Abstract
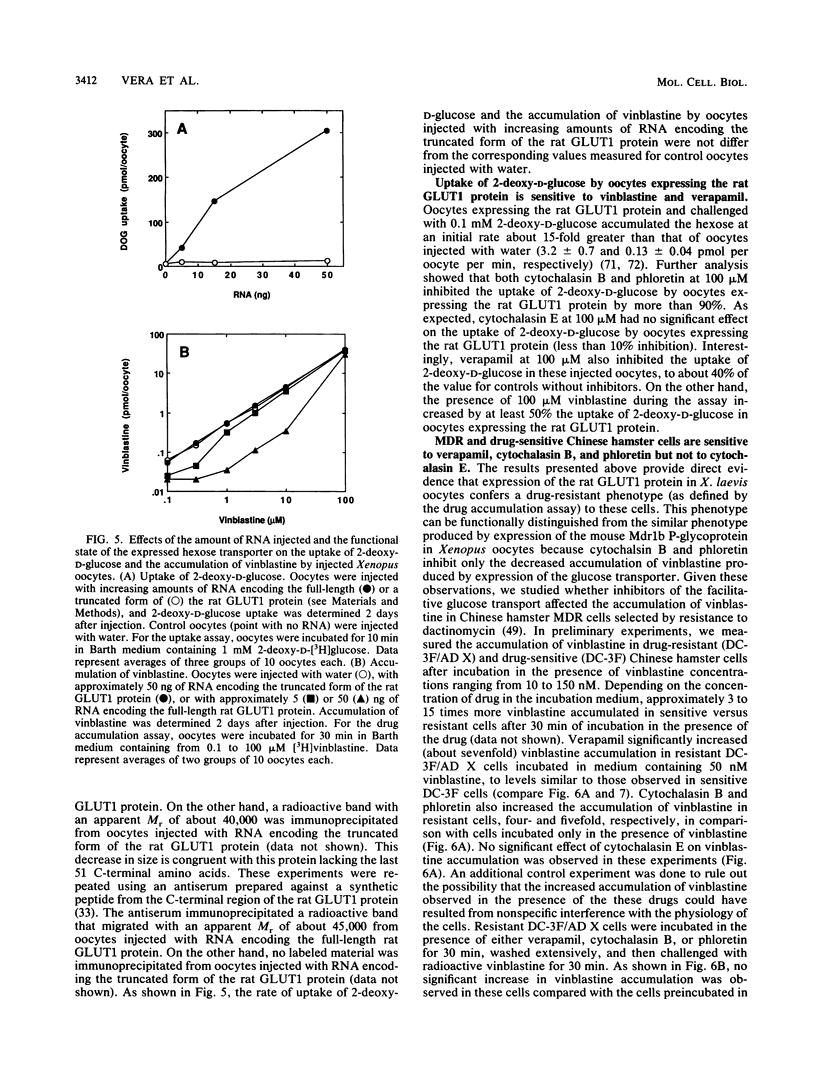
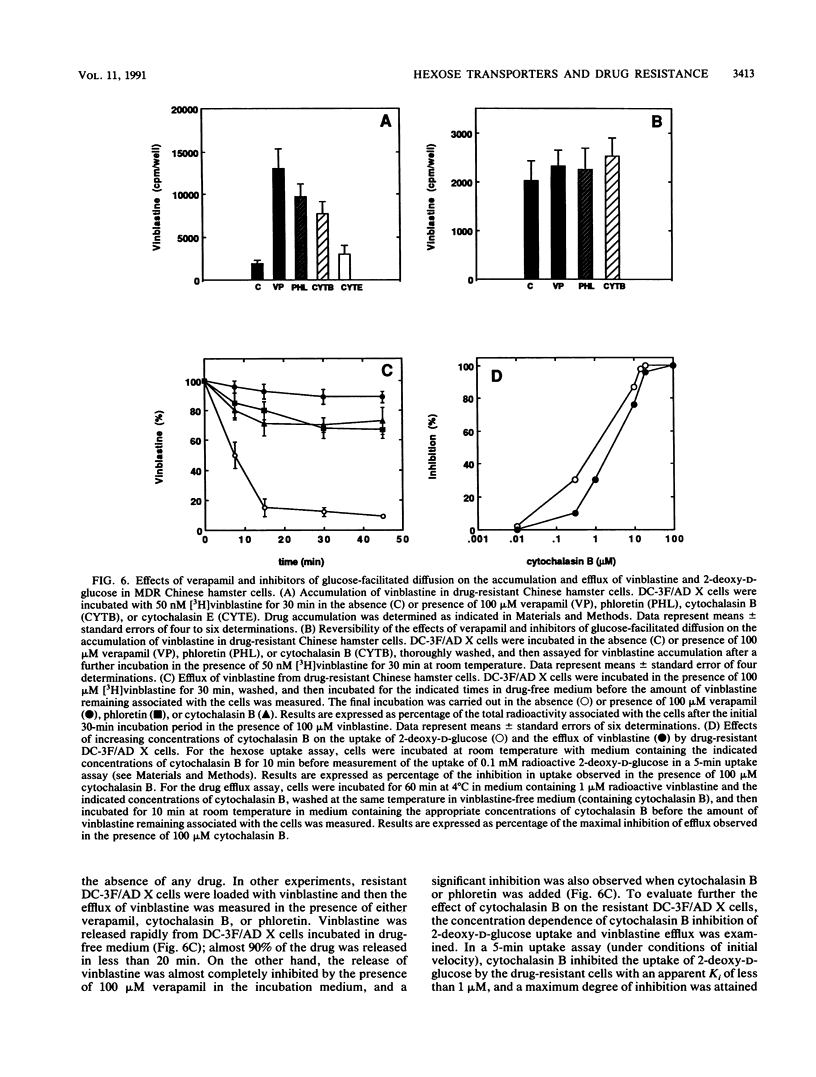
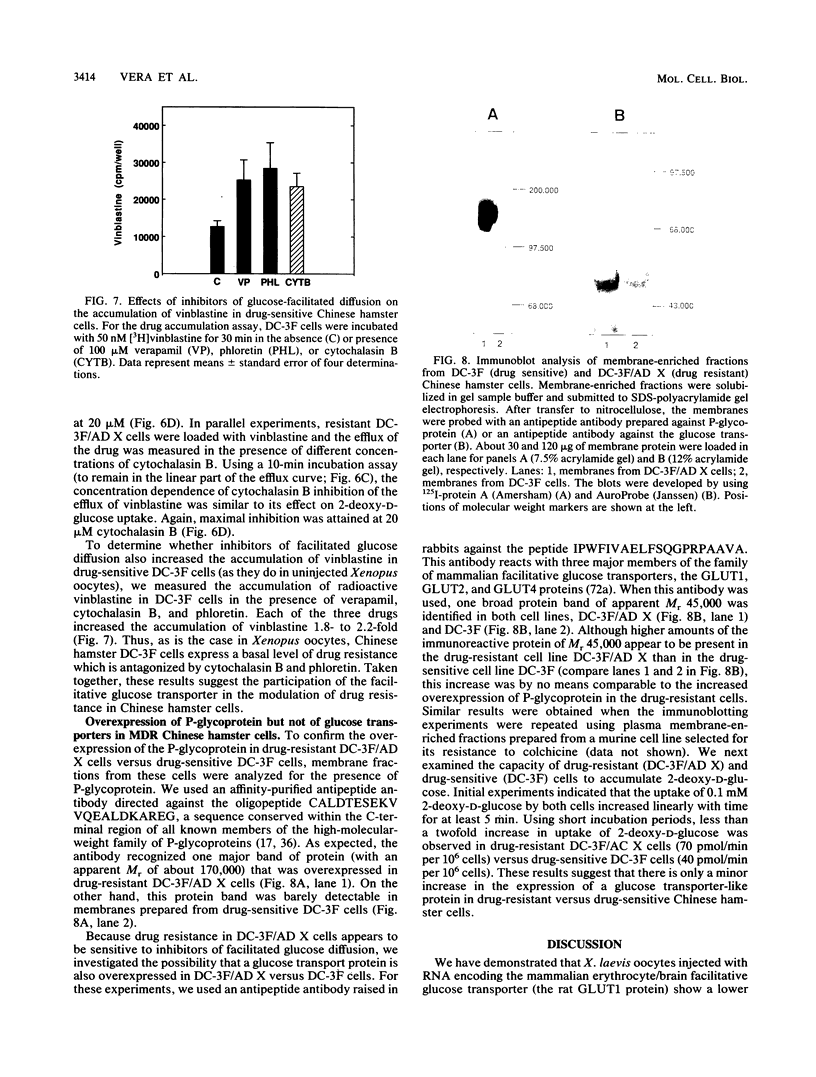
We show that D- but not L-hexoses modulate the accumulation of radioactive vinblastine in injected Xenopus laevis oocytes expressing the murine Mdr1b P-glycoprotein. We also show that X. laevis oocytes injected with RNA encoding the rat erythroid/brain glucose transport protein (GLUT1) and expressing the corresponding functional transporter exhibit a lower accumulation of [3H]vinblastine and show a greater capacity to extrude the drug than do control oocytes not expressing the rat GLUT1 protein. Cytochalasin B and phloretin, two inhibitors of the mammalian facilitative glucose transporters, can overcome the reduced drug accumulation conferred by expression of the rat GLUT1 protein in Xenopus oocytes but have no significant effect on the accumulation of drug by Xenopus oocytes expressing the mouse Mdr1b P-glycoprotein. These drugs also increase the accumulation of [3H]vinblastine in multidrug-resistant Chinese hamster ovary cells. Cytochalasin E, an analog of cytochalasin B that does not affect the activity of the facilitative glucose transporter, has no effect on the accumulation of vinblastine by multidrug-resistant Chinese hamster cells or by oocytes expressing either the mouse Mdr1b P-glycoprotein or the GLUT1 protein. In all three cases, the drug verapamil produces a profound effect on the cellular accumulation of vinblastine. Interestingly, although immunological analysis indicated the presence of massive amounts of P-glycoprotein in the multidrug-resistant cells, immunological and functional studies revealed only a minor increase in the expression of a hexose transporter-like protein in resistant versus drug-sensitive cells. Taken together, these results suggest the participation of the mammalian facilitative glucose transporter in the development of drug resistance.
