Abstract
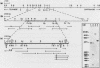
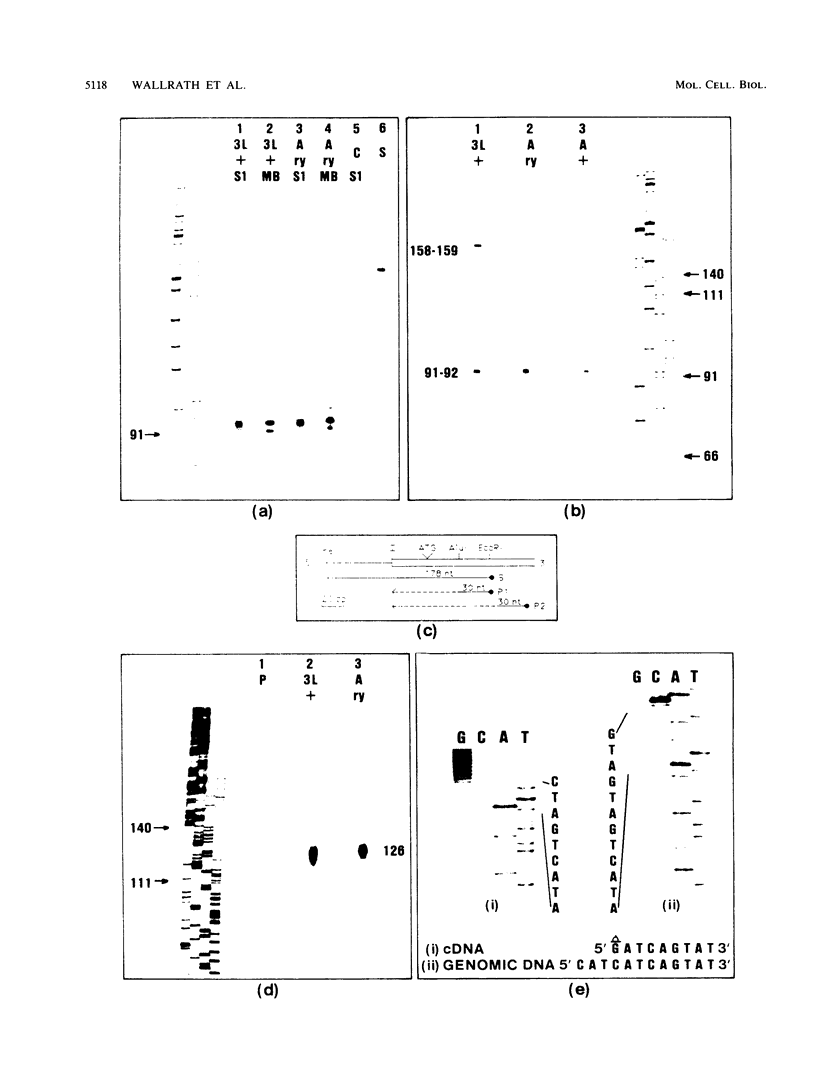
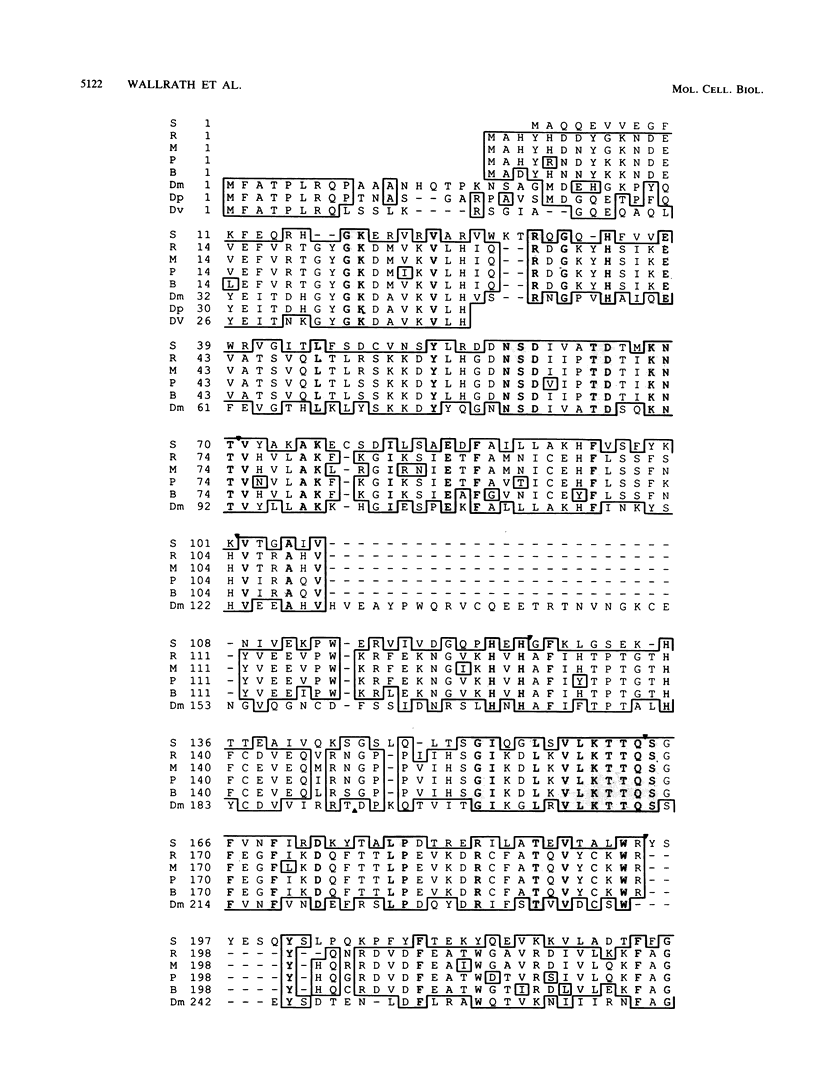
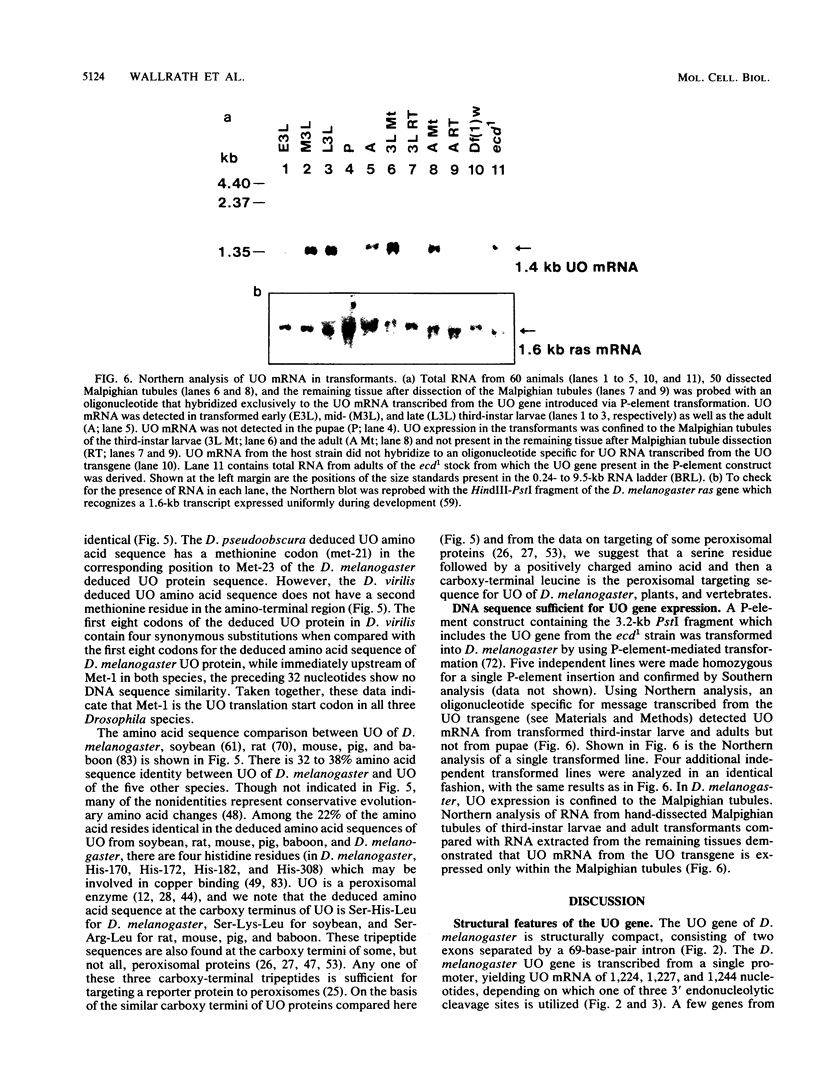
The urate oxidase (UO) gene of Drosophila melanogaster is expressed during the third-instar larval and adult stages, exclusively within a subset of cells of the Malpighian tubules. The UO gene contains a 69-base-pair intron and encodes mature mRNAs of 1,224, 1,227, and 1,244 nucleotides, depending on the site of 3' endonucleolytic cleavage prior to polyadenylation. A direct repeat, 5'-AAGTGAGAGTGAT-3', is the proposed cis-regulatory element involved in 20-hydroxyecdysone repression of the UO gene. The deduced amino acid sequences of UO of D. melanogaster, rat, mouse, and pig and uricase II of soybean show 32 to 38% identity, with 22% of amino acid residues identical in all species. With use of P-element-mediated germ line transformation, 826 base pairs 5' and approximately 1,200 base pairs 3' of the D. melanogaster UO transcribed region contain all of the cis elements allowing for appropriate temporal regulation and Malpighian tubule-specific expression of the UO gene.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bender W., Spierer P., Hogness D. S. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983 Jul 25;168(1):17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bray S. J., Johnson W. A., Hirsh J., Heberlein U., Tjian R. A cis-acting element and associated binding factor required for CNS expression of the Drosophila melanogaster dopa decarboxylase gene. EMBO J. 1988 Jan;7(1):177–188. doi: 10.1002/j.1460-2075.1988.tb02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Schulz R. A., Koehler M. M., Savakis C., Cherbas P. Structure of the Eip28/29 gene, an ecdysone-inducible gene from Drosophila. J Mol Biol. 1986 Jun 20;189(4):617–631. doi: 10.1016/0022-2836(86)90492-4. [DOI] [PubMed] [Google Scholar]
- Chovnick A., Finnertty V., Schalet A., Duck P. Studies on genetic organization in higher organisms. I. Analysis of a complex gene in Drosophila melanogaster. Genetics. 1969 May;62(1):145–160. doi: 10.1093/genetics/62.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Denome R. M., Cole C. N. Patterns of polyadenylation site selection in gene constructs containing multiple polyadenylation signals. Mol Cell Biol. 1988 Nov;8(11):4829–4839. doi: 10.1128/mcb.8.11.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesen T. D., Johnson D. H., Henikoff S. