Abstract
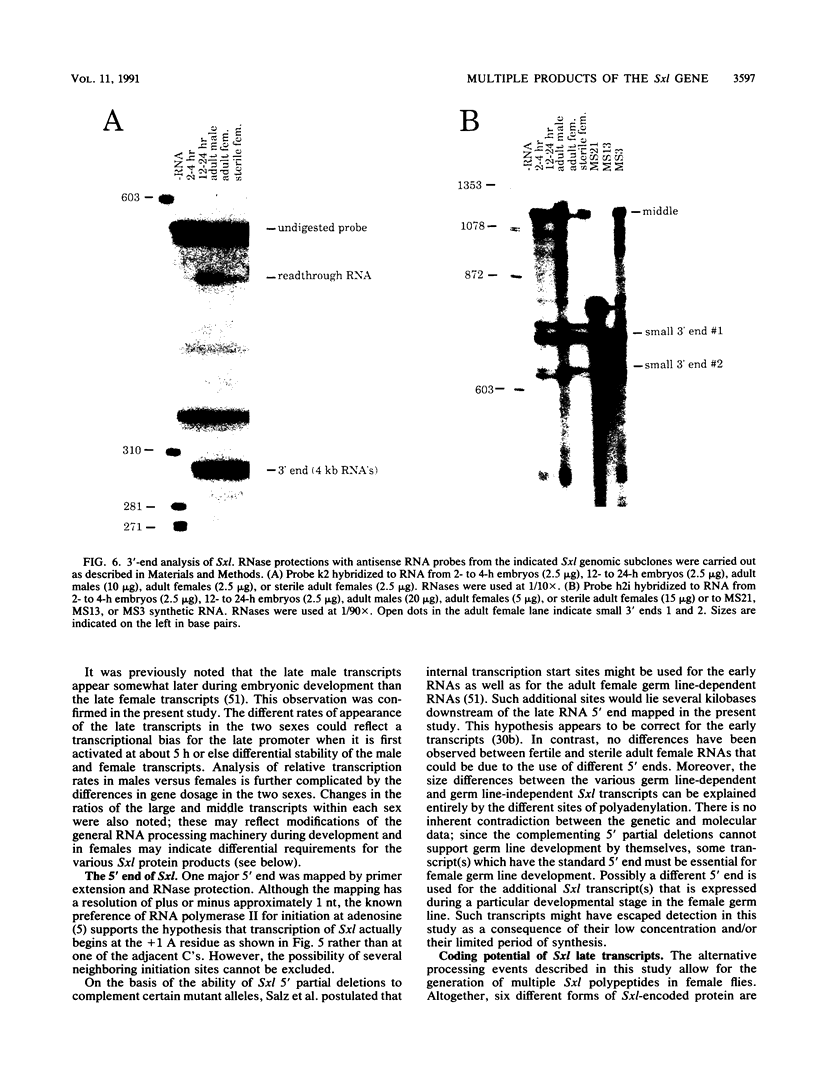
Sex-lethal (Sxl), a key sex determination gene in Drosophila melanogaster, is known to express a set of three early transcripts arising during early embryogenesis and a set of seven late transcripts occurring from midembryogenesis through adulthood. Among the late transcripts, male-specific mRNAs were distinguished from their female counterparts by the presence of an extra exon interrupting an otherwise long open reading frame (ORF). We have now analyzed the structures of the late Sxl transcripts by cDNA sequencing, Northern (RNA) blotting, primer extension, and RNase protection. The late transcripts appear to use a common 5' end but differ at their 3' ends by the use of alternative polyadenylation sites. Two of these sites lack canonical AATAAA sequences, and their use correlates in females with the presence of a functional germ line, suggesting possible tissue-specific polyadenylation. Besides the presence of the male-specific exon, no additional sex-specific splicing events were detected, although a number of non-sex-specific splicing variants were observed. In females, the various forms of late Sxl transcript potentially encode up to six slightly different polypeptides. All of the protein-coding differences occur outside the previously defined ribonucleoprotein motifs. One class of Sxl mRNAs also includes a second long ORF in the same frame as the first ORF but separated from it by a single ochre codon. The function of this second ORF is unknown. Significant amounts of apparently partially processed Sxl RNAs were observed, consistent with the hypothesis that the regulated Sxl splices occur relatively slowly.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amasino R. M. Acceleration of nucleic acid hybridization rate by polyethylene glycol. Anal Biochem. 1986 Feb 1;152(2):304–307. doi: 10.1016/0003-2697(86)90413-6. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Belote J. M. Sex determination and dosage compensation in Drosophila melanogaster. Annu Rev Genet. 1983;17:345–393. doi: 10.1146/annurev.ge.17.120183.002021. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Nagoshi R. N., Burtis K. C. Molecular genetic aspects of sex determination in Drosophila. Bioessays. 1987 Feb;6(2):66–70. doi: 10.1002/bies.950060206. [DOI] [PubMed] [Google Scholar]
- Baker B. S. Sex in flies: the splice of life. Nature. 1989 Aug 17;340(6234):521–524. doi: 10.1038/340521a0. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Ziff E. B. Promoters and heterogeneous 5' termini of the messenger RNAs of adenovirus serotype 2. J Mol Biol. 1981 Jun 25;149(2):189–221. doi: 10.1016/0022-2836(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Horabin J. I., Schedl P., Cline T. W. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991 Apr 19;65(2):229–239. doi: 10.1016/0092-8674(91)90157-t. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Maine E. M., Schedl P., Cline T. W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988 Dec 23;55(6):1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Belote J. M. Male-Specific Lethal Mutations of DROSOPHILA MELANOGASTER . II. Parameters of Gene Action during Male Development. Genetics. 1983 Dec;105(4):881–896. doi: 10.1093/genetics/105.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Kubli E., Kohli J., de Henau S., Grosjean H. Nonsense suppression in eukaryotes: the use of the Xenopus oocyte as an in vivo assay system. Nucleic Acids Res. 1980 Nov 25;8(22):5169–5178. doi: 10.1093/nar/8.22.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Bopp D., Bell L. R., Cline T. W., Schedl P. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev. 1991 Mar;5(3):403–415. doi: 10.1101/gad.5.3.403. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Busson D., Gans M., Komitopoulou K., Masson M. Genetic Analysis of Three Dominant Female-Sterile Mutations Located on the X Chromosome of DROSOPHILA MELANOGASTER. Genetics. 1983 Oct;105(2):309–325. doi: 10.1093/genetics/105.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler B., Pirrotta V., Irminger-Finger I., Nöthiger R. The sex-determining gene tra of Drosophila: molecular cloning and transformation studies. EMBO J. 1986 Dec 20;5(13):3607–3613. doi: 10.1002/j.1460-2075.1986.tb04689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W. A male-specific lethal mutation in Drosophila melanogaster that transforms sex. Dev Biol. 1979 Oct;72(2):266–275. doi: 10.1016/0012-1606(79)90117-9. [DOI] [PubMed] [Google Scholar]