Full text
PDF

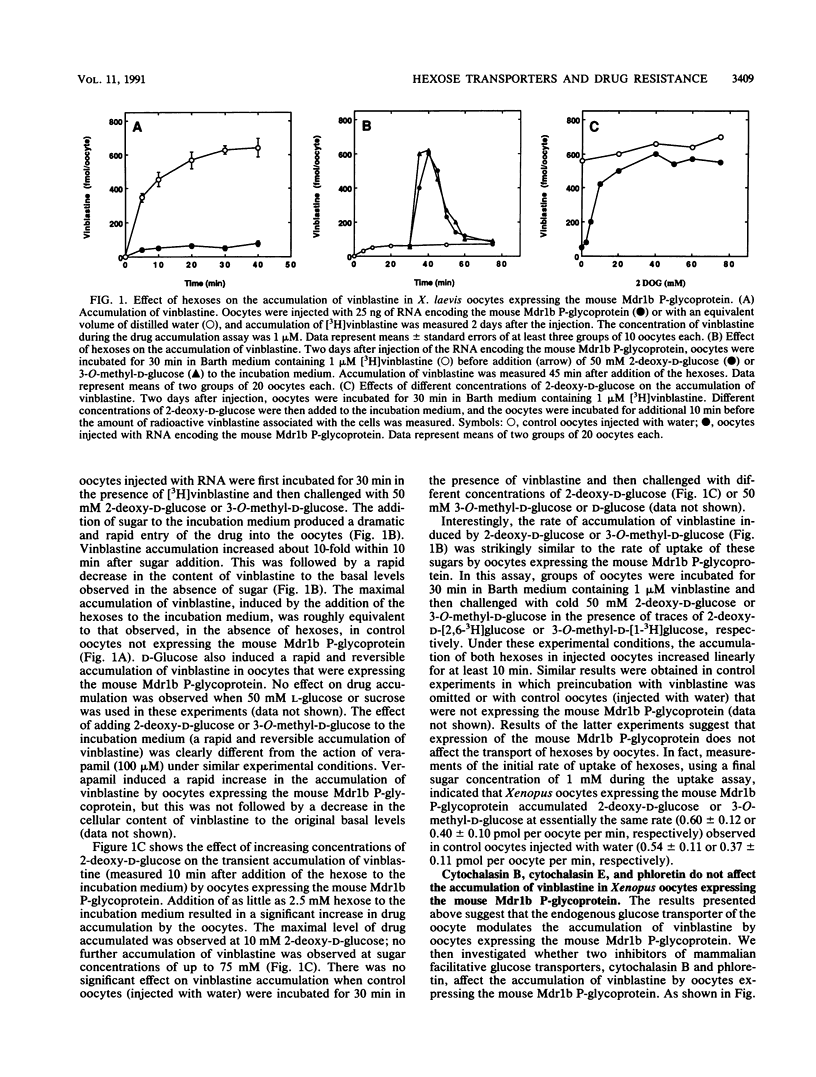
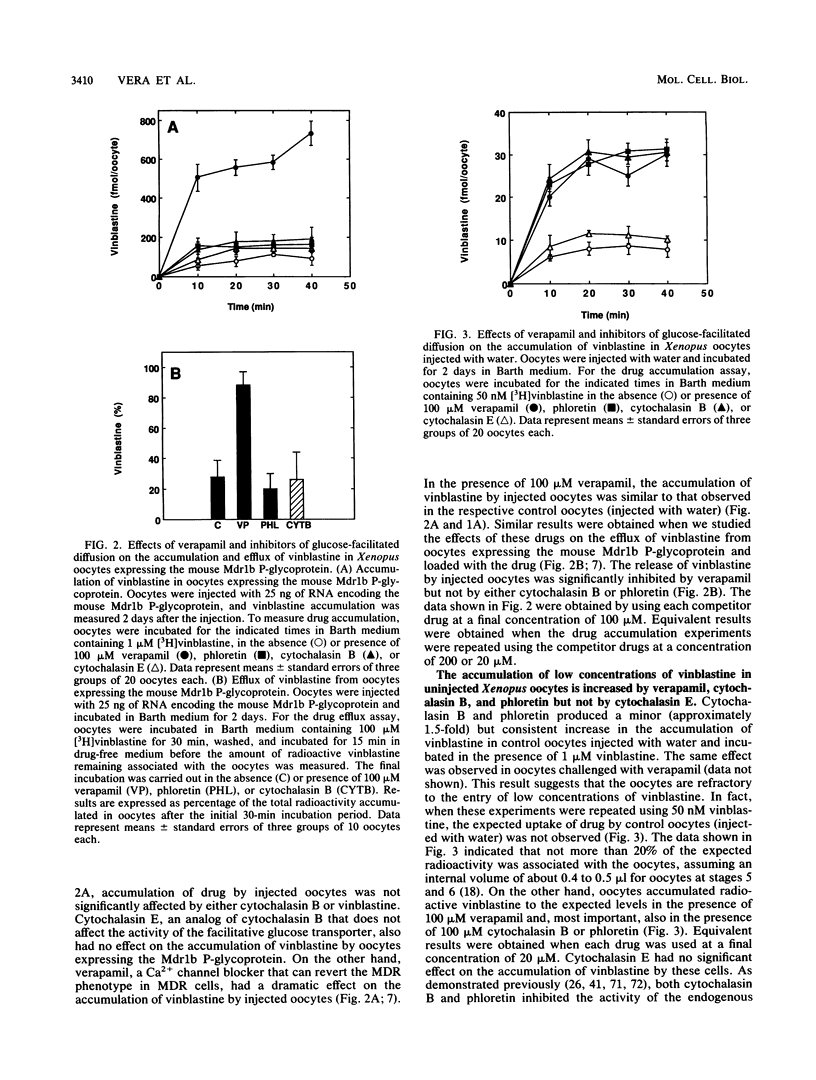
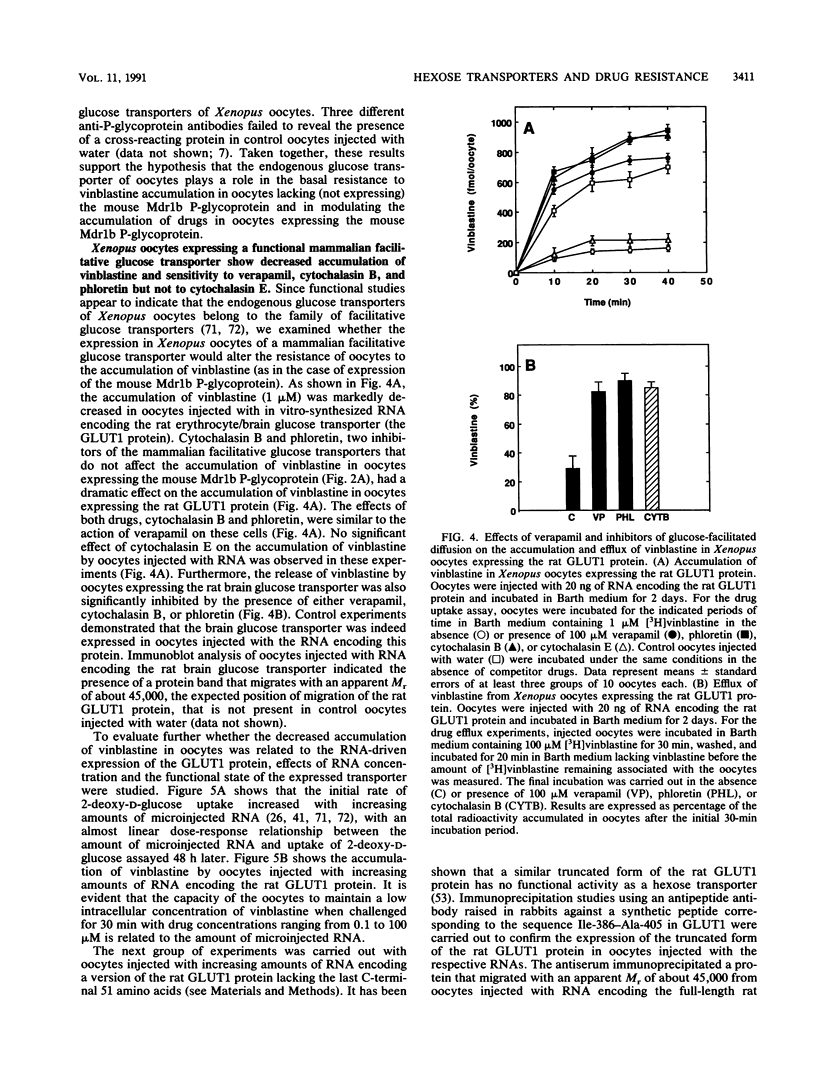




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Asano T., Shibasaki Y., Ohno S., Taira H., Lin J. L., Kasuga M., Kanazawa Y., Akanuma Y., Takaku F., Oka Y. Rabbit brain glucose transporter responds to insulin when expressed in insulin-sensitive Chinese hamster ovary cells. J Biol Chem. 1989 Feb 25;264(6):3416–3420. [PubMed] [Google Scholar]
- Azzaria M., Schurr E., Gros P. Discrete mutations introduced in the predicted nucleotide-binding sites of the mdr1 gene abolish its ability to confer multidrug resistance. Mol Cell Biol. 1989 Dec;9(12):5289–5297. doi: 10.1128/mcb.9.12.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers A., Helgerson A. L. The human erythrocyte sugar transporter is also a nucleotide binding protein. Biochemistry. 1989 Oct 17;28(21):8337–8346. doi: 10.1021/bi00447a011. [DOI] [PubMed] [Google Scholar]
- Castillo G., Vera J. C., Yang C. P., Horwitz S. B., Rosen O. M. Functional expression of murine multidrug resistance in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4737–4741. doi: 10.1073/pnas.87.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Gottesman M. M., Pastan I. H. Increased vinblastine binding to membrane vesicles from multidrug-resistant KB cells. J Biol Chem. 1986 Jun 15;261(17):7921–7928. [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Cornwell M. M., Safa A. R., Felsted R. L., Gottesman M. M., Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore T., Cheung M. O., Duronio V., Lo T. C. Stimulation of hexose transport in L6 rat myoblasts by antibody and by glucose starvation. Biochem J. 1986 Sep 15;238(3):831–836. doi: 10.1042/bj2380831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Debenham P. G., Kartner N., Siminovitch L., Riordan J. R., Ling V. DNA-mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol. 1982 Aug;2(8):881–889. doi: 10.1128/mcb.2.