Abstract
Opioid abuse and dependency are international problems. Studies have shown that neuronal inflammation and degeneration might be related to the development of opioid addiction. Thus, using neuroprotective agents might be beneficial for treating opioid addiction. Memantine, an Alzheimer's disease medication, has neuroprotective effects in vitro and in vivo. In this study, we evaluated whether a low dose of memantine prevents opioid-induced drug-seeking behavior in rats and analyzed its mechanism. A conditioned-place-preference test was used to investigate the morphine-induced drug-seeking behaviors in rats. We found that a low-dose (0.2–1 mg/kg) of subcutaneous memantine significantly attenuated the chronic morphine-induced place-preference in rats. To clarify the effects of chronic morphine and low-dose memantine, serum and brain levels of cytokines and brain-derived neurotrophic factor (BDNF) were measured. After 6 days of morphine treatment, cytokine (IL-1β, IL-6) levels had significantly increased in serum; IL-1β and IL-6 mRNA levels had significantly increased in the nucleus accumbens and medial prefrontal cortex, both addiction-related brain areas; and BDNF levels had significantly decreased, both in serum and in addiction-related brain areas. Pretreatment with low-dose memantine significantly attenuated chronic morphine-induced increases in serum and brain cytokines. Low-dose memantine also significantly potentiated serum and brain BDNF levels. We hypothesize that neuronal inflammation and BDNF downregulation are related to the progression of opioid addiction. We hypothesize that the mechanism low-dose memantine uses to attenuate morphine-induced addiction behavior is its anti-inflammatory and neurotrophic effects.
Keywords: addiction, BDNF, cytokine, memantine, morphine
Introduction
Commonly abused substances, such as morphine, which is frequently prescribed as an analgesic for chronic severe pain, cocaine, heroin, cannabis, methamphetamine, amphetamine, and ketamine, have become major medical and social issues. The opioids morphine and heroin in particular have long been abused in Taiwan. Long-term opioid use causes both physiological and psychological dependence on and tolerance for the drug. These side effects cause opioid users to tend to increase their dose and make it difficult for them to abstain from using the drug. Therefore, there is a need to develop therapeutic interventions that minimize these side-effects, especially for opioid addiction.
Opioid addiction is related to activation of the mesolimbic dopaminergic pathway, which project from the ventral tegmental area (VTA) of the midbrain to the nucleus accumbens (NAc) and then to the prefrontal cortex (PFC) (Koob and Nestler, 1997). However, the neuronal basis of opioid addiction of these brain areas remains unclear.
Recently, the role of cytokines in developing morphine tolerance, physical dependence, and the opioid-induced rewarding effect has been extensively studied. In vitro studies have shown that acute morphine treatment altered the production of various cytokines, including tumor necrosis factor (TNF)-α (Kapasi et al., 2000), interleukin (IL)-1β (Pacifici et al., 2000), and IL-6 (Zubelewicz et al., 2000). Glia cell overactivation and increased proinflammatory cytokine secretion were observed following chronic morphine treatment on the lumbar spinal cord of rats (Raghavendra et al., 2002). Inhibiting glia cell activation or antagonizing the activity of the proinflammatory cytokines IL-1β, IL-6, and TNF-α attenuated the development of morphine tolerance in rats (Song and Zhao, 2001). In humans, long-term intrathecally administered morphine caused an increase of IL-6 in cerebrospinal fluid (Zin et al., 2010). In the brains of opioid addicts, inducible nitric oxide synthetase (iNOS) and cytokine expression was detected in noradrenergic locus coeruleus (LC) cells (Dyuizen and Lamash, 2009). Thus, chronic opioid treatment might activate glia cells in the drug addiction-related brain area and be involved in morphine addiction.
In addition, neurotrophic factors may be important in opioid-induced neuronal degeneration and addiction. Brain-derived neurotrophic factor (BDNF), is a member of the nerve growth factor family involved in neuronal survival, differentiation (Mizuno et al., 1994), synaptogenesis, and maintenance (Peng et al., 2003; Carrasco et al., 2007; Ohira and Hayashi, 2009). BDNF has promoted the survival of a wide range of neurons, for example, the dopaminergic neurons of the substania nigra (Hyman et al., 1991), cerebellar granule neurons (Segal et al., 1992), motor neurons (Oppenheim et al., 1992), and retinal ganglion cells (Johnson et al., 1986). Because BDNF also acts on primary sensory and cholinergic neurons of the basal forebrain, its dysfunction might also be associated with drug addiction (Alderson et al., 1990). One study (Angelucci et al., 2007) associated chronic heroin and cocaine abuse with decreased serum concentrations of BDNF. However, other studies have reported that chronic morphine treatment significantly increased BDNF gene expression in the locus coeruleus (Numan et al., 1998) and nucleus paragigantocellularis in rats (Hatami et al., 2007). An in vitro study (Takayama and Ueda, 2005) reported morphine-induced chemotaxis and BDNF expression in microglia. The role of BDNF and neuronal degeneration in long-term opioid use requires additional study.
Studies had showed that some agents with anti-inflammatory and neuroprotective effects attenuated chronic morphine-induced addictive behavior. For example, dextro-morphine (Zhang et al., 2004), dextromethorphan (Huang et al., 2003), carnosine (Gong et al., 2007) attenuated the conditioned place-preference produced by chronic morphine (Gong et al., 2007; Huang et al., 2003; Wu et al., 2007), which suggests that they and other neuroprotective agents might be beneficial in opioid addiction treatment.
Memantine is an N-methyl-D-aspartate (NMDA) receptor antagonist and an Alzheimer's disease medication that is neuroprotective because it increases glia cell-derived neurotrophic factor (GDNF) production (Wu et al., 2009) and protects rodents against amphetamine derivative-induced neurotoxic damage in vitro and in vivo (Chipana et al., 2008). In a clinical study (unpublished data), we found that the methadone replacement dose required for heroin-addicted patients treated with a low dose (5 mg/day) of oral memantine was significantly lower than for those not treated with memantine. Thus, we hypothesized that (a) chronic opioid treatment induces cytokine expression and downregulates BDNF expression, subsequently causing the neuronal degeneration related to drug-seeking behavior, and that (b) low-dose memantine decreases cytokine expression and increases BDNF expression, subsequently dampening the intensity of neuronal damage and chronic morphine-induced addictive effects. To verify the effects of memantine, we used a chronic-morphine-induced condition place-preference (CPP) animal model to investigate the effects of memantine on chronic morphine-induced drug-seeking behavior. To clarify the mechanism of memantine, we analyzed cytokine and BDNF expression in the serum and the addiction-related brain areas of rats.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing between 300 and 350 g were used in this study. They were maintained in a room at 24 ± 0.5°C with a 12-h light/dark cycle. Food and water were available ad libitum. All experiments were approved by and conformed to the guidelines of the Animal Care Committee of National Chung Kung University.