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol Cell Biol. 1988 Dec;8(12):5206–5215. doi: 10.1128/mcb.8.12.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J., Charron J., Gagner J. P., Jeannotte L., Nemer M., Plante R. K., Wrange O. Pro-opiomelanocortin gene: a model for negative regulation of transcription by glucocorticoids. J Cell Biochem. 1987 Dec;35(4):293–304. doi: 10.1002/jcb.240350404. [DOI] [PubMed] [Google Scholar]
- Edgar B. A., O'Farrell P. H. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989 Apr 7;57(1):177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Rubin G. M. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev. 1990 Mar;4(3):444–463. doi: 10.1101/gad.4.3.444. [DOI] [PubMed] [Google Scholar]
- Friedman T. B., Johnson D. H. Autonomous timer in malpighian tubules. Science. 1978 Jun 9;200(4346):1186–1186. doi: 10.1126/science.200.4346.1186. [DOI] [PubMed] [Google Scholar]
- Friedman T. B., Johnson D. H. Temporal control of urate oxidase activity in Drosophila: evidence of an autonomous timer in malpighian tubules. Science. 1977 Jul 29;197(4302):477–479. doi: 10.1126/science.406675. [DOI] [PubMed] [Google Scholar]
- Friedman T. B. Observations on the regulation of uricase activity during development of Drosophila melanogaster. Biochem Genet. 1973 Jan;8(1):37–45. doi: 10.1007/BF00485555. [DOI] [PubMed] [Google Scholar]
- Garbe J. C., Bendena W. G., Pardue M. L. Sequence evolution of the Drosophila heat shock locus hsr omega. I. The nonrepeated portion of the gene. Genetics. 1989 Jun;122(2):403–415. doi: 10.1093/genetics/122.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman E. Genetic regulation of xanthine dehydrogenase in Drosophila melanogaster. Fed Proc. 1965 Sep-Oct;24(5):1243–1251. [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Schneider M., Howell S. H., Garrard L. J., Goodman J. M., Distel B., Tabak H., Subramani S. Peroxisomal protein import is conserved between yeast, plants, insects and mammals. EMBO J. 1990 Jan;9(1):85–90. doi: 10.1002/j.1460-2075.1990.tb08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Subramani S. Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J Cell Biol. 1988 Sep;107(3):897–905. doi: 10.1083/jcb.107.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Taya K., Suga T., Niinobe S. Studies on peroxisomes. VI. Relationship between the peroxisomal core and urate oxidase. J Biochem. 1976 May;79(5):1029–1034. doi: 10.1093/oxfordjournals.jbchem.a131143. [DOI] [PubMed] [Google Scholar]