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars K. L., Du R. P., Naik M., Evernden-Porelle D., Kartner N., van der Bliek A. M., Ling V. Expression of hamster P-glycoprotein and multidrug resistance in DNA-mediated transformants of mouse LTA cells. Mol Cell Biol. 1987 Feb;7(2):718–724. doi: 10.1128/mcb.7.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Gros P. Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol. 1990 Apr;10(4):1652–1663. doi: 10.1128/mcb.10.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fine R. L., Patel J., Chabner B. A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):582–586. doi: 10.1073/pnas.85.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J., Kuang K. Y., Vera J. C., Arant S., Silverstein S. C., Loike J., Rosen O. M. Glucose transporters serve as water channels. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3244–3247. doi: 10.1073/pnas.87.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Gould G. W., Lienhard G. E. Expression of a functional glucose transporter in Xenopus oocytes. Biochemistry. 1989 Nov 28;28(24):9447–9452. doi: 10.1021/bi00450a030. [DOI] [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Raymond M., Bell J., Housman D. Cloning and characterization of a second member of the mouse mdr gene family. Mol Cell Biol. 1988 Jul;8(7):2770–2778. doi: 10.1128/mcb.8.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Functional role for the 170- to 180-kDa glycoprotein specific to drug-resistant tumor cells as revealed by monoclonal antibodies. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7785–7789. doi: 10.1073/pnas.83.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J Biol Chem. 1988 Jan 25;263(3):1454–1458. [PubMed] [Google Scholar]
