Background: Low binding affinity of product UMP is used to argue against substrate distortion contributing to orotidine-5′-monophosphate decarboxylase catalysis.
Results: Atomic resolution structure and surface plasmon resonance analysis reveal strong repulsion between active site residue and UMP.
Conclusion: Low UMP affinity does not disprove contribution of substrate distortion to catalysis.
Significance: Substrate distortion can still be considered as a mechanistic feature of this most proficient enzyme.
Keywords: Crystal Structure, Decarboxylase, Enzyme Mechanisms, Pyrimidine, Surface Plasmon Resonance (SPR), ODCase, OMPDC, Atomic Resolution Structure, Substrate Distortion
Abstract
Orotidine 5′-monophosphate decarboxylase (ODCase) accelerates the decarboxylation of its substrate by 17 orders of magnitude. One argument brought forward against steric/electrostatic repulsion causing substrate distortion at the carboxylate substituent as part of the catalysis has been the weak binding affinity of the decarboxylated product (UMP). The crystal structure of the UMP complex of ODCase at atomic resolution (1.03 Å) shows steric competition between the product UMP and the side chain of a catalytic lysine residue. Surface plasmon resonance analysis indicates that UMP binds 5 orders of magnitude more tightly to a mutant in which the interfering side chain has been removed than to wild-type ODCase. These results explain the low affinity of UMP and counter a seemingly very strong argument against a contribution of substrate distortion to the catalytic reaction mechanism of ODCase.
Introduction
Orotidine 5′-monophosphate decarboxylase (ODCase)3 is one of the most proficient enzymes known (1). It decarboxylates orotidine 5′-monophosphate (OMP) and produces uridine 5′-monophosphate (UMP) in the final step of the de novo pyrimidine biosynthesis pathway (Fig. 1A). It also accelerates the reaction by 17 orders of magnitude, as compared with the spontaneous reaction in water at neutral pH, without employing cofactors or metal ions (1–5).
FIGURE 1.
ODCase catalyzing reaction and its reaction center. A, substrates and products of decarboxylation and cyano-converting reactions. B, reaction center of MtODCase in complex with 6-hydroxy-UMP (BMP, Protein Data Bank (PDB): 1lor (29)). Transparent spheres indicate the van der Waals radii of the four charged side chains Lys-42, Asp 70, Lys-72, and Asp-75′ (′ indicates that the residue belongs to the second subunit of the dimeric enzyme). Numbers beside the dotted lines represent the distances between the connected atoms in Angstroms.
The reaction mechanism of this enzyme has been the subject of extensive investigations. More than 170 crystal structures have been determined, and numerous kinetic assays at various conditions have been performed. These experiments established that an electrostatic residue network composed of the charged side chains of two aspartate and two lysine residues, all completely conserved, plays a dominant role in catalysis (Fig. 1B) (2–5). As with all enzymes, the reaction acceleration provided by ODCase is explained in part by transition state stabilization (6). Lys-72 (the sequence numbers in this study correspond to those of the ODCase from Methanothermobacter thermautotrophicus (MtODCase)) is considered to be the key residue to stabilize the intermediate vinyl anion (6, 7). However, there is still no general agreement on all the details of the reaction mechanism. In particular, the observation that ODCase also converts 6-cyano-UMP into 6-hydroxy-UMP at the same site where the decarboxylation reaction occurs (Fig. 1A) (8) complicates the scenario. The environment required to stabilize the vinyl anion does not seem suitable to support, at the same time, the intermediate of the side reaction because the release of a negatively charged cyano group would leave a positive intermediate, the opposite of a carbanion.
A possible contributor to the acceleration of a broader array of reactions may be substrate distortion (5, 9, 10). Such a distortion effect was observed in crystal structures of various ODCase-ligand complexes, such as OMP, 6-aceto-UMP, and 6-cyano-UMP, with both MtODCase and human ODCase (11–14). For 6-cyano-UMP, such a distortion receives strong support from Raman spectroscopy, which indicates bond bending of about 20° (15). The crystal structure of ODCase from Plasmodium falciparum in complex with OMP (16) implied that charge repulsion between the two carboxylates of Asp-70 and OMP is a prime candidate for causing such a distortion.
The binding affinity of UMP, however, is significantly weaker than that of the substrate OMP and other UMP derivatives with negatively charged substituents at C6 (17–20), an obviously serious argument against such an interpretation (18, 21). The low affinity of UMP seems inconsistent with the substrate distortion mechanism because UMP has lost its carboxylate group and with it the main candidate for repulsion with Asp-70. To investigate this inconsistency, we determined the crystal structure of the complex of MtODCase and UMP at atomic resolution, hypothesizing that the reason why UMP does not strongly bind to ODCase should be reflected in the molecular structure. We also undertook surface plasmon resonance analyses of wild-type ODCase and both the K72A and the K42A mutants of ODCase to further probe the roles of residues that played in catalysis in the context of the atomic resolution structure. The results of the combined investigations revealed a repulsive interaction between the side chain of Lys-72 and UMP, explaining the low affinity of the reaction product and removing a strong argument against significant contributions of substrate distortion to the enormous rate acceleration observed in ODCase catalysis.