Conditioned place-preference (CPP) test in rats
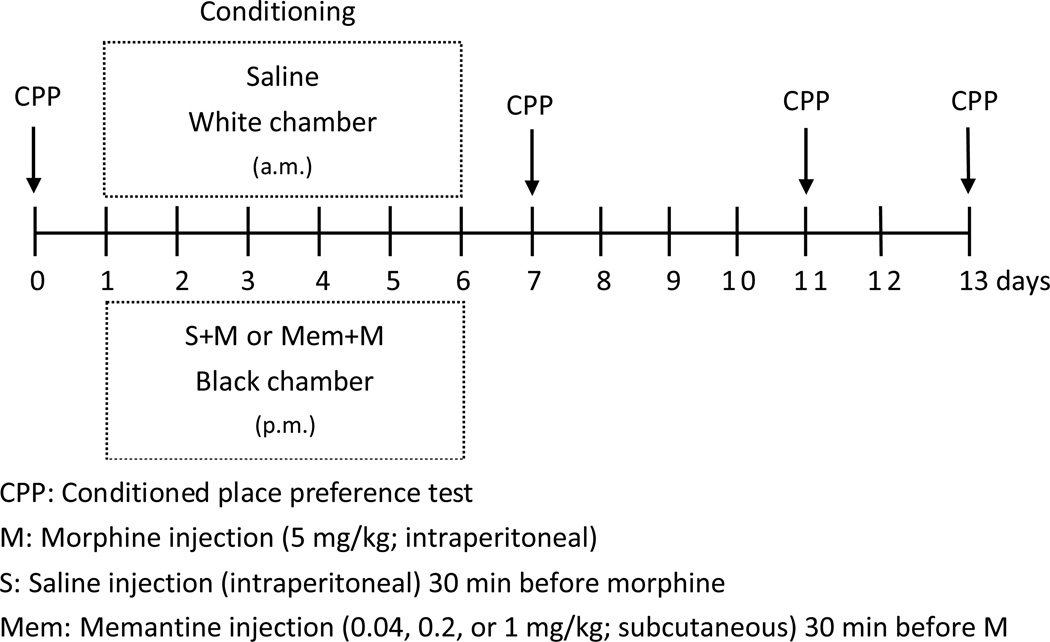
We used the CPP test to evaluate drug-induced addictive (drug-seeking) behavior in rats. The rats were intraperitoneally (i.p.) injected with morphine once daily for 6 days and evaluated for drug-induced place preference (Fig 1). The CPP test apparatus was a two-compartment box (60 × 29.2 × 29.2 cm) with a transparent plexiglass front separated by a gray cylinder platform (10.3 cm in diameter and 12 cm high), as previously described (Terashvili et al., 2008). One compartment was white with a textured floor and a red light, and the other was black with a smooth floor and a blue light. For CPP conditioning, the rats were given saline at 9 a.m. and morphine (M) (5 mg/kg; i.p.) at 4 p.m. for 6 days. The two distinctive compartments were paired repeatedly, one with the morphine injections and the other with the saline injections. The rats were kept for 40 min in the corresponding compartment with the guillotine doors closed. The place preference before conditioning and on the days after conditioning (days 0, 7, 11, and 13) was determined. Each rat was placed on a neutral (gray) platform and allowed to step down from the platform to either the white or black compartment. A sliding wall was then put down on the platform and the rat was free to access either compartment through two openings (9.5 × 12 cm each) on each side of the platform. The amount of time spent in the black or white compartment was manually measured for 15 min. That this was drug-seeking behavior was determined by the increase in the time spent in the compartment previously paired with the morphine injection than in the compartment previously paired with saline. To evaluate the effect of memantine, it (0, 0.04, 0.2, or 1 mg/kg, s.c.) was given 30 min before each morphine injection (days 1 to 6) once daily for 6 days (Fig 1).
Fig 1. The experimental protocol.
Measuring serum cytokine and BDNF levels
To evaluate the effects of morphine and memantine on peripheral cytokine expression in the rats, a whole blood sample was drawn from each rat’s tail vein on days 0, 1, and 6, two hours after the drug had been injected. Serum was isolated from the whole blood after the blood had been centrifuged at 3000 g at 4°C for 15 min, and then it was immediately stored at −80°C. The serum levels of IL-1β, IL-6, and BDNF were measured using an ELISA kit (Quantikine; R&D Systems, Minneapolis, MN, USA).
Measuring brain cytokine and BDNF levels
To evaluate the effects of drugs on cytokine and BDNF expression in the central nervous system, the rat brain was removed and the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc) were separately dissected. To measure cytokine expression, brain tissue samples were weighed and then homogenized (100 mg/1000 µl) with RIPA buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4 (sodium orthovanadate), 1 µg/ml leupeptin, and 1 mM PMSF [pH 7.5]) and centrifuged at 4°C (13,000 g) for 20 min. The supernatants were collected and immediately stored at −80°C. Brain levels of IL-6, IL-1β, and BDNF expression were measured using an ELISA kit (R&D Systems).