- Hammond J. R., Johnstone R. M., Gros P. Enhanced efflux of [3H]vinblastine from Chinese hamster ovary cells transfected with a full-length complementary DNA clone for the mdr1 gene. Cancer Res. 1989 Jul 15;49(14):3867–3871. [PubMed] [Google Scholar]
- Haspel H. C., Rosenfeld M. G., Rosen O. M. Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem. 1988 Jan 5;263(1):398–403. [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. I., Lothstein L., Horwitz S. B. Differential overexpression of three mdr gene family members in multidrug-resistant J774.2 mouse cells. Evidence that distinct P-glycoprotein precursors are encoded by unique mdr genes. J Biol Chem. 1989 Jul 15;264(20):12053–12062. [PubMed] [Google Scholar]
- Inaba M., Kobayashi H., Sakurai Y., Johnson R. K. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979 Jun;39(6 Pt 1):2200–2203. [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Keller K., Strube M., Mueckler M. Functional expression of the human HepG2 and rat adipocyte glucose transporters in Xenopus oocytes. Comparison of kinetic parameters. J Biol Chem. 1989 Nov 15;264(32):18884–18889. [PubMed] [Google Scholar]
- Kitagawa T., Tanaka M., Akamatsu Y. Regulation of glucose transport activity and expression of glucose transporter mRNA by serum, growth factors and phorbol ester in quiescent mouse fibroblasts. Biochim Biophys Acta. 1989 Mar 27;980(1):100–108. doi: 10.1016/0005-2736(89)90205-8. [DOI] [PubMed] [Google Scholar]