EXPERIMENTAL PROCEDURES
Materials
Uridine 5′-monophosphate disodium salt was purchased from Nacalai Tesque (Kyoto, Japan). 6-Azauridine 5′-monophosphate (6azaUMP) was purchased from Carbosynth (Compton, UK). HBS-N buffer and series S sensor chip CM5 for Biacore were purchased from GE Healthcare UK Ltd. (Little Chalfont, UK). All other reagents were analytical grade.
Protein Preparation
Wild-type MtODCase (WT-MtODCase) and its mutants (K42A and K72A) were expressed in Escherichia coli and purified as described using nickel-nitrilotriacetic acid and gel filtration chromatography (14, 22). Purified samples were dialyzed against buffer A (20 mm HEPES-NaOH, pH 7.5, 150 mm NaCl, 5 mm DTT).
Crystallography
Crystallization was performed at 293 K using the hanging-drop vapor diffusion method. A solution of buffer A containing 10 mg/ml WT-MtODCase and 10 mm uridine 5′-monophosphate (UMP) was mixed with equal amounts of precipitant solution composed of 1.28 m sodium citrate, 5% (v/v) dioxane, and 0.1 m HEPES-Na, pH 7.5. Crystals grew as clusters, and several cycles of microseeding were necessary to obtain a single crystal. Crystals were dipped in a cryoprotectant buffer consisting of 1.2 m sodium citrate, 15% glycerol, and 0.1 m MES-Na, pH 6.5, before being flash-frozen in a stream of nitrogen at ∼100 K.
Various attempts to obtain an atomic resolution dataset of MtODCase with UMP were taken at beamlines of the Photon Factory and SPring-8, Japan, as well as at the Advanced Photon Source. The dataset eventually used in structure refinement was collected at beamline 14BM-C of the BioCARS sector at the Advanced Photon Source. The diffraction data were integrated and scaled using HKL2000 (23). The crystals displayed the same space group and unit cell constants as the previously determined structure of the K72A complex of 6-aminoUMP, which was phased by the molecular replacement method with the program MOLREP (39) using the WT in complex with 6azaUMP (1dvj, Ref. 5). Thus, the coordinates of K72A and 6-aminoUMP complex were used as the initial model for refinement. Small changes to the model were accomplished using the program package COOT (24). The refinement was started using the program REFMAC (25), and the program SHELEX (26) was applied at the later stages. Anisotropic B factors were assigned for all non-hydrogen atoms including solvent molecules. The bonds and angles of the side chains of Lys-42, Asp-70, Asp-75, and the pyrimidine ring of the ligand were unrestrained at the final refinement stage. The restraint parameters for Lys-72 were not changed from the default values due to its assuming dual conformations. The occupancies of the conformations A and B of Lys-72 were refined in the program SHELEX at the final stage of the refinement, whereas those of other residues were estimated based on the corresponding electron densities. The refined structure was validated using MolProbity (27). Statistics are summarized in Table 1.
TABLE 1.
Data and refinement statistics
| WT-UMP | |
|---|---|
| Data | |
| Wavelength (Å) | 0.9000 |
| Space group | C2221 |
| Cell parameters (a/b/c (Å)) | 57.94/103.23/73.57 |
| Resolution (Å)a | 50-1.03 (1.04-1.03) |
| No. of observed reflections | 1,146,202 |
| No. of unique reflections | 108,363 |
| Completeness (%)a | 99.9 (97.1) |
| I/σ(I)a | 46.3 (1.8) |
| Rmerge (%)a,b | 5.3 (80.6) |
| Refinement | |
| Resolution (Å) | 10-1.03 |
| R/Rfree (%)c | 11.77/13.86 |
| No. of non-hydrogen atoms (protein/ligand/solvent) | 1937/21/174 |
| No. of hydrogens | 688 |
| r.m.s.d.d (bond/angle/plane) | 0.015/0.030/0.03 |
| Ramachandran (favored/allowed (%))e | 99.1/100.0 |
| PDB ID | 3W07 |
a Values for the outermost resolution shell are shown in parentheses.
b Rmerge = Σ|Ii − 〈Ii〉|/Σ〈Ii〉, where Ii is the observed intensity and 〈Ii〉 is the average intensity over symmetry equivalent measurements.
c r = Σ‖Fobs| − |Fcalc‖/Σ|Fobs|. Rfree is the same as R, but for a 5% subset of all reflections that were never used in crystallographic refinement.
d r.m.s.d. (root mean square distances) from ideal values in Å. The angle value indicates the deviation of the angle distances, which are the distances between two atoms that are both bonded to the same atom.
e Ramachandran analysis performed by MolProbity.