Quantifying the mRNA of cytokines and BDNF in drug addiction related brain areas
The rats were decapitated after 6 days of drug injections. The drug-addiction-related brain areas, the mPFC and NAc, were dissected and stored at −80°C. The expression levels of mRNAs for IL-1β, IL-6, and BDNF in the two brain area were measured using a real-time quantitative polymerase chain reaction (QPCR). Total RNA was extracted from each tissue sample using a total RNA isolation kit (SV 96, Z3500; Promega, Madison, WI, USA) and synthesized into first-strand cDNA using a high-efficacy cDNA synthesis kit (SuperScript III; Invitrogen, Carlsbad, CA, USA). An aliquot (500 ng) of first-strand cDNA was quantified on a real-time QPCR system (StepOne; Applied Biosystems, Foster City, CA, USA) using TaqMan gene expression master mix (Applied Biosystems) with specific primers to amplify IL-1β, IL-6, BDNF and GADPH. For these genes, probe/primer kits were purchased from Applied Biosystems (TaqMan Gene Expression Assay ID: Rn00580432-ml for IL-1β, ID: Rn01410330-ml for IL-6, ID: Rn02531967-sl for BDNF, and ID: Rn99999916-s1 for GADPH). IL-1β, IL-6 and BDNF mRNA expression in the same brain areas was analyzed and calculated with the relative quantity change percentage (%) compared with the saline group.
Drugs and chemicals
Morphine hydrochloride was purchased from the National Bureau of Controlled Drugs, National Health Administration, Taipei, Taiwan. Except where otherwise noted, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Data analysis and statistics
GraphPad Prism 5 was used to analyze data. Data are means ± standard error of the mean (S.E.M.). One-way analysis of variance (ANOVA) and then the Newman-Keuls test were used for statistical evaluations. Significance was set at p < 0.05.
Results
Rats treated with chronic morphine showed significant CPP
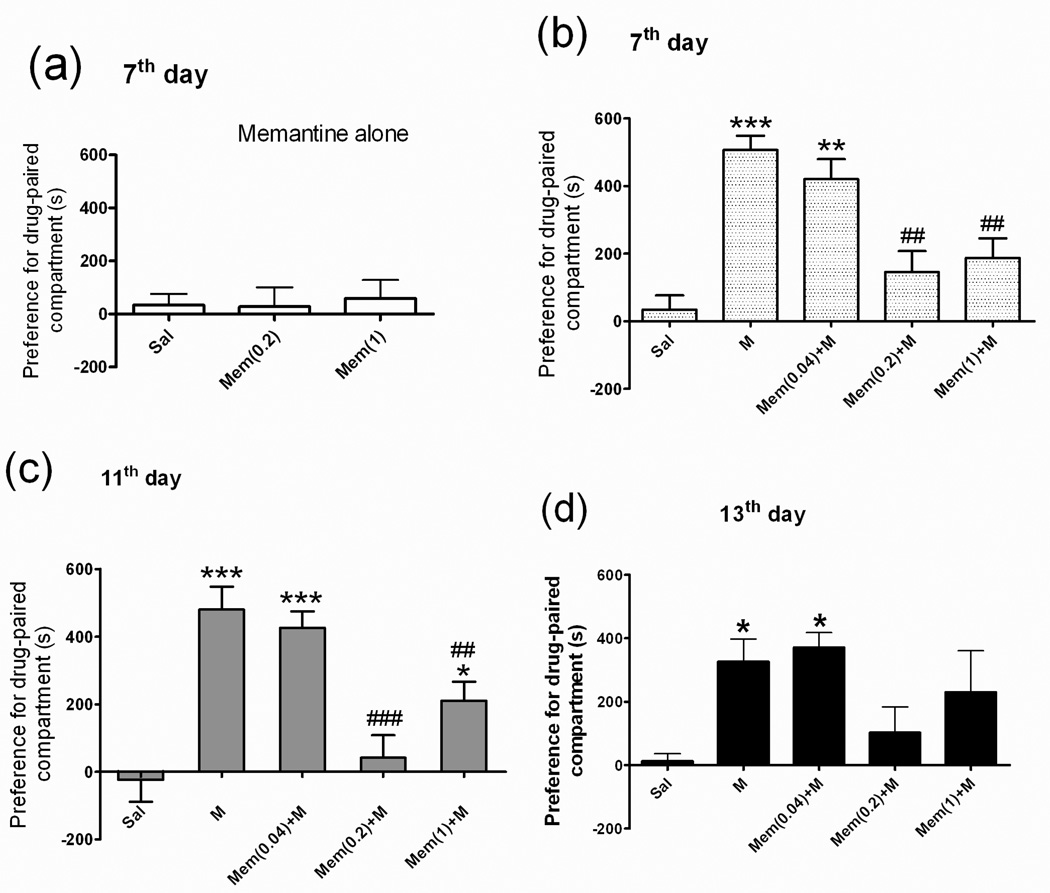
Rats were injected with morphine (M) (5 mg/kg; i.p.) or saline vehicle and then immediately placed in the CPP box for place conditioning. CPP was tested both before (day 0) and after six days of drug-paired conditioning (days 7, 11, and 13). In the saline (Sal) group, the rats did not stay significantly longer in the drug-paired compartment on day 7 (Fig 2, Sal group). In the morphine-treated group, the rats stayed significantly longer in the drug-paired compartment than did the rats in the saline group on days 7 (465.9 ± 58.3 s vs. 27.4 ± 60.7 s), 11 (476.8 ± 77.0 s vs. 22.71 ± 65.7 s), and 13 (325.7 ± 72 s vs. 12.3 ± 23.6 s) (Fig 2b, 2c, 2d, M group).
Fig 2. Place preference behavior in rats.
The rats were divided into 7 groups (n = 7–14 each): the saline (Sal) group was given a daily intraperitoneal injection of saline only; the two memantine (Mem) groups were given either a daily subcutaneous injection of 0.2 or 1.0 mg/kg of memantine only; the morphine (M) group a daily intraperitoneal injection of 5 mg/kg of morphine only; the three Mem+M groups were given a daily subcutaneous injection of Mem (0.04, 0.2, or 1.0 mg/kg) plus a daily intraperitoneal injection of 5 mg/kg of morphine. After 6 days of these treatments, the rats were placed in a conditioned place preference (CPP) box for the drug-paired condition. CPP tests were done on days 7, 11, and 13. CPP data (a) for Sal and Mem-only (0.2 or 1) groups on day 7; (b) for Sal, M-only, and Mem+M groups on day 7; (c) for Sal, M-only, and Mem+M groups on day 11; and (d) for Sal, M-only, and Mem+M groups on day 13. Data are means ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001 vs. Sal group. ##p < 0.01, ###p < 0.001 vs. M group (one-way ANOVA).
