Abstract
MicroRNA-21 (miR-21) is a highly expressed microRNA (miRNA) in cardiovascular system. Recent studies have revealed that its expression is deregulated in heart and vasculature under cardiovascular disease conditions such as proliferative vascular disease, cardiac hypertrophy and heart failure, and ischemic heart disease. miR-21 is found to play important roles in vascular smooth muscle cell proliferation and apoptosis, cardiac cell growth and death, and cardiac fibroblast functions. Accordingly, miR-21 is proven to be involved in the pathogenesis of the above-mentioned cardiovascular diseases as demonstrated by both loss-of-function and gain-of-function approaches. Programmed cell death 4 (PDCD4), phosphatase and tensin homology deleted from chromosome 10 (PTEN), sprouty1 (SPRY1), and sprouty2 (SPRY2) are the current identified target genes of miR-21 that are involved in miR-21-mediated cardiovascular effects. miR-21 might be a novel therapeutic target in cardiovascular diseases. This review article summarizes the research progress regarding the roles of miR-21 in cardiovascular disease.
Keywords: MicroRNA, MicroRNA-21, Gene Expression, Cardiovascular Disease
Introduction
MicroRNAs (miRNAs) are endogenous, noncoding, single-stranded RNAs of ~22 nucleotides encoded by short inverted repeats within the genome. The first miRNA, lin-4, was discovered in Caenorhabditis elegans in 1993 [1, 2]. The presence of miRNAs in vertebrates was confirmed in 2001 [3]. Currently, more than 800 miRNAs have been cloned and sequenced in humans [4], and the estimated number of miRNA genes is as high as 1,000 in the human genome [5]. miRNAs regulate their target gene expression by degradation, translational inhibition, or translational activation of their target mRNAs [6, 7]. As a group, miRNAs may directly regulate more than 30% of the genes in a cell [8].
The biological functions of some miRNAs including miR-21 have been well investigated during the past 5 years [4, 9]. miR-21 is encoded by a single gene and displays a strong evolutionary conservation across a wide range of vertebrate species. The human miR-21 gene is mapped to chromosome 17q23.2, where it overlaps with the protein-coding gene VMP1 (or TMEM49). However, the primary transcript containing miR-21 (i.e., pri-miR-21) is independently transcribed from a conserved promoter that is located within the intron of the overlapping protein-coding gene [10, 11]. Unlike some other selectively expressed miRNAs, miR-21 is universally expressed in mammal organ systems such as the heart, the spleen, the small intestine and the colon [12]. The biological roles of miR-21 are well demonstrated in tumor studies [9, 10]. Indeed, miR-21 has been found to be overexpressed in many tumor samples. Moreover, many functional studies of miR-21 have identified that miR-21 has oncogenic activity and can be classed as an oncomir.
More recently, the roles of miR-21 in cardiovascular biology and disease have received significant attention [13]. miR-21 has been found to be highly expressed in all main types of cardiovascular cells, including vascular smooth muscle cell (VSMC) [14], endothelial cell [15], cardiomyocyte [16], and cardiac fibroblast [17]. Interestingly, miR-21 is aberrantly expressed in many cardiovascular diseases [13]. Moreover, miR-21 has been found to play important roles in these cardiovascular disorders in recent studies by both loss-of-function and gain-of-function approaches. Furthermore, the potential target genes involved in miR-21-mediated cardiovascular effects have started to be identified. This review article summarizes the research progress regarding the roles of miR-21 in cardiovascular disease.