- Koch G., Smith M., Twentyman P., Wright K. Identification of a novel calcium-binding protein (CP22) in multidrug-resistant murine and hamster cells. FEBS Lett. 1986 Jan 20;195(1-2):275–279. doi: 10.1016/0014-5793(86)80176-4. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Martinsson T., Dahllöf B., Wettergren Y., Leffler H., Levan G. Pleiotropic drug resistance and gene amplification in a SEWA mouse tumor cell line. Complex relations revealed by drug uptake data, and lipid and protein analysis. Exp Cell Res. 1985 Jun;158(2):382–394. doi: 10.1016/0014-4827(85)90463-x. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Meyers M. B., Rittmann-Grauer L., O'Brien J. P., Safa A. R. Characterization of monoclonal antibodies recognizing a Mr 180,000 P-glycoprotein: differential expression of the Mr 180,000 and Mr 170,000 P-glycoproteins in multidrug-resistant human tumor cells. Cancer Res. 1989 Jun 15;49(12):3209–3214. [PubMed] [Google Scholar]
- Meyers M. B., Spengler B. A., Chang T. D., Melera P. W., Biedler J. L. Gene amplification-associated cytogenetic aberrations and protein changes in vincristine-resistant Chinese hamster, mouse, and human cells. J Cell Biol. 1985 Feb;100(2):588–597. doi: 10.1083/jcb.100.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd L. M., Werner H., Shen-Orr Z., Roberts C. T., Jr, LeRoith D., Haspel H. C., Raizada M. K. Regulation of rat brain/HepG2 glucose transporter gene expression by phorbol esters in primary cultures of neuronal and astrocytic glial cells. Endocrinology. 1990 Jan;126(1):545–549. doi: 10.1210/endo-126-1-545. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Katagiri H., Akanuma Y., Takaku F. C-terminal truncated glucose transporter is locked into an inward-facing form without transport activity. Nature. 1990 Jun 7;345(6275):550–553. doi: 10.1038/345550a0. [DOI] [PubMed] [Google Scholar]
- Peterson R. H., Biedler J. L. Plasma membrane proteins and glycoproteins from Chinese hamster cells sensitive and resistant to actinomycin D. J Supramol Struct. 1978;9(3):289–298. doi: 10.1002/jss.400090302. [DOI] [PubMed] [Google Scholar]
