment and engulfment of pathogens, a recent study has shown that TLRs promote the clearance of bacteria by upregulating phagocytic activity (50). Thyroid cells show strong phagocytosis under TSH stimulation (51). Thus, active engulfment of bacteria to demonstrated in FRTL-5 cells in this study (Fig. 3) may be partly due to upregulation by TSH.
Iodide is one of the risk factors for the development of AITD. It has been reported that high concentrations of iodide induced thyroid cell injury and subsequent inflammation with production of reactive oxygen species in NOD.H-2h4 mice (36). The excess iodide uptake results in chemokine production that recruits immunocompetent cells to the thyroid (31), which may contribute to the initiation of thyroid autoimmunity. It seems that iodide preferentially induces chemokines rather than other inflammatory cytokines as shown in this study, suggesting the possibility that the iodide-induced intracellular signaling cascade may be different from that of PRRs. In this study, we have shown that iodide induces TGFB1 expression. It has been shown that TGF-β inhibits TSH-induced SLC5A5 mRNA and protein levels in a dose-dependent manner (42). TGF-β modulates the function of recruited lymphocytes depending on the cytokine milieu, such as IL-2 and IL-6 produced by peripheral tissue and immune competent cells (43,52,53).
The recent identification of PRRs has revealed that endogenous molecules (DAMPs) produced by dying cells can also induce innate immune responses (23,29). Cell damage can be induced by various insults such as ischemia/reperfusion injury, trauma, or other harmful stimuli that induce apoptosis or necrosis. In such situations, fragments of genomic DNA may be released and are recognized by cytosolic histone H2B, which in turn activates innate and acquired immune systems (26). We have shown in the present study that surrounding myocytes stimulated with nucleic acids, LPS, or IFN-γ also express Ifnb1 and several chemokines, suggesting that surrounded mesenchymal cells also contribute to immune activation in local thyroid tissue. Moreover, the differences between thyrocytes and myocytes in response to several ligands suggest that these cells have distinctive potentials in their response to PAMPs and DAMPs, activating genes related to innate and acquired immune responses.
In summary, we have demonstrated that thyroid cells can respond to PAMPs, DAMPs, and high concentrations of iodide. In their response, the cells produce inflammatory cytokines, chemokines, and type I IFNs, resulting in innate immune activation. Surrounding myocytes and recruited immunocompetent cells also contributed to immune responses in the thyroid gland. These results provide new details on the mechanisms underlying immune activation in the thyroid. This knowledge further contributes to our understanding of processes triggering autoimmune reactions in this important organ.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Takao Obara (Tokyo Women's Medical University) for providing thyroid tissue and Drs. Kazue Takano (Tokyo Women's Medical University), Emiko Yamada and Tetsu Yamada (both in Kanaji Hospital) for valuable support. This work was supported in part by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (#15390296 and #21591187 to K.S.).
Author Disclosure Statement
The authors declare that no conflicts of interest exist.
References
- 1.Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–340. doi: 10.1080/08916930410001705394. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida H. Amino N. Yagawa K. Uemura K. Satoh M. Miyai K. Kumahara Y. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab. 1978;46:859–862. doi: 10.1210/jcem-46-6-859. [DOI] [PubMed] [Google Scholar]
- 3.Raspe E. Costagliola S. Ruf J. Mariotti S. Dumont JE. Ludgate M. Identification of the thyroid Na+/I− cotransporter as a potential autoantigen in thyroid autoimmune disease. Eur J Endocrinol. 1995;132:399–405. doi: 10.1530/eje.0.1320399. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida A. Hisatome I. Taniguchi S. Shirayoshi Y. Yamamoto Y. Miake J. Ohkura T. Akama T. Igawa O. Shigemasa C. Kamijo K. Ikuyama S. Caturegli P. Suzuki K. Pendrin is a novel autoantigen recognized by patients with autoimmune thyroid diseases. J Clin Endocrinol Metab. 2009;94:442–448. doi: 10.1210/jc.2008-1732. [DOI] [PubMed] [Google Scholar]
- 5.Ealey PA. Kohn LD. Ekins RP. Marshall NJ. Characterization of monoclonal antibodies derived from lymphocytes from Graves' disease patients in a cytochemical bioassay for thyroid stimulators. J Clin Endocrinol Metab. 1984;58:909–914. doi: 10.1210/jcem-58-5-909. [DOI] [PubMed] [Google Scholar]