Effect of low-dose memantine on CPP in rats treated with chronic morphine
To test the effect of low-dose memantine on morphine-induced drug-seeking behavior, the rats were subcutaneously treated with different low doses of memantine (Mem 0.2 or 1 mg/kg; s.c.) alone, or memantine (Mem 0.04, 0.2, or 1.0 mg/kg; s.c.)+M, and were then placed in the CPP box for 6 days of place conditioning. The CPP in rats treated with memantine only (Mem 0.2 or 1 mg/kg; s.c.) was not affected (Fig 2a). However, the CPP in rats pretreated with 0.2 or 1 mg/kg of memantine, but not in rats treated with 0.04 mg/kg, before each dose of morphine was significantly attenuated, even on days 7, 11, and 13 [Fig 2b, 2c, 2d, Mem (0.2)+M, Mem (1)+M), Mem (0.04)+M)].
Peripheral and central cytokine expression in rats treated with chronic morphine
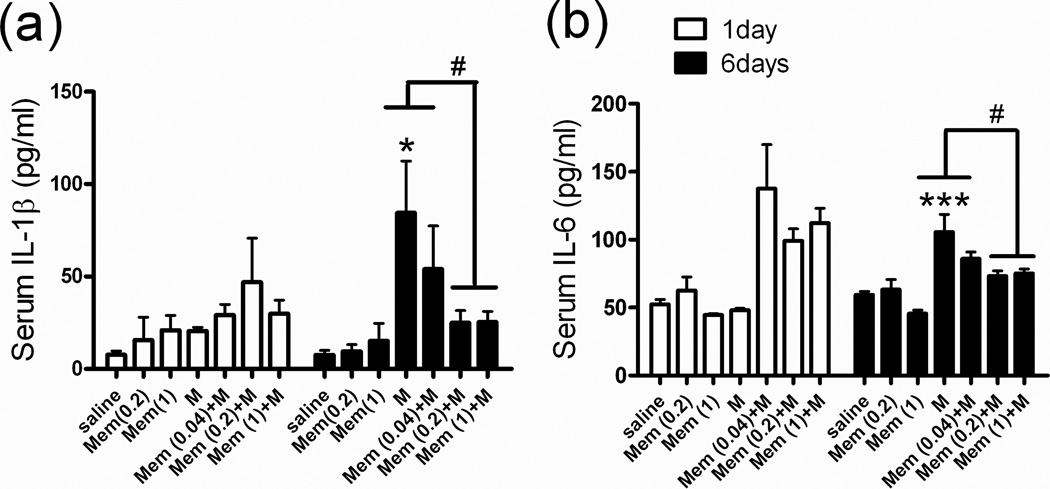
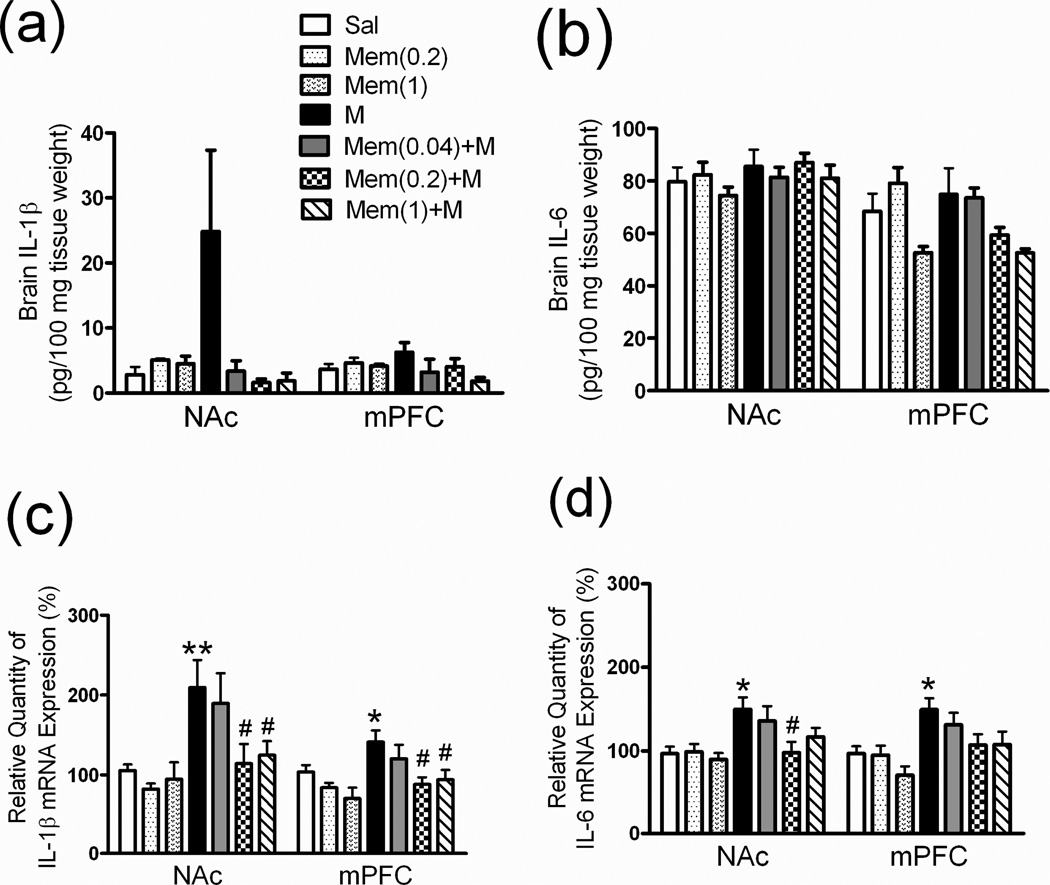
Serum IL-1β and IL-6 levels were significantly higher in M-group rats after they had been treated with morphine (M 5 mg/kg; i.p.) for 6 days (Fig 3a, 3b, M group), which suggested the possibility of opioid-induced production of cytokines in the rat peripheral system. We also analyzed cytokine expression in the addiction-related brain areas. After 6 days of morphine injections, the IL-1β level in the NAc (Fig 4a, Sal vs. M: 2.8 ± 1.2 vs. 24.9 ± 12.5 pg/100 mg tissue weight) and the mPFC (Fig 4a, Sal vs. M: 3.6 ± 0.8 vs. 6.3 ± 1.5 pg/100 mg tissue weight) tended to be higher, but not significantly different from those in the saline group. In addition, the IL-6 level in the NAc and mPFC was not higher in the chronic morphine treatment group (Fig 4b, Sal vs. M group). Therefore, we used QPCR to measure the cytokine mRNA expression in the NAc and mPFC. IL-1β mRNA levels were significantly higher in the NAc (Fig 4c, M group: 209.5 ± 34.2 %, p < 0.01) and the mPFC (Fig 4c, M group: 141.3 ± 14.5 %, p < 0.05) as well as IL-6 mRNA expression levels in the NAc (Fig 4d, M group: 149.9 ± 14.2 %, p < 0.05) and the mPFC (Fig 4d, M group: 149.6 ± 13.6 %, p < 0.05) after 6 days of morphine treatment.