- Hentschel C., Irminger J. C., Bucher P., Birnstiel M. L. Sea urchin histone mRNA termini are located in gene regions downstream from putative regulatory sequences. Nature. 1980 May 15;285(5761):147–151. doi: 10.1038/285147a0. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Morgan B. A., Scholnick S. B. Delimiting regulatory sequences of the Drosophila melanogaster Ddc gene. Mol Cell Biol. 1986 Dec;6(12):4548–4557. doi: 10.1128/mcb.6.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Hung M. C., Wensink P. C. Sequence and structure conservation in yolk proteins and their genes. J Mol Biol. 1983 Mar 15;164(4):481–492. doi: 10.1016/0022-2836(83)90046-3. [DOI] [PubMed] [Google Scholar]
- Ito M., Suzuki M., Takagi Y. Nucleotide sequence of cDNA and predicted amino acid sequence of rat liver uricase. Eur J Biochem. 1988 Apr 15;173(2):459–463. doi: 10.1111/j.1432-1033.1988.tb14021.x. [DOI] [PubMed] [Google Scholar]
- Johnson D. H., Edström J. E., Burnett J. B., Friedman T. B. Cloning of a Drosophila melanogaster adenine phosphoribosyltransferase structural gene and deduced amino acid sequence of the enzyme. Gene. 1987;59(1):77–86. doi: 10.1016/0378-1119(87)90268-x. [DOI] [PubMed] [Google Scholar]
- Johnson D. H., Friedman T. B. Purine-resistant Drosophila melanogaster result from mutations in the adenine phosphoribosyltransferase structural gene. Proc Natl Acad Sci U S A. 1983 May;80(10):2990–2994. doi: 10.1073/pnas.80.10.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J. A., Desplan C., Wright D. K., O'Farrell P. H. Evolutionary conservation of homeodomain-binding sites and other sequences upstream and within the major transcription unit of the Drosophila segmentation gene engrailed. Mol Cell Biol. 1989 Oct;9(10):4304–4311. doi: 10.1128/mcb.9.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of Drosophila pre-mRNAs. Nucleic Acids Res. 1985 Jul 11;13(13):4971–4981. doi: 10.1093/nar/13.13.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Gehring W. J. Sequence requirement for expression of the Drosophila melanogaster heat shock protein hsp22 gene during heat shock and normal development. Mol Cell Biol. 1986 Jun;6(6):2011–2019. doi: 10.1128/mcb.6.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral L. G., Johnson D. H., Burnett J. B., Friedman T. B. Cloning a cDNA for Drosophila melanogaster urate oxidase. Gene. 1986;45(2):131–137. doi: 10.1016/0378-1119(86)90247-7. [DOI] [PubMed] [Google Scholar]