- 6.Aderem A. Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T. Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 8.Kawashima A. Tanigawa K. Akama T. Yoshihara A. Ishii N. Suzuki K. Innate immune activation and thyroid autoimmunity. J Clin Endocrinol Metab. 2011;96:3661–3671. doi: 10.1210/jc.2011-1568. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K. Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 10.Bekeredjian-Ding I. Jego G. Toll-like receptors-sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinman DM. Yi AK. Beaucage SL. Conover J. Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jelinek I. Leonard JN. Price GE. Brown KN. Meyer-Manlapat A. Goldsmith PK. Wang Y. Venzon D. Epstein SL. Segal DM. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol. 2011;186:2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartholomeu DC. Ropert C. Melo MB. Parroche P. Junqueira CF. Teixeira SM. Sirois C. Kasperkovitz P. Knetter CF. Lien E. Latz E. Golenbock DT. Gazzinelli RT. Recruitment and endo-lysosomal activation of TLR9 in dendritic cells infected with Trypanosoma cruzi. J Immunol. 2008;181:1333–1344. doi: 10.4049/jimmunol.181.2.1333. [DOI] [PubMed] [Google Scholar]
- 14.Prantner D. Darville T. Nagarajan UM. Stimulator of IFN gene is critical for induction of IFN-beta during Chlamydia muridarum infection. J Immunol. 2010;184:2551–2560. doi: 10.4049/jimmunol.0903704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi O. Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Kufer TA. Sansonetti PJ. NLR functions beyond pathogen recognition. Nat Immunol. 2011;12:121–128. doi: 10.1038/ni.1985. [DOI] [PubMed] [Google Scholar]
- 17.Munz C. Lunemann JD. Getts MT. Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol. 2010;11:28–35. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
- 19.Harii N. Lewis CJ. Vasko V. McCall K. Benavides-Peralta U. Sun X. Ringel MD. Saji M. Giuliani C. Napolitano G. Goetz DJ. Kohn LD. Thyrocytes express a functional toll-like receptor 3: overexpression can be induced by viral infection and reversed by phenylmethimazole and is associated with Hashimoto's autoimmune thyroiditis. Mol Endocrinol. 2005;19:1231–1250. doi: 10.1210/me.2004-0100. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki K. Suzuki K. Yamada E. Yamada T. Takeshita F. Matsumoto M. Mitsuhashi T. Obara T. Takano K. Sato K. Suppression of iodide uptake and thyroid hormone synthesis with stimulation of the type I interferon system by double-stranded ribonucleic acid in cultured human thyroid follicles. Endocrinology. 2007;148:3226–3235. doi: 10.1210/en.2006-1638. [DOI] [PubMed] [Google Scholar]
- 21.Nicola JP. Nazar M. Mascanfroni ID. Pellizas CG. Masini-Repiso AM. NF-kappaB p65 subunit mediates lipopolysaccharide-induced Na(+)/I(−) symporter gene expression by involving functional interaction with the paired domain transcription factor Pax8. Mol Endocrinol. 2010;24:1846–1862. doi: 10.1210/me.2010-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicola JP. Velez ML. Lucero AM. Fozzatti L. Pellizas CG. Masini-Repiso AM. Functional toll-like receptor 4 conferring lipopolysaccharide responsiveness is expressed in thyroid cells. Endocrinology. 2009;150:500–508. doi: 10.1210/en.2008-0345. [DOI] [PubMed] [Google Scholar]
- 23.Kono H. Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 25.Green DR. Ferguson T. Zitvogel L. Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawashima A. Tanigawa K. Akama T. Wu H. Sue M. Yoshihara A. Ishido Y. Kobiyama K. Takeshita F. Ishii KJ. Hirano H. Kimura H. Sakai T. Ishii N. Suzuki K. Fragments of genomic DNA released by injured cells activate innate immunity and suppress endocrine function in the thyroid. Endocrinology. 2011;152:1702–1712. doi: 10.1210/en.2010-1132. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki K. Mori A. Saito J. Moriyama E. Ullianich L. Kohn LD. Follicular thyroglobulin suppresses iodide uptake by suppressing expression of the sodium/iodide symporter gene. Endocrinology. 1999;140:5422–5430. doi: 10.1210/endo.140.11.7124. [DOI] [PubMed] [Google Scholar]
- 28.Ishii KJ. Suzuki K. Coban C. Takeshita F. Itoh Y. Matoba H. Kohn LD. Klinman DM. Genomic DNA released by dying cells induces the maturation of APCs. J Immunol. 2001;167:2602–2607. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 29.Ishii KJ. Coban C. Kato H. Takahashi K. Torii Y. Takeshita F. Ludwig H. Sutter G. Suzuki K. Hemmi H. Sato S. Yamamoto M. Uematsu S. Kawai T. Takeuchi O. Akira S. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson EM. Tomer Y. The genetic basis of thyroid autoimmunity. Thyroid. 2007;17:949–961. doi: 10.1089/thy.2007.0153. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki K. Tanigawa K. Suzuki K. Yamada E. Yamada T. Takano K. Obara T. Sato K. Iodide-induced chemokines and genes related to immunological function in cultured human thyroid follicles in the presence of thyrotropin. Thyroid. 2010;20:67–76. doi: 10.1089/thy.2009.0242. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K. Mori A. Ishii KJ. Saito J. Singer DS. Klinman DM. Krause PR. Kohn LD. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc Natl Acad Sci USA. 1999;96:2285–2290. doi: 10.1073/pnas.96.5.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanigawa K. Suzuki K. Kimura H. Takeshita F. Wu H. Akama T. Kawashima A. Ishii N. Tryptophan aspartate-containing coat protein (CORO1A) suppresses Toll-like receptor signalling in Mycobacterium leprae infection. Clin Exp Immunol. 2009;156:495–501. doi: 10.1111/j.1365-2249.2009.03930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeshita F. Suzuki K. Sasaki S. Ishii N. Klinman DM. Ishii KJ. Transcriptional regulation of the human TLR9 gene. J Immunol. 2004;173:2552–2561. doi: 10.4049/jimmunol.173.4.2552. [DOI] [PubMed] [Google Scholar]