Fig 3. Serum cytokine (IL-1β, IL-6) expression profiles in rats.
See Figure 2 legend for groups and treatments. Serum (a) IL-1β and (b) IL-6 levels after 1–6 days of treatment in all groups. Data are means ± S.E.M. **p < 0.01, ***p < 0.001 vs. Sal group. #p < 0.05 vs. M group (one-way ANOVA).
Fig 4. Brain cytokine (IL-1β, IL-6) and mRNA expression profiles in rats.
See Figure 2 legend for groups and treatments. The (a) IL-1β and (b) IL-16 levels in two addiction-related brain areas (NAc: nucleus accumbens, mPFC: medial prefrontal cortex). The (c) IL-1β mRNA and (d) IL-6 mRNA expression levels in the NAc and mPFC mRNA expression was measured using quantitative polymerase chain reaction (QPCR) analysis and calculated as the relative quantity change percentage (%)compared with the saline group. Data are means ± S.E.M. *p < 0.05, **p < 0.01 vs. Sal group. #p < 0.05 vs. M group (one-way ANOVA).
Effect of low-dose memantine on the peripheral and central nervous system cytokine secretions in rats treated with chronic morphine
Treatment with memantine only (Mem 0.2 or 1 mg/kg) for 6 days did not influence the IL-1β (Fig 3a, Mem 0.2 or 1) and IL-6 (Fig 3b, Mem 0.2 or 1) baseline level in rat serum. In rats pretreated with low-dose memantine (Mem 0.2 or 1.0 mg/kg; s.c.) 30 min before each morphine injection, morphine-induced increases in serum IL-1β and IL-6 were significantly attenuated in rats treated with Mem (0.2 or 1.0)+M (Fig 3a, 3b). In the NAc and mPFC, treatment with memantine alone did not influence IL-1β (Fig 4a) or IL-6 (Fig 4b) baseline levels or their mRNA expression levels (4c, 4d). However, in the NAc and mPFC of rats treated with Mem (0.2 or 1.0)+M, IL-1β mRNA (Fig 4c) and IL-6 mRNA (Fig 4d) were significantly lower than in rats treated with morphine only.
Effect of chronic morphine treatment on peripheral and central BDNF expression in rats
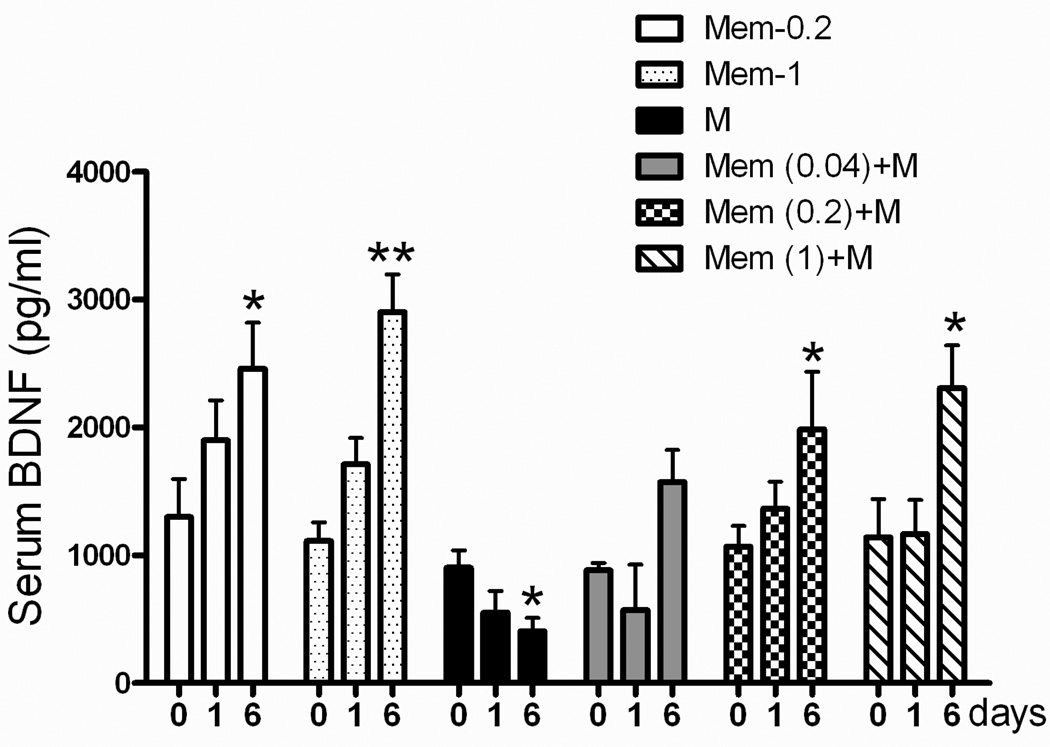
Serum BDNF were significantly lower in rats treated with morphine only (5 mg/kg; i.p.) for 6 days (Fig 5, black bar), which suggested the possibility of opioid-induced inhibition of BDNF in the rat peripheral system. BDNF expression in the NAc (Fig 6a, black bar) and mPFC (Fig 6b, black bar) was also significantly lower in rats treated with morphine only (5 mg/kg; i.p.) for 6 days than in rats treated with saline only.
Fig 5. Serum BDNF expression profiles in rats.
See Figure 2 legend for groups and treatments. Data are means ± S.E.M. *p < 0.05, **p < 0.01 vs. pretreatment data (day 0) within the same group (one-way ANOVA).