- Laird-Offringa I. A., Elfferich P., Knaken H. J., de Ruiter J., van der Eb A. J. Analysis of polyadenylation site usage of the c-myc oncogene. Nucleic Acids Res. 1989 Aug 25;17(16):6499–6514. doi: 10.1093/nar/17.16.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lepesant J. A., Kejzlarova-Lepesant J., Garen A. Ecdysone-inducible functions of larval fat bodies in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5570–5574. doi: 10.1073/pnas.75.11.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Lewin A. S., Hines V., Small G. M. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol. 1990 Apr;10(4):1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Martin M., Giangrande A., Ruiz C., Richards G. Induction and repression of the Drosophila Sgs-3 glue gene are mediated by distinct sequences in the proximal promoter. EMBO J. 1989 Feb;8(2):561–568. doi: 10.1002/j.1460-2075.1989.tb03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T., Ohno K., Miura S., Fujiki Y. Peroxisome targeting signal of rat liver acyl-coenzyme A oxidase resides at the carboxy terminus. Mol Cell Biol. 1989 Jan;9(1):83–91. doi: 10.1128/mcb.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M., Gehring W. J. Expression of the caudal gene in the germ line of Drosophila: formation of an RNA and protein gradient during early embryogenesis. Cell. 1987 Feb 13;48(3):465–478. doi: 10.1016/0092-8674(87)90197-8. [DOI] [PubMed] [Google Scholar]
- Moriyama E. N., Gojobori T. Evolution of nested genes with special reference to cuticle proteins in Drosophila melanogaster. J Mol Evol. 1989 May;28(5):391–397. doi: 10.1007/BF02603074. [DOI] [PubMed] [Google Scholar]
- Motojima K., Kanaya S., Goto S. Cloning and sequence analysis of cDNA for rat liver uricase. J Biol Chem. 1988 Nov 15;263(32):16677–16681. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer B., Marlor R., Parkhurst S., Corces V. Characterization and developmental expression of a Drosophila ras oncogene. Mol Cell Biol. 1985 Apr;5(4):885–889. doi: 10.1128/mcb.5.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem. 1986 Oct;158(1):165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Zelechowska M., Foster V., Bergmann H., Verma D. P. Primary structure of the soybean nodulin-35 gene encoding uricase II localized in the peroxisomes of uninfected cells of nodules. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5040–5044. doi: 10.1073/pnas.82.15.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J. M., McLean J. R., Reynolds E. R. Molecular and developmental genetics of the Punch locus, a pterin biosynthesis gene in Drosophila melanogaster. Dev Genet. 1989;10(3):273–286. doi: 10.1002/dvg.1020100316. [DOI] [PubMed] [Google Scholar]