miR-21 in Proliferative Vascular Disease
Neointimal lesion formation is a common pathological lesion found in diverse proliferative vascular diseases, such as atherosclerosis, coronary heart disease, postangioplasty restenosis, and transplantation arteriopathy. Using a well-established neointimal formation model, we determined the miRNA expression profile in rat carotid artery after angioplasty [14]. We found that multiple miRNAs were differentially expressed in rat carotid arteries with neointimal growth after angioplasty. Among the aberrantly expressed miRNAs, miR-21 expression had more than a fivefold increase in balloon-injured arteries compared with that in normal control vessels. Knock-down of the overexpressed miR-21in balloon-injured rat carotid arteries inhibited neointimal lesion growth significantly [14]. In cultured VSMCs in vitro and in rat carotid arteries in vivo, we identified that anti-apoptotic and proliferative effects on VSMCs were the major cellular mechanisms involved in miR-21-mediated vascular neointimal growth. In respect to the molecular mechanisms, phosphatase and tensin homology deleted from chromosome 10 (PTEN) [14] and programmed cell death 4 (PDCD4) [18] were two major target genes responsible for miR-21-mediated cellular effects on VSMCs. It is well known that PTEN is expressed in cardiomyocytes, fibroblasts, endothelial cells, and vascular smooth muscle cells where it modulates cell survival/apoptosis, hypertrophy, contractility, and metabolism via its target molecules, phosphoinositide-3 kinases (PI3Ks) and Akt [19]. Thus, PTEN is a critical molecule in the development of many cardiovascular diseases [19]. PDCD4 has been known as a tumor suppressor gene and potential target for anticancer therapies for several years [20]. Recent studies have revealed that PDCD4 is also involved in cardiovascular biology by regulating the apoptosis of vascular smooth muscle cells and cardiac cells [18, 21]. The upregulation of miR-21 in proliferative vessels was further confirmed in a mouse ligation model [22]. These observations indicate clearly that miR-21 is involved in the pathogenesis of proliferative vascular disease (Fig. 1) [23].
Fig. 1.
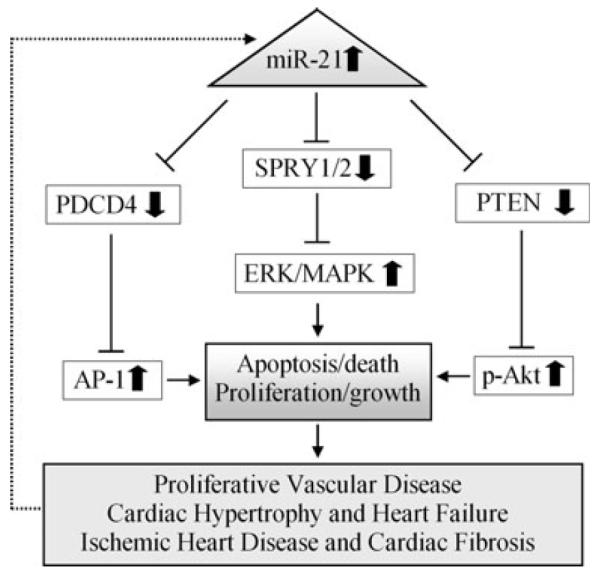
The roles of miR-21in cardiovascular disease and their potential mechanisms
miR-21 in Cardiac Hypertrophy and Heart Failure
Cardiac hypertrophy is a common pathological response to a number of cardiovascular diseases, such as hypertension, ischemic heart disease, vascular disease, and endocrine disorder [24]. Cardiac hypertrophy often leads to heart failure, and is a major determinant of mortality and morbidity in cardiovascular diseases. miRNAs are important regulators for the differentiation and growth of cardiac cells, and it is therefore reasonable to hypothesize that miRNAs play important roles in cardiac hypertrophy and heart failure. The miRNA expression profiles were well demonstrated in mouse hearts in which cardiac hypertrophy was induced by transverse aortic constriction (TAC) or calcineurin transgenic approach [16, 25–28]. In these studies, miR-21 was found to be significantly upregulated in hypertrophic animal hearts.
The biological role of miR-21 in cardiac hypertrophy has been determined by several recent studies, although the results are still controversial [16, 28–32] (Fig. 1). Thum et al. reported that miR-21 was not highly expressed in cardiac myocytes [29]. However, our studies revealed that miR-21 was still abundant in cardiac cells [16, 21, 31]. One of the potential reasons for the controversial results is that the expression of miR-21 in cardiac myocytes is related to the development. Indeed, the expression of miR-21 in neonatal rat cardiac myocytes is much higher than that in adult cardiac myocytes [29]. Additional experiments using the adult cardiac myocytes will be required in miR-21 studies. In cultured cardiac cells, we identified that inhibition of miR-21 had a significant negative effect on cardiomyocyte hypertrophy [16]. The finding was further confirmed by another group [30]. More recently, one excellent study had demonstrated that miR-21 was a critical regulator for cardiac cell outgrowth, which is a morphological change that accompanies cardiac hypertrophy [32]. These investigators revealed that miR-21 inhibition could block β-adrenergic receptor (βAR) stimulation-induced cardiomyocyte outgrowth. In contrast, the cardiomyocyte outgrowth was significantly enhanced by overexpression of miR-21. They further identified that miR-21-mediated cardiomyocyte outgrowth was related to its target gene SPRY2. The in vivo effect of miR-21 on cardiac hypertrophy was reported by Thum et al. [29]. These investigators demonstrated that downregulation of miR-21 in adult mouse hearts by an antagomir approach was able to reduce cardiomyocyte size and the heart weight under the hypertrophy-inducing condition.