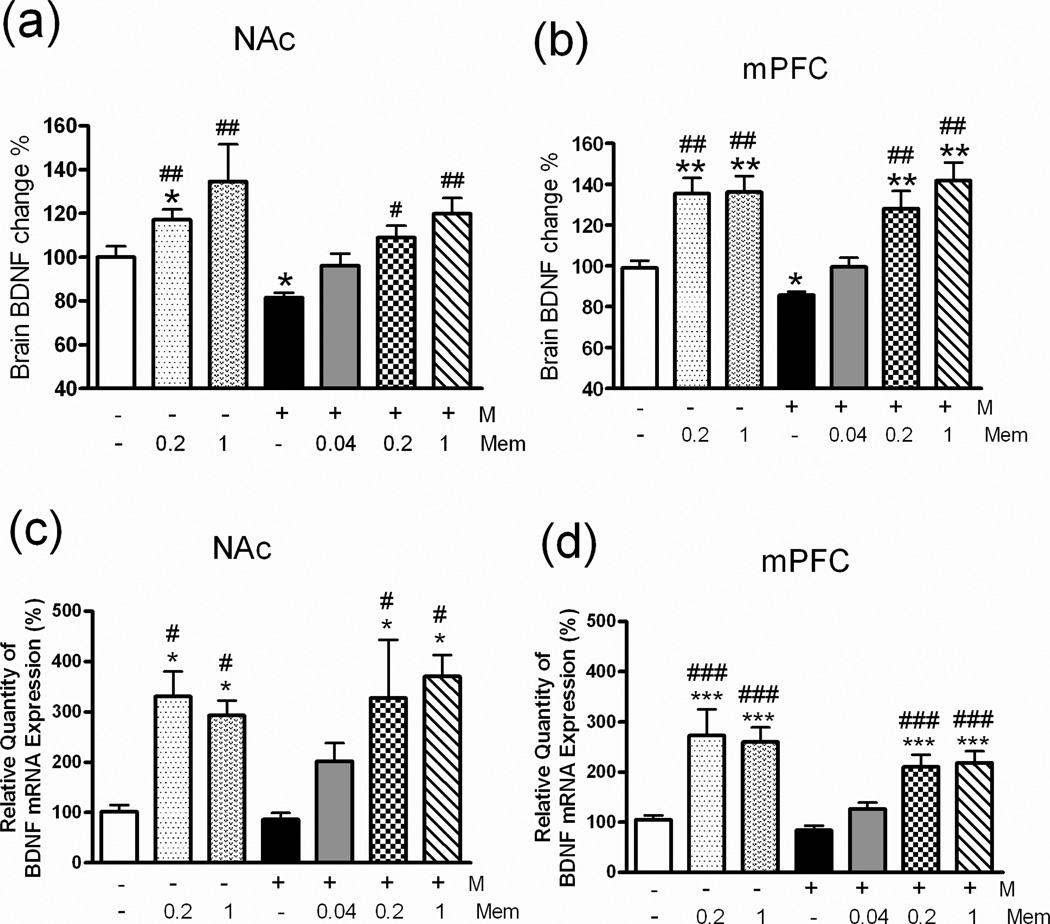
Fig 6. Brain BDNF expression profiles in rats.
See Figure 2 legend for groups and treatments. The BDNF expression profile was calculated as the relative quantity change percentage (%) compared with the saline group of the same brain area. BDNF protein expression levels in (a) the NAc (nucleus accumbens), (b) the mPFC (medial prefrontal cortex), and BDNF mRNA relative expression levels in (c) the NAc and (d) the mPFC of all groups. *p < 0.05, **p < 0.01, ***p < 0.001 vs. saline group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. M group (one-way ANOVA).
Effect of low-dose memantine on BDNF production in rats treated with chronic morphine
In rats treated with memantine only (Mem 0.2 or 1 mg/kg) for 6 days, serum BDNF levels were significantly higher (Fig 5, Memantine only group), as were BDNF (Fig 6a, 6b) and BDNF mRNA levels in the NAc (Fig 6c) and mPFC (Fig 6d). In rats treated with Mem (0.2 or 1.0)+M, serum BDNF expression was significantly higher than in all other groups (Fig 5), as was BDNF expression (Fig 6a, 6b) and BDNF mRNA expression (Fig 6c, 6d) in the NAc and mPFC, but the latter were not significantly higher in rats treated with Mem (0.04)+M.
Discussion
In this study, we showed the significant anti-addictive, anti-inflammatory, and neurotrophic effects of low-dose memantine in rats. Our findings suggest that low-dose memantine is neuroprotective in morphine-addicted rats. We found that IL-1β and IL-6 levels had significantly increased in the peripheral system of rats that had been given 6 days of morphine treatment. We also found the IL-1β levels increased in the drug addiction-related brain areas, the NAc and mPFC; however, brain cytokine levels were not significantly different from those in the saline group. In contrast, IL-1β and IL-6 mRNA levels in the NAc and mPFC were significantly different between morphine-addicted and saline-treated rats. In addition, there was a significant discrepancy between BDNF mRNA and protein levels: brain BDNF mRNA increased about 250–340% in memantine-treated groups, and brain BDNF protein level increased about 120–140%. The small increase of cytokine mRNA (150–200%) in the brain area might have caused only a minor change in cytokine expression in the central nervous system, perhaps because the dose of morphine was not high enough or the 6-day treatment duration not long enough to induce a dramatic increase in brain cytokine expression. However, a significant increase in brain cytokine mRNA expression might have a negative effect on neurons after chronic morphine treatment. A treatment period significantly longer than 6 days may be needed in future studies to clarify the effect of brain cytokine expression after long-term morphine use.
Our findings support those of studies which report that opioids synergistically stimulate cytokine production in the peripheral and central nervous systems in vivo. In the peripheral nervous system, morphine may indirectly affect immunocytes via the central nervous system. Animal studies (Bertolucci et al., 1996) have suggested that subcutaneous or intracerebroventricular administration of morphine elevates serum IL-6 expression, thereby activating the hypothalamus-pituitary-adrenal and autonomic nervous systems. However, the mechanism of opioid-induced cytokine production in the central nervous system is not clear. In vitro studies have reported that morphine potentiates TNF-α production in murine (Chao et al., 1994) and human (Peterson et al., 1994) glia cells.