Surface Plasmon Resonance Analysis
Surface plasmon resonance assays were performed using a Biacore T100 (GE Healthcare). WT and mutant MtODCase proteins were immobilized on sensor chips (Series S sensor chip CM5) using the amine coupling method and following the standard protocol (28). The MtODCase solutions were diluted to 0.1 mg/ml in 10 mm acetate buffer, pH 5 and 4.75 for WT and mutants, respectively, prior to immobilization. The sensor chips were activated by treating them with a mixture of 1-ethyl-3-(3-dimethylamino-propyl)-carbodiimide and N-hydroxysuccinimide. The protein solutions were then brought into contact with the sensor chip for 420 s at a flow rate of 10 μl/min followed by an ethanolamine hydrochloride solution treatment. 2,000–4,000 resonance units of MtODCases were immobilized on the sensor chips.
The binding assays were performed at 25 °C and at a flow rate of 30 μl/min using HBS-N buffer composed of 10 mm HEPES-Na, pH 7.4, and 150 mm NaCl. The measurements were performed in triplicate for each combination of WT/K42A/K72A and UMP as well as WT and 6azaUMP. The KD values for WT and K42A mutant were calculated with the affinity analysis option using Biacore T100 evaluation software version 1.1.1. The KD values for the K72A mutant were estimated with the kinetics evaluation option of the same program.
RESULTS AND DISCUSSION
The crystal structure of the ODCase-product complex was determined at an atomic resolution of 1.03 Å (the highest resolution of all ODCase structures determined today) and refined to Rcryst and Rfree factors of 11.8 and 13.9%, respectively (Table 1). Double conformers were assigned in 57 out of 215 modeled residues. Anisotropic B values of all individual atoms were refined, and 688 out of a possible 1,658 hydrogen atoms were added to the model structure. The side chains of Lys-42, Asp-70, and Asp-75 and the pyrimidine ring of UMP were unrestrained to avoid bias in the analysis of atoms in the ligand-binding site. The root mean square distances between the present and previously determined WT-MtODCase-UMP complex structures (1LOQ (29) and 3G1D (9), both at 1.5 Å resolution) are 0.32 Å (all 209 Cα) and 0.43 Å (all 215 Cα), respectively.
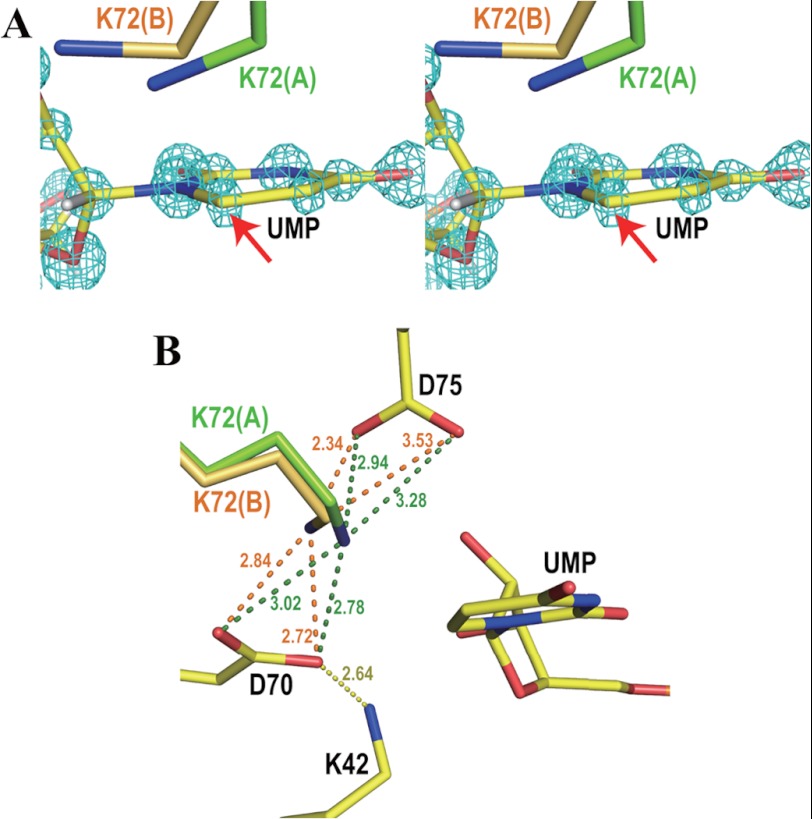
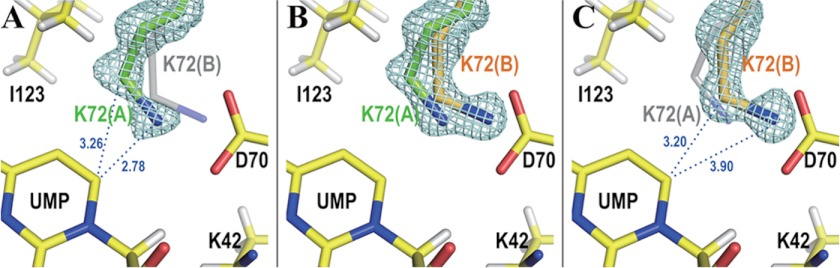