- O'Tousa J. E., Baehr W., Martin R. L., Hirsh J., Pak W. L., Applebury M. L. The Drosophila ninaE gene encodes an opsin. Cell. 1985 Apr;40(4):839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Steller H., Bozzetti M. P. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985 Dec 16;4(13A):3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O. Ecdysteroid-regulated gene expression in Drosophila melanogaster. Eur J Biochem. 1988 Aug 1;175(2):199–204. doi: 10.1111/j.1432-1033.1988.tb14184.x. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Quan F., Forte M. A. Two forms of Drosophila melanogaster Gs alpha are produced by alternate splicing involving an unusual splice site. Mol Cell Biol. 1990 Mar;10(3):910–917. doi: 10.1128/mcb.10.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. G., Nemali M. R., Reddy M. K., Reddy M. N., Yuan P. M., Yuen S., Laffler T. G., Shiroza T., Kuramitsu H. K., Usuda N. Isolation and sequence determination of a cDNA clone for rat peroxisomal urate oxidase: liver-specific expression in the rat. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9081–9085. doi: 10.1073/pnas.85.23.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G., Cassab A., Bourouis M., Jarry B., Dissous C. The normal developmental regulation of a cloned sgs3 'glue' gene chromosomally integrated in Drosophila melanogaster by P element transformation. EMBO J. 1983;2(12):2137–2142. doi: 10.1002/j.1460-2075.1983.tb01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M. A., Haffley L., Kaufman T. C. Characterization of amalgam: a member of the immunoglobulin superfamily from Drosophila. Cell. 1988 Nov 18;55(4):589–600. doi: 10.1016/0092-8674(88)90217-6. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Joh T., Tsuzuki T., Shimada K., Matsukado Y. Structural organization of the mouse mitochondrial malate dehydrogenase gene. J Mol Biol. 1988 Mar 5;200(1):1–11. doi: 10.1016/0022-2836(88)90328-2. [DOI] [PubMed] [Google Scholar]
- Török I., Karch F. Nucleotide sequences of heat shock activated genes in Drosophila melanogaster. I. Sequences in the regions of the 5' and 3' ends of the hsp 70 gene in the hybrid plasmid 56H8. Nucleic Acids Res. 1980 Jul 25;8(14):3105–3123. doi: 10.1093/nar/8.14.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. W., Lee C. C., Muzny D. M., Caskey C. T. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9412–9416. doi: 10.1073/pnas.86.23.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]