Cardiac fibrosis is a pathological component of cardiac hypertrophy and heart failure. The expression of miR-21 in cardiac fibroblasts is much higher than that in cardiomyocytes. Interestingly, Thum et al. found that miR-21 was significantly upregulated in cardiac fibroblasts of the failing hearts [29]. Upregulation of miR-21 in response to cardiac stress was shown to enhance ERK-MAP kinase signaling, leading to fibroblast proliferation and fibrosis. Conversely, silencing of miR-21 using an antagomir in a mouse cardiac hypertrophy and failure model reduced cardiac ERK-MAP kinase activity, inhibited interstitial fibrosis, and attenuated cardiac dysfunction. Remarkably, miR-21 inhibition not only prevented cardiac hypertrophy but also was capable of reversing cardiac remodeling in response to stress. SPRY1, a potent inhibitor of the Ras/MEK/ERK pathway, was found to be a direct target of miR-21, and mediated the effect of miR-21 on cardiac fibroblasts (Fig. 1) [29].
miR-21 in Ischemic Heart Disease
Acute myocardial infarction (AMI) has long been the leading cause of morbidity and mortality in developed countries. The expression signature of miRNAs in the late phase of AMI (3 and 14 days after AMI) was identified by van Rooij et al [33]. These investigators found that the expression of miR-21, miR-214, and miR-223 was increased in the border zone of the infarcted hearts. In our recent study, we detected the cardiac miRNA expression profile in the early phase of AMI [31]. We found that miR-21 expression was significantly down-regulated in infarcted areas, but was upregulated in border areas in the infarcted rat hearts at 6 h and 24 h after AMI. Interestingly, overexpression of miR-21 via adenovirus-mediated gene transfer decreased myocardial infarct size at 24 h after AMI. The protective effect of miR-21 against ischemia-induced cardiac cell death and myocardial infarction was mediated, at least in part, by its target gene, PDCD4 and its downstream molecule, activator protein 1 (AP-1; Fig. 1).
Yin et al. identified a protective effect of miR-21 against ischemia/reperfusion (I/R)-induced heart damages in an in vitro mouse model [34, 35]. They found that myocardial infarct size was significantly reduced by treatment with heat-shock- or preconditioning-induced miRNAs including miR-21. They further demonstrated that upregulation of heat-shock protein 70 (HSP70) and of the endothelial and inducible nitric oxide synthases might be responsible for these miRNAs-mediated cardiac protection [34, 35]. However, it is still unclear that how injection of mature miRNAs could achieve their therapeutic effects, because our unpublished data suggested that administration of some other mature miRNAs might fail to regulate their target genes. In contrast, these target genes were successfully regulated by either their pre-miRNAs or adenovirus-expressing these miRNAs. Thus, the effect of mature miRNAs may vary based on individual miRNAs. The effect of mature miR-21 on its target genes should be verified in future studies. In addition, unlike exosomes-linked or chemically modified miRNAs, the naked miRNAs are not stable in circulating blood. The therapeutic effect of miR-21 might not be induced by naked miR-21. Nevertheless, more studies should be performed in order to identify the mechanisms involved in these mature miRNAs-induced therapeutic effects in vivo. For the upregulation of a miRNA in vivo, one promising approach is to use adeno-associated virus (AAV) as demonstrated by a recent report [36].