Our high resolution structure reveals the binding mode of UMP and its interactions with the protein matrix in close detail. As shown in Fig. 2, the side chain of Lys-72 assumes two conformations. In the first conformation (occupancy 54%, green in Fig. 2), the Cϵ and Nζ atoms of the residue are located 3.26 and 2.78 Å, respectively, from C6 of UMP, whereas in the second conformation (occupancy 46%, orange in Fig. 2), the corresponding distances are 3.20 and 3.90 Å. Both distances in conformation A are shorter than the sum of the van der Waals radii, as is the contact involving Cϵ in conformation B. In the second conformation, UMP even seems to push Lys-72 out of its original position. In addition, the pyrimidine ring structure of UMP is slightly distorted with the C6 atom located out of the plane of the pyrimidine ring by 0.10 Å, whereas the remaining five ring atoms deviate by less than 0.015 Å (Fig. 3A). The Lys-42-Asp-70-Lys-72-Asp-75′ network shown in Fig. 1B is kept intact in both of the two conformations (Fig. 3B). The structural features imply that UMP and Lys-72 compete to take their favorite positions of lowest energy in the active site of the enzyme.
FIGURE 2.
Omit electron density maps of Lys-72 superposed on its double conformations. Hydrogen atoms are drawn in white. Numbers beside the blue dotted lines represent the distances between the two connected atoms in Angstroms. A, conformation A. The light blue mesh shows the Fo − Fc omit electron density map of conformation A of Lys-72 contoured at 3.5 σ. Conformations A and B of Lys-72 are drawn in green and transparent gray, respectively. B, both conformations A and B. The light blue mesh represents the Fo − Fc omit electron density map of Lys-72 contoured at 4.5 σ. Conformations A and B of Lys-72 are drawn in green and orange, respectively. C, conformation B. The light blue mesh represents the Fo − Fc omit electron density map of conformation B of Lys-72 contoured at 3.5 σ. Conformations A and B of Lys-72 are drawn in transparent gray and orange, respectively.
FIGURE 3.
Ligand binding site. A, stereo view of UMP and both conformations of Lys-72 superposed on the Fo − Fc omit electron density map of UMP contoured at 12 σ. Conformations A and B of Lys-72 are drawn in green and orange, respectively. A red arrow points to the C6 atom of UMP, which is displaced from the plane of the pyrimidine ring by 0.10 Å. B, the Lys-42-Asp-70-Lys-72-Asp-75′ network in the present atomic resolution structure. Numbers beside the dotted lines represent the distances between the two connected atoms in Angstroms. The color code is the same as in panel A.
This finding encouraged us to investigate the binding affinity between the K72A mutant of ODCase and UMP. So far, no Ki for UMP had been reported for this mutant because of its extremely low enzymatic activity (30). To overcome this problem, we performed KD assays using the surface plasmon resonance technique.