- Cline T. W. Autoregulatory functioning of a Drosophila gene product that establish es and maintains the sexually determined state. Genetics. 1984 Jun;107(2):231–277. doi: 10.1093/genetics/107.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W. Evidence that sisterless-a and sisterless-b are two of several discrete "numerator elements" of the X/A sex determination signal in Drosophila that switch Sxl between two alternative stable expression states. Genetics. 1988 Aug;119(4):829–862. doi: 10.1093/genetics/119.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W. The interaction between daughterless and sex-lethal in triploids: a lethal sex-transforming maternal effect linking sex determination and dosage compensation in Drosophila melanogaster. Dev Biol. 1983 Feb;95(2):260–274. doi: 10.1016/0012-1606(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Feng Y. X., Hatfield D. L., Rein A., Levin J. G. Translational readthrough of the murine leukemia virus gag gene amber codon does not require virus-induced alteration of tRNA. J Virol. 1989 May;63(5):2405–2410. doi: 10.1128/jvi.63.5.2405-2410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen J. P. Dosage Compensation in Drosophila: Evidence That daughterless and Sex-lethal Control X Chromosome Activity at the Blastoderm Stage of Embryogenesis. Genetics. 1987 Nov;117(3):477–485. doi: 10.1093/genetics/117.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Novel mechanisms of translational control in Saccharomyces cerevisiae. Trends Genet. 1988 Jun;4(6):169–174. doi: 10.1016/0168-9525(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Drosophila sex determination: a cascade of regulated splicing. Cell. 1989 Mar 24;56(6):905–906. doi: 10.1016/0092-8674(89)90619-3. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Sex determination compared in Drosophila and Caenorhabditis. Nature. 1990 Apr 19;344(6268):721–728. doi: 10.1038/344721a0. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hoshijima K., Sakamoto H., Shimura Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature. 1990 Mar 29;344(6265):461–463. doi: 10.1038/344461a0. [DOI] [PubMed] [Google Scholar]
- Kohli J., Kwong T., Altruda F., Söll D., Wahl G. Characterization of a UGA-suppressing serine tRNA from Schizosaccharomyces pombe with the help of a new in vitro assay system for eukaryotic suppressor tRNAs. J Biol Chem. 1979 Mar 10;254(5):1546–1551. [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Saint R. B., Beachy P. A., Harte P. J., Peattie D. A., Hogness D. S. Structure and expression of a family of Ultrabithorax mRNAs generated by alternative splicing and polyadenylation in Drosophila. Genes Dev. 1989 Feb;3(2):243–258. doi: 10.1101/gad.3.2.243. [DOI] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Lucchesi J. C., Manning J. E. Gene dosage compensation in Drosophila melanogaster. Adv Genet. 1987;24:371–429. doi: 10.1016/s0065-2660(08)60013-9. [DOI] [PubMed] [Google Scholar]
- Lucchesi J. C., Skripsky T. The link between dosage compensation and sex differentiation in Drosophila melanogaster. Chromosoma. 1981;82(2):217–227. doi: 10.1007/BF00286106. [DOI] [PubMed] [Google Scholar]
- Maine E. M., Salz H. K., Schedl P., Cline T. W. Sex-lethal, a link between sex determination and sexual differentiation in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1985;50:595–604. doi: 10.1101/sqb.1985.050.01.072. [DOI] [PubMed] [Google Scholar]