- Peterson R. H., Meyers M. B., Spengler B. A., Biedler J. L. Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res. 1983 Jan;43(1):222–228. [PubMed] [Google Scholar]
- Raymond M., Rose E., Housman D. E., Gros P. Physical mapping, amplification, and overexpression of the mouse mdr gene family in multidrug-resistant cells. Mol Cell Biol. 1990 Apr;10(4):1642–1651. doi: 10.1128/mcb.10.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979 Dec 25;254(24):12701–12705. [PubMed] [Google Scholar]
- Roninson I. B., Chin J. E., Choi K. G., Gros P., Housman D. E., Fojo A., Shen D. W., Gottesman M. M., Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Shen D. W., Cardarelli C., Hwang J., Cornwell M., Richert N., Ishii S., Pastan I., Gottesman M. M. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986 Jun 15;261(17):7762–7770. [PubMed] [Google Scholar]
- Shen J., Hughes C., Chao C., Cai J., Bartels C., Gessner T., Subjeck J. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3278–3282. doi: 10.1073/pnas.84.10.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Rorsman P., Ashcroft F. M. Modulation of dihydropyridine-sensitive Ca2+ channels by glucose metabolism in mouse pancreatic beta-cells. Nature. 1989 Nov 30;342(6249):550–553. doi: 10.1038/342550a0. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Iida H. Effects of cytochalasins and colchicine on the accumulation and retention of daunomycin and vincristine in drug resistant tumor cells. Biochem Pharmacol. 1986 Apr 1;35(7):1087–1090. doi: 10.1016/0006-2952(86)90143-7. [DOI] [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Ten Houte de Lange T., Kooiman P. M., Van der Velde-Koerts T., Borst P. The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J. 1987 Nov;6(11):3325–3331. doi: 10.1002/j.1460-2075.1987.tb02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Functional expression of mammalian glucose transporters in Xenopus laevis oocytes: evidence for cell-dependent insulin sensitivity. Mol Cell Biol. 1989 Oct;9(10):4187–4195. doi: 10.1128/mcb.9.10.