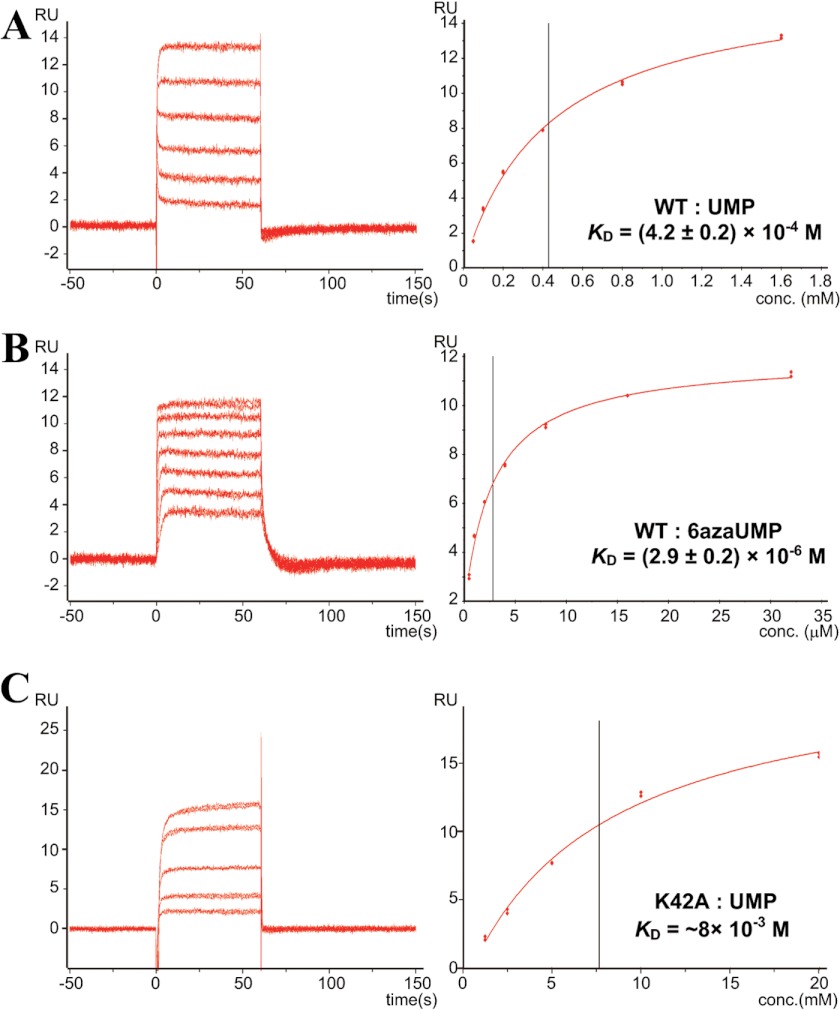
WT-MtODCase was immobilized on a Biacore sensor chip. UMP and 6azaUMP were applied individually to the sensor chip. These compounds resulted in the sensorgrams shown in Fig. 4, A and B, left panels; the corresponding KD values were calculated with the affinity analysis option of the Biacore T100 evaluation software. They are displayed in Fig. 4, A and B, right panels. The estimated value for UMP is 4.2 ± 0.2 × 10−4 m (Table 2), which is comparable with the published Ki for UMP of 3.3 ± 0.1 × 10−4 m at 55 °C (20). The KD value for 6azaUMP is 2.9 ± 0.2 × 10−6 m, approximately one-fourth of the published Ki value for this compound (1.24 ± 0.07 × 10−5 m at 55 °C (20)) (Table 2). These numbers show that KD analysis using Biacore is able to at least estimate the order of magnitude of Ki values.
FIGURE 4.
Typical sensorgrams and affinity analysis of WT and K42A MtODCase. The left and right panels represent sensorgrams and the corresponding analyses, respectively. RU stands for resonance unit. The vertical black lines in the right panels indicate the KD values. A, wild-type MtODCase is immobilized, and binding of UMP is analyzed. B, wild-type MtODCase is immobilized, and binding of 6azaUMP is analyzed. C, K42A MtODCase is immobilized, and binding of UMP is analyzed.
TABLE 2.
Binding affinity of various ligands to MtODCase
| Binding affinity | Temperature (reference) | |
|---|---|---|
| m | ||
| KD values | ||
| WT-UMP | (4.2 ± 0.2) × 10−4 | 25 °C |
| WT-6azaUMP | (2.9 ± 0.2) × 10−6 | 25 °C |
| K72A-UMP | (4.1 ± 0.3) × 10−9 | 25 °C |
| K42A-UMPa | ∼8 × 10−3 | 25 °C |
| Ki values | ||
| WT-UMP | (3.3 ± 0.1) × 10−4 | 55 °C (20) |
| WT-6azaUMP | (1.24 ± 0.07) × 10−5 | 55 °C (20) |
a Only a rough estimate is possible because of the very low affinity pushing the analytical limits of Biacore analysis.
Next, we determined the KD of UMP for the MtODCase K42A and K72A mutants. The KD of UMP for the K42A mutant is ∼8 mm (Fig. 4C and Table 2), which is an order of magnitude larger than the one seen with the WT enzyme. This value is reasonable because removal of the Lys-42 side chain leads to loss of the interaction between enzyme and the 2′-OH of ribose (9, 29). In contrast, the KD of UMP for the K72A mutant is 4.1 ± 0.3 × 10−9 m, 5 orders of magnitude lower than the WT value (4.2 × 10−4 m, Fig. 5 and Table 2). This huge difference indicates that the side chain of Lys-72 interferes with UMP binding and thereby significantly weakens the affinity between WT-ODCase and its product, consequently supporting product release. However, when lysine is replaced by alanine in the K72A mutant, the binding strength of UMP is second only to that of 6-hydroxy-UMP, the best ODCase inhibitor known (9, 20, 31–36). This adds strong product inhibition to loss of transition state stabilization as explanations for the high reduction in catalytic potency of the K72A mutant enzyme.