- Manley J. L. Polyadenylation of mRNA precursors. Biochim Biophys Acta. 1988 May 6;950(1):1–12. doi: 10.1016/0167-4781(88)90067-x. [DOI] [PubMed] [Google Scholar]
- McKeown M., Belote J. M., Baker B. S. A molecular analysis of transformer, a gene in Drosophila melanogaster that controls female sexual differentiation. Cell. 1987 Feb 13;48(3):489–499. doi: 10.1016/0092-8674(87)90199-1. [DOI] [PubMed] [Google Scholar]
- McKeown M., Belote J. M., Boggs R. T. Ectopic expression of the female transformer gene product leads to female differentiation of chromosomally male Drosophila. Cell. 1988 Jun 17;53(6):887–895. doi: 10.1016/s0092-8674(88)90369-8. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi R. N., McKeown M., Burtis K. C., Belote J. M., Baker B. S. The control of alternative splicing at genes regulating sexual differentiation in D. melanogaster. Cell. 1988 Apr 22;53(2):229–236. doi: 10.1016/0092-8674(88)90384-4. [DOI] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'hare K., Levis R., Rubin G. M. Transcription of the white locus in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6917–6921. doi: 10.1073/pnas.80.22.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S., Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986 Jul;6(7):2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Mohler D., Engstrom L., Mahowald A. P. X-linked female-sterile loci in Drosophila melanogaster. Genetics. 1986 Jul;113(3):695–712. doi: 10.1093/genetics/113.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Salz H. K., Cline T. W., Schedl P. Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics. 1987 Oct;117(2):221–231. doi: 10.1093/genetics/117.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., Maine E. M., Keyes L. N., Samuels M. E., Cline T. W., Schedl P. The Drosophila female-specific sex-determination gene, Sex-lethal, has stage-, tissue-, and sex-specific RNAs suggesting multiple modes of regulation. Genes Dev. 1989 May;3(5):708–719. doi: 10.1101/gad.3.5.708. [DOI] [PubMed] [Google Scholar]
- Schüpbach T. Normal female germ cell differentiation requires the female X chromosome to autosome ratio and expression of sex-lethal in Drosophila melanogaster. Genetics. 1985 Mar;109(3):529–548. doi: 10.1093/genetics/109.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnowski B. A., Belote J. M., McKeown M. Sex-specific alternative splicing of RNA from the transformer gene results from sequence-dependent splice site blockage. Cell. 1989 Aug 11;58(3):449–459. doi: 10.1016/0092-8674(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann-Zwicky M., Amrein H., Nöthiger R. Genetic control of sex determination in Drosophila. Adv Genet. 1990;27:189–237. doi: 10.1016/s0065-2660(08)60026-7. [DOI] [PubMed] [Google Scholar]
- Swanson M. S., Nakagawa T. Y., LeVan K., Dreyfuss G. Primary structure of human nuclear ribonucleoprotein particle C proteins: conservation of sequence and domain structures in heterogeneous nuclear RNA, mRNA, and pre-rRNA-binding proteins. Mol Cell Biol. 1987 May;7(5):1731–1739. doi: 10.1128/mcb.7.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenoyama T., Fukunaga A., Ioshi K. Studies on the sex-specific lethals of Drosophila melanogaster. V. Sex transformation caused by interactions between a female-specific lethal, Sxlf 1, and the male-specific lethals mle(3)132, msl-2(27), and mle. Genetics. 1982 Oct;102(2):233–243. doi: 10.1093/genetics/102.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle R. P., Morch M. D. Stop making sense: or Regulation at the level of termination in eukaryotic protein synthesis. FEBS Lett. 1988 Aug 1;235(1-2):1–15. doi: 10.1016/0014-5793(88)81225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Petti L., Braun D., Seung S., Kieff E. A bicistronic Epstein-Barr virus mRNA encodes two nuclear proteins in latently infected, growth-transformed lymphocytes. J Virol. 1987 Apr;61(4):945–954. doi: 10.1128/jvi.61.4.945-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]