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Reconstitution of an insulin signaling pathway in Xenopus laevis oocytes: coexpression of a mammalian insulin receptor and three different mammalian hexose transporters. Mol Cell Biol. 1990 Feb;10(2):743–751. doi: 10.1128/mcb.10.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Cornwell M. M., Cardarelli C. O., Gottesman M. M., Pastan I. Single cell analysis of daunomycin uptake and efflux in multidrug-resistant and -sensitive KB cells: effects of verapamil and other drugs. Cancer Res. 1986 Nov;46(11):5941–5946. [PubMed] [Google Scholar]
- Wilson C. M., Serrano A. E., Wasley A., Bogenschutz M. P., Shankar A. H., Wirth D. F. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989 Jun 9;244(4909):1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- Yamada K., Tillotson L. G., Isselbacher K. J. Regulation of hexose transporters in chicken embryo fibroblasts: stimulation by the phorbol ester TPA leads to increased numbers of functioning transporters. J Cell Physiol. 1986 May;127(2):211–215. doi: 10.1002/jcp.1041270204. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Van der Bliek A. M., Biedler J. L., Borst P. Differential amplification and disproportionate expression of five genes in three multidrug-resistant Chinese hamster lung cell lines. Mol Cell Biol. 1986 Dec;6(12):4717–4722. doi: 10.1128/mcb.6.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Kooiman P. M., Schneider C., Borst P. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene. 1988 Nov 30;71(2):401–411. doi: 10.1016/0378-1119(88)90057-1. [DOI] [PubMed] [Google Scholar]