FIGURE 5.
Typical sensorgrams and kinetic analysis of WT and K72A MtODCase. The upper panel represents the experimental (red) and simulated (black) sensorgrams to evaluate the KD value. The lower panel shows the residuals, which indicate the differences between the experimental and the simulated sensorgrams. RU stands for resonance unit.
The most remarkable acceleration of the decarboxylation it catalyzes has made the detailed chemical mechanism of ODCase a target of intense scrutiny. Several of the underlying catalytic features have been well established, e.g. the strong contribution of the 5′-phosphate group (30, 37, 38) or the vinyl carbanion nature of a reaction intermediate (6, 7). It is also generally assumed that Lys-72, which is completely conserved in all known ODCase sequences (16), plays an important role in balancing the negative charge of the vinyl carbanion and thereby lowers the energy of the transition state. The amino head group of the residue closely approaches the C6 atom of the pyrimidine rings of ligands (2–5, 11, 29), and its mutation to an alanine decreases the decarboxylation rate by 5 orders of magnitude (30). We now identify a close interaction between UMP and Lys-72 in the active site of the enzyme as the structural basis for the low affinity of WT-ODCase for its product. This finding counters a seemingly strong argument brought forward against the participation of substrate distortion in ODCase catalysis. As several earlier studies also support such a contribution (5, 9, 14, 15), it is highly probable that ODCase uses both transition state stabilization and substrate distortion (plus potentially other still unknown mechanistic features), with all of them needed to achieve the extraordinary rate acceleration of the enzyme.
It is reasonable to assume that ODCase makes use of a number of catalytic effects to achieve its high proficiency in decarboxylation reaction. In contrast, with an active site not evolved to support the generation of cationic intermediates, the enzyme is restricted to the use of substrate distortion when catalyzing the cyano-converting reaction described earlier (8), resulting in a rather slow rate for this reaction. Further investigations, such as theoretical studies based on the present experimental results, promise to shed more light on the mechanistic features of substrate distortion and how exactly it might contribute to ODCase catalysis.
Acknowledgments
We are grateful to the staff members at the beamlines of the Photon Factory, SPring-8, and the BioCARS sector of the Advanced Photon Source for the help with data collection, to Wanda Gillon for sample preparation, and to Marie-Louise Hjaersen for help with crystallization experiments. Use of beamlines at the Photon Factory and SPring-8 was approved by the Photon Factory Advisory Committee (Proposal 2006G164) and by the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal 2006B1212), respectively. Use of the Advanced Photon Source was supported by the United States Department of Energy, Basic Energy Sciences, Office of Science, under Contract DE-AC02-06CH11357. Use of the BioCARS Sector 14 was supported by the National Institutes of Health, National Institute of General Medical Sciences grant P41GM103543 (formerly National Center for Research Resources P41RR007707).
This work was supported in part by Grants-in-aid for Young Scientists (B) (20770081 and 2277012) and Scientific Research (C) (24570130) from the Japan Society for the Promotion of Science (JSPS) and the Uehara Memorial Foundation (to M. F.) as well as the Canadian Institutes for Health Research, the Canada Research Chairs Program, and the Ontario Ministry of Health and Long Term Care (to E. F. P.).
The atomic coordinates and structure factors (code 3W07) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- ODCase
- orotidine 5′-monophosphate decarboxylase
- MtODCase
- ODCase from M. thermautotrophicus
- OMP
- orotidine 5′-monophosphate
- 6azaUMP
- 6-azauridine 5′-monophosphate.