- 35.Leoni SG. Kimura ET. Santisteban P. De la Vieja A. Regulation of thyroid oxidative state by thioredoxin reductase has a crucial role in thyroid responses to iodide excess. Mol Endocrinol. 2011;25:1924–1935. doi: 10.1210/me.2011-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Many MC. Maniratunga S. Varis I. Dardenne M. Drexhage HA. Denef JF. Two-step development of Hashimoto-like thyroiditis in genetically autoimmune prone non-obese diabetic mice: effects of iodine-induced cell necrosis. J Endocrinol. 1995;147:311–320. doi: 10.1677/joe.0.1470311. [DOI] [PubMed] [Google Scholar]
- 37.Sharma R. Traore K. Trush MA. Rose NR. Burek CL. Intracellular adhesion molecule-1 up-regulation on thyrocytes by iodine of non-obese diabetic.H2(h4) mice is reactive oxygen species-dependent. Clin Exp Immunol. 2008;152:13–20. doi: 10.1111/j.1365-2249.2008.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golstein J. Dumont JE. Cytotoxic effects of iodide on thyroid cells: difference between rat thyroid FRTL-5 cell and primary dog thyrocyte responsiveness. J Endocrinol Invest. 1996;19:119–126. doi: 10.1007/BF03349847. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K. Kimura H. Wu H. Kudo N. Kim WB. Suzuki S. Yoshida A. Caturegli P. Kohn LD. Excess iodide decreases transcription of NIS and VEGF genes in rat FRTL-5 thyroid cells. Biochem Biophys Res Commun. 2010;393:286–290. doi: 10.1016/j.bbrc.2010.01.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura A. Wakabayashi Y. Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem. 2010;147:781–792. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pisarev MA. Thomasz L. Juvenal GJ. Role of transforming growth factor beta in the regulation of thyroid function and growth. Thyroid. 2009;19:881–892. doi: 10.1089/thy.2007.0303. [DOI] [PubMed] [Google Scholar]
- 42.Costamagna E. Garcia B. Santisteban P. The functional interaction between the paired domain transcription factor Pax8 and Smad3 is involved in transforming growth factor-beta repression of the sodium/iodide symporter gene. J Biol Chem. 2004;279:3439–3446. doi: 10.1074/jbc.M307138200. [DOI] [PubMed] [Google Scholar]
- 43.Nicolussi A. D'Inzeo S. Santulli M. Colletta G. Coppa A. TGF-beta control of rat thyroid follicular cells differentiation. Mol Cell Endocrinol. 2003;207:1–11. doi: 10.1016/s0303-7207(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 44.Pupjalis D. Goetsch J. Kottas DJ. Gerke V. Rescher U. Annexin A1 released from apoptotic cells acts through formyl peptide receptors to dampen inflammatory monocyte activation via JAK/STAT/SOCS signalling. EMBO Mol Med. 2011;3:102–114. doi: 10.1002/emmm.201000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeshita F. Tanaka T. Matsuda T. Tozuka M. Kobiyama K. Saha S. Matsui K. Ishii KJ. Coban C. Akira S. Ishii N. Suzuki K. Klinman DM. Okuda K. Sasaki S. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J Virol. 2006;80:6218–6224. doi: 10.1128/JVI.00121-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobiyama K. Takeshita F. Jounai N. Sakaue-Sawano A. Miyawaki A. Ishii KJ. Kawai T. Sasaki S. Hirano H. Ishii N. Okuda K. Suzuki K. Extrachromosomal histone H2B mediates innate antiviral immune responses induced by intracellular double-stranded DNA. J Virol. 2010;84:822–832. doi: 10.1128/JVI.01339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baccala R. Hoebe K. Kono DH. Beutler B. Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 48.Diebold SS. Montoya M. Unger H. Alexopoulou L. Roy P. Haswell LE. Al-Shamkhani A. Flavell R. Borrow P. Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 49.Kato H. Takeuchi O. Sato S. Yoneyama M. Yamamoto M. Matsui K. Uematsu S. Jung A. Kawai T. Ishii KJ. Yamaguchi O. Otsu K. Tsujimura T. Koh CS. Reis e Sousa C. Matsuura Y. Fujita T. Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 50.Kong L. Ge BX. MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 2008;18:745–755. doi: 10.1038/cr.2008.65. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki K. Lavaroni S. Mori A. Yamamoto K. Yi X. Miyagi E. Katoh R. Kohn LD. Kawaoi A. Thyroglobulin: a master regulator of follicular function via transcriptional suppression of thyroid specific genes. Acta Histochem Cytochem. 1999;32:111–119. [Google Scholar]
- 52.Davidson TS. DiPaolo RJ. Andersson J. Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 53.Li MO. Wan YY. Sanjabi S. Robertson AK. Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.