In the brains of human opioid addicts, iNOS and cytokine expression has been detected in noradrenergic locus coeruleus cells (Dyuizen and Lamash, 2009). The presence of opioid receptors on rat astrocytes (Ruzicka et al., 1995) and the ability of morphine to stimulate primary glia cells to increase their production of cytokines (Chao et al., 1994; Peterson et al., 1998) suggest a possible interaction of morphine with glia cells in the central nervous system. Moreover, Qin et al. (2007) showed that a systemically administered single dose of lipopolysaccharide, which cannot not readily reach the brain, induced early (1–9 h) serum and liver TNF-α expression and long-lasting (7–30 days) brain TNF-α expression, and, subsequently, caused neurodegeneration (Qin et al., 2007). Peripheral inflammation may initiate or contribute to neuroinflammation and neurodegeneration in the central nervous system (Simard and Rivest, 2005); thus, chronic morphine use may stimulate cytokine production in both the peripheral and central nervous systems, cause neuro-inflammation, and subsequently damage neurons that are associated with or exaggerate drug-seeking behavior.
Second, we found BDNF dysfunction in the peripheral and central nervous systems of rats given chronic morphine treatment. It was reported (Pan et al., 1998) that BDNF could cross the blood brain barrier and that BDNF levels in the brain and plasma underwent similar changes during the maturation and aging processes in rats (Karege et al., 2002) and rhesus macaques (Cirulli et al., 2009), all of which suggests that plasma BDNF levels may reflect BDNF levels in the brain. In addition, decreased plasma BDNF protein levels in humans might reflect the degree of neuronal degeneration in Alzheimer's disease (Laske et al., 2006). Furthermore, chronic morphine treatment has resulted in a large (30%) reduction in the dopaminergic neurons of the ventral tegmental area (VTA) (Chu et al., 2008; Sklair-Tavron et al., 1996). In rat cortical neuron cultures, a combination of heroin and cocaine caused significant neurotoxicity (Cunha-Oliveira et al., 2010), which suggested the possibility that chronic morphine use causes neuronal damage and degeneration. In our study, we found the same profile of decreased BDNF in rat serum and brain area after 6 days of morphine treatment. The decreased serum and brain BDNF level might indicate a certain degree of neuronal degeneration caused by long-term opioid abuse in vivo. Taking all these findings together, using therapeutic agents with anti-inflammatory or neurotrophic effects may inhibit the neurodegeneration caused by chronic opioid use and provide a beneficial adjuvant treatment for opioid addiction.
Memantine in higher doses (7.5–20 mg/kg; s.c.) was considered an NMDA receptor antagonist and an inhibitor of morphine-induced tolerance, physical dependence, and drug seeking effects in animal models (Popik and Skolnick, 1996; Ribeiro Do Couto et al., 2004). However, we recently showed (Lin et al., 2011) that using a low dose of memantine (0.02–2.0 mg/kg) abolished cocaine-induced CPP behavior in rats via its IL-6-modulating effect in the medial prefrontal cortex. In our clinic, 5 mg (0.1 mg/kg) of oral memantine added to the methadone replacement therapy given to heroin abusers significantly attenuated the methadone replacement dose and the combined heroin use (unpublished data). The human plasma memantine concentration was about 10–50 ng/ml (0.05–0.2 µM). This low dose of memantine was not high enough to blockade the NMDA receptors (IC50: 2–3 µM) (Parsons et al., 1999). In this study, we used low doses (0.2 and 1.0 mg/kg; s.c.) of memantine that do not have the NMDA blocking effect but showed significant inhibition of CPP in morphine-addicted rats. These data suggest a non-NMDA or biphasic effect of low-dose memantine. Memantine was recently shown to be neurotrophic and neuroprotective through its upregulation of BDNF (Meisner et al., 2008) and GDNF (Wu et al., 2009). In the present study, we found that low-dose memantine decreased cytokine expression and upregulated BDNF expression in the serum and drug-addiction-related brain areas in rats, which indicates the possible anti-inflammatory and neurotrophic effects of low-dose memantine on the limbic dopaminergic system. As a consequence, the anti-addictive mechanism of low dose memantine may be its anti-inflammation effects, upregulation of BDNF production, and neuronal protection effects.
In conclusion, our results suggest the possibility that chronic morphine use may induce neuronal degeneration in the brain areas associated with addictive behaviors. Using the neuroprotection agent memantine at a low dose may provide adjuvant therapy for chronic opioid-induced addiction.
Acknowledgments
This study was supported in part by grant NSC 98-2627-B-006-017 from the Taiwan National Science Council (to RBL), and by a grant from the National Cheng Kung University Project to Promote Academic Excellence and Develop a World Class Research Center.
Footnotes
Conflict of interest
All authors declare that they have no conflicts of interest.