REFERENCES
- 1. Radzicka A., Wolfenden R. (1995) A proficient enzyme. Science 267, 90–93 [DOI] [PubMed] [Google Scholar]
- 2. Appleby T. C., Kinsland C., Begley T. P., Ealick S. E. (2000) The crystal structure and mechanism of orotidine 5′-monophosphate decarboxylase. Proc. Natl. Acad. Sci. U.S.A. 97, 2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris P., Navarro Poulsen J. C., Jensen K. F., Larsen S. (2000) Structural basis for the catalytic mechanism of a proficient enzyme: orotidine 5′-monophosphate decarboxylase. Biochemistry 39, 4217–4224 [DOI] [PubMed] [Google Scholar]
- 4. Miller B. G., Hassell A. M., Wolfenden R., Milburn M. V., Short S. A. (2000) Anatomy of a proficient enzyme: the structure of orotidine 5′-monophosphate decarboxylase in the presence and absence of a potential transition state analog. Proc. Natl. Acad. Sci. U.S.A. 97, 2011–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu N., Mo Y., Gao J., Pai E. F. (2000) Electrostatic stress in catalysis: structure and mechanism of the enzyme orotidine monophosphate decarboxylase. Proc. Natl. Acad. Sci. U.S.A. 97, 2017–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amyes T. L., Wood B. M., Chan K., Gerlt J. A., Richard J. P. (2008) Formation and stability of a vinyl carbanion at the active site of orotidine 5′-monophosphate decarboxylase: pKa of the C-6 proton of enzyme-bound UMP. J. Am. Chem. Soc. 130, 1574–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toth K., Amyes T. L., Wood B. M., Chan K., Gerlt J. A., Richard J. P. (2010) Product deuterium isotope effects for orotidine 5′-monophosphate decarboxylase: effect of changing substrate and enzyme structure on the partitioning of the vinyl carbanion reaction intermediate. J. Am. Chem. Soc. 132, 7018–7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujihashi M., Bello A. M., Poduch E., Wei L., Annedi S. C., Pai E. F., Kotra L. P. (2005) An unprecedented twist to ODCase catalytic activity. J. Am. Chem. Soc. 127, 15048–15050 [DOI] [PubMed] [Google Scholar]
- 9. Chan K. K., Wood B. M., Fedorov A. A., Fedorov E. V., Imker H. J., Amyes T. L., Richard J. P., Almo S. C., Gerlt J. A. (2009) Mechanism of the orotidine 5′-monophosphate decarboxylase-catalyzed reaction: evidence for substrate destabilization. Biochemistry 48, 5518–5531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao J. (2003) Catalysis by enzyme conformational change as illustrated by orotidine 5′-monophosphate decarboxylase. Curr. Opin. Struct. Biol. 13, 184–192 [DOI] [PubMed] [Google Scholar]
- 11. Wu N., Gillon W., Pai E. F. (2002) Mapping the active site-ligand interactions of orotidine 5′-monophosphate decarboxylase by crystallography. Biochemistry 41, 4002–4011 [DOI] [PubMed] [Google Scholar]
- 12. Wittmann J. G., Heinrich D., Gasow K., Frey A., Diederichsen U., Rudolph M. G. (2008) Structures of the human orotidine-5′-monophosphate decarboxylase support a covalent mechanism and provide a framework for drug design. Structure 16, 82–92 [DOI] [PubMed] [Google Scholar]
- 13. Heinrich D., Diederichsen U., Rudolph M. G. (2009) Lys314 is a nucleophile in non-classical reactions of orotidine-5′-monophosphate decarboxylase. Chemistry 15, 6619–6625 [DOI] [PubMed] [Google Scholar]
- 14. Fujihashi M., Wei L., Kotra L. P., Pai E. F. (2009) Structural characterization of the molecular events during a slow substrate-product transition in orotidine 5′-monophosphate decarboxylase. J. Mol. Biol. 387, 1199–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Callahan B. P., Bell A. F., Tonge P. J., Wolfenden R. (2006) A Raman-active competitive inhibitor of OMP decarboxylase. Bioorg. Chem. 34, 59–65 [DOI] [PubMed] [Google Scholar]
- 16. Tokuoka K., Kusakari Y., Krungkrai S. R., Matsumura H., Kai Y., Krungkrai J., Horii T., Inoue T. (2008) Structural basis for the decarboxylation of orotidine 5′-monophosphate (OMP) by Plasmodium falciparum OMP decarboxylase. J. Biochem. 143, 69–78 [DOI] [PubMed] [Google Scholar]
- 17. Krungkrai S. R., DelFraino B. J., Smiley J. A., Prapunwattana P., Mitamura T., Horii T., Krungkrai J. (2005) A novel enzyme complex of orotate phosphoribosyltransferase and orotidine 5′-monophosphate decarboxylase in human malaria parasite Plasmodium falciparum: physical association, kinetics, and inhibition characterization. Biochemistry 44, 1643–1652 [DOI] [PubMed] [Google Scholar]
- 18. Lewis C. A., Jr., Wolfenden R. (2007) Indiscriminate binding by orotidine 5′-phosphate decarboxylase of uridine 5′-phosphate derivatives with bulky anionic c6 substituents. Biochemistry 46, 13331–13343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller B. G., Hassell A. M., Milburn M. V., Short S. A. (2000) Crystallization of native and selenomethionyl yeast orotidine 5′-phosphate decarboxylase. Acta Crystallogr. D Biol. Crystallogr. 56, 472–474 [DOI] [PubMed] [Google Scholar]