Reference
- Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Ricci V, Pomponi M, Conte G, Mathe AA, Attilio Tonali P, Bria P. Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. Journal of psychopharmacology (Oxford, England) 2007;21:820–825. doi: 10.1177/0269881107078491. [DOI] [PubMed] [Google Scholar]
- Bertolucci M, Perego C, De Simoni MG. Central opiate modulation of peripheral IL-6 in rats. Neuroreport. 1996;7:1181–1184. doi: 10.1097/00001756-199604260-00017. [DOI] [PubMed] [Google Scholar]
- Carrasco MA, Castro P, Sepulveda FJ, Tapia JC, Gatica K, Davis MI, Aguayo LG. Regulation of glycinergic and GABAergic synaptogenesis by brain-derived neurotrophic factor in developing spinal neurons. Neuroscience. 2007;145:484–494. doi: 10.1016/j.neuroscience.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK. Priming effect of morphine on the production of tumor necrosis factor-alpha by microglia: implications in respiratory burst activity and human immunodeficiency virus-1 expression. J Pharmacol Exp Ther. 1994;269:198–203. [PubMed] [Google Scholar]
- Chipana C, Torres I, Camarasa J, Pubill D, Escubedo E. Memantine protects against amphetamine derivatives-induced neurotoxic damage in rodents. Neuropharmacology. 2008;54:1254–1263. doi: 10.1016/j.neuropharm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Chu NN, Xia W, Yu P, Hu L, Zhang R, Cui CL. Chronic morphine-induced neuronal morphological changes in the ventral tegmental area in rats are reversed by electroacupuncture treatment. Addict Biol. 2008;13:47–51. doi: 10.1111/j.1369-1600.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Branchi I, Antonucci MT, Aloe L, Suomi SJ, Alleva E. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009;34:172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR. Neurotoxicity of heroin-cocaine combinations in rat cortical neurons. Toxicology. 2010;276:11–17. doi: 10.1016/j.tox.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Dyuizen I, Lamash NE. Histo- and immunocytochemical detection of inducible NOS and TNF-alpha in the locus coeruleus of human opiate addicts. J Chem Neuroanat. 2009;37:65–70. doi: 10.1016/j.jchemneu.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Gong YX, Wang HJ, Zhu YP, Zhang WP, Dai HB, Zhang SH, Wei EQ, Chen Z. Carnosine ameliorates morphine-induced conditioned place preference in rats. Neurosci Lett. 2007;422:34–38. doi: 10.1016/j.neulet.2007.05.048. [DOI] [PubMed] [Google Scholar]
- Hatami H, Oryan S, Semnanian S, Kazemi B, Bandepour M, Ahmadiani A. Alterations of BDNF and NT-3 genes expression in the nucleus paragigantocellularis during morphine dependency and withdrawal. Neuropeptides. 2007;41:321–328. doi: 10.1016/j.npep.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Huang EY, Liu TC, Tao PL. Co-administration of dextromethorphan with morphine attenuates morphine rewarding effect and related dopamine releases at the nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:386–392. doi: 10.1007/s00210-003-0803-7. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6:3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi AA, Gibbons N, Mattana J, Singhal PC. Morphine stimulates mesangial cell TNF-alpha and nitrite production. Inflammation. 2000;24:463–476. doi: 10.1023/a:1007016329300. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G, Schott K. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm. 2006;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- Lin KY, Cherng CG, Yang FR, Lin LC, Lu RB, Yu L. Memantine abolishes the formation of cocaine-induced conditioned place preference possibly via its IL-6-modulating effect in medial prefrontal cortex. Behav Brain Res. 2011;220:126–131. doi: 10.1016/j.bbr.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Meisner F, Scheller C, Kneitz S, Sopper S, Neuen-Jacob E, Riederer P, ter Meulen V, Koutsilieri E. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: a novel pharmacological action of memantine. Neuropsychopharmacology. 2008;33:2228–2236. doi: 10.1038/sj.npp.1301615. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–10708. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Hayashi M. A New Aspect of the TrkB Signaling Pathway in Neural Plasticity. Current neuropharmacology. 2009;7:276–285. doi: 10.2174/157015909790031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW, Yin QW, Prevette D, Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 1992;360:755–757. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- Pacifici R, di Carlo S, Bacosi A, Pichini S, Zuccaro P. Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int J Immunopharmacol. 2000;22:603–614. doi: 10.1016/s0192-0561(00)00023-0. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Peng HB, Yang JF, Dai Z, Lee CW, Hung HW, Feng ZH, Ko CP. Differential effects of neurotrophins and schwann cell-derived signals on neuronal survival/growth and synaptogenesis. J Neurosci. 2003;23:5050–5060. doi: 10.1523/JNEUROSCI.23-12-05050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HH, Jr, Chao CC. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol. 1994;50:167–175. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Popik P, Skolnick P. The NMDA antagonist memantine blocks the expression and maintenance of morphine dependence. Pharmacol Biochem Behav. 1996;53:791–797. doi: 10.1016/0091-3057(95)02163-9. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Minarro J. Effects of NMDA receptor antagonists (MK-801 and memantine) on the acquisition of morphine-induced conditioned place preference in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1035–1043. doi: 10.1016/j.pnpbp.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express mu, delta and kappa opioid receptor mRNA. Brain Res Mol Brain Res. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- Segal RA, Takahashi H, McKay RD. Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron. 1992;9:1041–1052. doi: 10.1016/0896-6273(92)90064-k. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Do pathogen exposure and innate immunity cause brain diseases? Neurol Res. 2005;27:717–725. doi: 10.1179/016164105X49526. [DOI] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Takayama N, Ueda H. Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. J Neurosci. 2005;25:430–435. doi: 10.1523/JNEUROSCI.3170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashvili M, Wu HE, Schwasinger ET, Hung KC, Hong JS, Tseng LF. (+)-Morphine attenuates the (−)-morphine-produced conditioned place preference and the mu-opioid receptor-mediated dopamine increase in the posterior nucleus accumbens of the rat. Eur J Pharmacol. 2008;587:147–154. doi: 10.1016/j.ejphar.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HE, Schwasinger ET, Terashvili M, Tseng LF. dextro-Morphine attenuates the morphine-produced conditioned place preference via the sigma (1) receptor activation in the rat. Eur J Pharmacol. 2007;562:221–226. doi: 10.1016/j.ejphar.2007.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Tzeng NS, Qian L, Wei SJ, Hu X, Chen SH, Rawls SM, Flood P, Hong JS, Lu RB. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology. 2009;34:2344–2357. doi: 10.1038/npp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hong JS, Kim HC, Zhang W, Block ML. Morphinan neuroprotection: new insight into the therapy of neurodegeneration. Crit Rev Neurobiol. 2004;16:271–302. doi: 10.1615/critrevneurobiol.v16.i4.30. [DOI] [PubMed] [Google Scholar]
- Zin CS, Nissen LM, O'Callaghan JP, Moore BJ, Smith MT. Preliminary Study of the Plasma and Cerebrospinal Fluid Concentrations of IL-6 and IL-10 in Patients with Chronic Pain Receiving Intrathecal Opioid Infusions by Chronically Implanted Pump for Pain Management. Pain Med. 2010 doi: 10.1111/j.1526-4637.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- Zubelewicz B, Muc-Wierzgon M, Harbuz MS, Brodziak A. Central single and chronic administration of morphine stimulates corticosterone and interleukin (IL)-6 in adjuvant-induced arthritis. J Physiol Pharmacol. 2000;51:897–906. [PubMed] [Google Scholar]