- 20. Poduch E., Wei L., Pai E. F., Kotra L. P. (2008) Structural diversity and plasticity associated with nucleotides targeting orotidine monophosphate decarboxylase. J. Med. Chem. 51, 432–438 [DOI] [PubMed] [Google Scholar]
- 21. Kamerlin S. C., Chu Z. T., Warshel A. (2010) On catalytic preorganization in oxyanion holes: highlighting the problems with the gas-phase modeling of oxyanion holes and illustrating the need for complete enzyme models. J. Org. Chem. 75, 6391–6401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu N., Christendat D., Dharamsi A., Pai E. F. (2000) Purification, crystallization, and preliminary X-ray study of orotidine 5′-monophosphate decarboxylase. Acta Crystallogr. D Biol. Crystallogr. 56, 912–914 [DOI] [PubMed] [Google Scholar]
- 23. Otwinowski Z., Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 24. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 25. Murshudov G. N., Vagin A. A., Lebedev A., Wilson K. S., Dodson E. J. (1999) Efficient anisotropic refinement of macromolecular structures using FFT. Acta Crystallogr. D Biol. Crystallogr. 55, 247–255 [DOI] [PubMed] [Google Scholar]
- 26. Sheldrick G. M. (2008) A short history of SHELX. Acta Crystallogr. A 64, 112–122 [DOI] [PubMed] [Google Scholar]
- 27. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lofas S., Johnsson B. (1990) A novel hydrogel matrix on gold surfaces in surface-plasmon resonance sensors for fast and efficient covalent immobilization of ligands. J. Chem. Soc. Chem. Commun. 1526–1528 [Google Scholar]
- 29. Wu N., Pai E. F. (2002) Crystal structures of inhibitor complexes reveal an alternate binding mode in orotidine-5′-monophosphate decarboxylase. J. Biol. Chem. 277, 28080–28087 [DOI] [PubMed] [Google Scholar]
- 30. Miller B. G., Butterfoss G. L., Short S. A., Wolfenden R. (2001) Role of enzyme-ribofuranosyl contacts in the ground state and transition state for orotidine 5′-phosphate decarboxylase: a role for substrate destabilization? Biochemistry 40, 6227–6232 [DOI] [PubMed] [Google Scholar]
- 31. Bello A. M., Konforte D., Poduch E., Furlonger C., Wei L., Liu Y., Lewis M., Pai E. F., Paige C. J., Kotra L. P. (2009) Structure-activity relationships of orotidine-5′-monophosphate decarboxylase inhibitors as anticancer agents. J. Med. Chem. 52, 1648–1658 [DOI] [PubMed] [Google Scholar]
- 32. Bello A. M., Poduch E., Liu Y., Wei L., Crandall I., Wang X., Dyanand C., Kain K. C., Pai E. F., Kotra L. P. (2008) Structure-activity relationships of C6-uridine derivatives targeting plasmodia orotidine monophosphate decarboxylase. J. Med. Chem. 51, 439–448 [DOI] [PubMed] [Google Scholar]
- 33. Lewis M., Meza-Avina M. E., Wei L., Crandall I. E., Bello A. M., Poduch E., Liu Y., Paige C. J., Kain K. C., Pai E. F., Kotra L. P. (2011) Novel interactions of fluorinated nucleotide derivatives targeting orotidine 5′-monophosphate decarboxylase. J. Med. Chem. 54, 2891–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meza-Avina M. E., Wei L., Liu Y., Poduch E., Bello A. M., Mishra R. K., Pai E. F., Kotra L. P. (2010) Structural determinants for the inhibitory ligands of orotidine-5′-monophosphate decarboxylase. Bioorg. Med. Chem. 18, 4032–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poduch E., Bello A. M., Tang S., Fujihashi M., Pai E. F., Kotra L. P. (2006) Design of inhibitors of orotidine monophosphate decarboxylase using bioisosteric replacement and determination of inhibition kinetics. J. Med. Chem. 49, 4937–4945 [DOI] [PubMed] [Google Scholar]
- 36. Thirumalairajan S., Mahaney B., Bearne S. L. (2010) Interrogation of the active site of OMP decarboxylase from Escherichia coli with a substrate analogue bearing an anionic group at C6. Chem. Commun. (Camb.) 46, 3158–3160 [DOI] [PubMed] [Google Scholar]
- 37. Amyes T. L., Ming S. A., Goldman L. M., Wood B. M., Desai B. J., Gerlt J. A., Richard J. P. (2012) Orotidine 5′-monophosphate decarboxylase: transition state stabilization from remote protein-phosphodianion interactions. Biochemistry 51, 4630–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desai B. J., Wood B. M., Fedorov A. A., Fedorov E. V., Goryanova B., Amyes T. L., Richard J. P., Almo S. C., Gerlt J. A. (2012) Conformational changes in orotidine 5′-monophosphate decarboxylase: a structure-based explanation for how the 5′-phosphate group activates the enzyme. Biochemistry 51, 8665–8678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vagin A. A., Isupov M. N. (2001) Spherically averaged phased translation function and its application to the search for molecules and fragments in electron-density maps. Acta Crystallogr. D Biol. Crystallogr. 57, 1451–1456 [DOI] [PubMed] [Google Scholar]