Cardiac fibrosis is a remodeling feature in hearts with ischemic heart disease. Roy et al. demonstrated that miR-21 had a predominant effect on cardiac fibroblasts during heart remodeling in response to I/R in the mouse hearts [17]. They identified that PTEN is a direct target of miR-21 in cardiac fibroblasts. Modulation of miR-21 had a significant effect on the expression of matrix metalloprotease-2 (MMP-2) via its target PTEN (Fig. 1) [17]. It should be noted that the biological roles of MMP-2 are not limited to fibrosis. In fact, MMP-2 has emerged as a key protease in various pathologies associated with oxidative stress, including myocardial ischemia–reperfusion, heart failure, atherosclerosis, aneurysm pathogenesis, angiogenesis [37–41].
miR-21 in Human Cardiovascular Disease
The sequence of mature miR-21 is conserved across a wide range of vertebrate species. However, the gene location of miR-21 in genome is different between human and other vertebrate species. For example, human miR-21 gene is located in chromosome 17, whereas in rat and mouse miR-21 gene is at chromosome 10, chromosome 21 respectively. Unlike human cancer tissues, human tissues with cardiovascular diseases are not easy to be obtained. Currently, only four studies have been performed to study the potential involvement of miR-21 in human cardiovascular diseases [26, 29, 30, 42]. Van Rooij et al. reported that no expression change of miR-21 was found in hearts from patients with heart failure, compared with that in control human hearts [26]. Using microarray analysis, Ikeda et al. also did not found any significant changes of miR-21 expression in human hearts with ischemic cardiomyopathy, dilated cardiomyopathy, or aortic stenosis [42]. In contrast, Thum et al. reported that the expression of miR-21 in human hearts from patients with heart failure was much higher than that in normal human control hearts [29, 30]. The reasons for the different results in human hearts are still unclear. The differences in patient selections, disease stages, and sensitivities of the miR-21 detecting methods might be responsible for the different results. More studies using the human cardiovascular tissues from patients with cardiovascular diseases should be performed in the future. Alternatively, other resources such as blood cells and circulating miRNAs could be used to determine the roles of miR-21 in cardiovascular diseases in humans.
Conclusion and Perspective
Both basic and clinical studies have revealed that miR-21 may play important roles in diverse cardiovascular diseases. Identification and validation of miRNA targets is of fundamental importance for the comprehensive understanding of miRNA functions and the potential therapeutic applications in cardiovascular diseases [43]. However, only four target genes of miR-21 have been described in cardiovascular system thus far (Fig. 1). The combination of computational analysis, bioinformatics, and cardiovascular experimental approaches will be needed for the miR-21 target gene study [44]. It should be noted that the major target genes of miR-21 may be cell-specific [45]. For example, Spry1 is not a miR-21 target gene in cardiomyocytes [32]; however, it is the target gene of miR-21 in cardiac fibroblasts [29]. miR-21 is also expressed in vascular endothelial cells and white blood cells. However, no studies have been performed in this research area. As endothelial cells and white blood cells are important in the development of cardiovascular disease, identifying the biological roles of miR-21 in these cells should be performed. It is well established that the expression of miRNAs is tightly controlled and is tissue, developmental stage, and disease specific [46]. However, we are currently unclear about how the expression miR-21 is regulated in diseased hearts and vessels. miR-21 is a broadly expressed miRNA in human tissues, development of new technologies and methods to specifically modulate miR-21 expression in cardiovascular cells will be critical for its therapeutic applications in cardiovascular diseases.
Acknowledgments
The author’s research was supported by a National Institutes of Health Grant (HL080133) and a grant from the American Heart Association (09GRNT2250567).
References
- 1.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 2.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 3.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 4.Friedman JM, Jones PA. MicroRNAs: critical mediators of differentiation, development and disease. Swiss Medical Weekly. 2008;139(33–34):466–472. doi: 10.4414/smw.2009.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, et al. Identification of hundreds of conserved and non-conserved human microRNAs. Nature Genetics. 2005;37(7):766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Pushparaj PN, Aarthi JJ, Kumar SD, Manikandan J. RNAi and RNAa—the yin and yang of RNAome. Bioinformation. 2008;2(6):235–237. doi: 10.6026/97320630002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. Journal of Cellular and Molecular Medicine. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochemical Society Transactions. 2009;37(Pt 4):918–925. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 11.Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, et al. miR-21 gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. Journal of Molecular Biology. 2008;378(3):492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific micro-RNAs from mouse. Current Biology. 2002;12(9):735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C. MicroRNomics: a newly emerging approach for disease biology. Physiological Genomics. 2008;33(2):139–147. doi: 10.1152/physiolgenomics.00034.2008. [DOI] [PubMed] [Google Scholar]
- 14.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circulation Research. 2007;100(11):1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 15.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circulation Research. 2007;100(8):1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? American Journal of Pathology. 2007;170(6):1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovascular Research. 2009;82(1):21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. Journal of Biological Chemistry. 2009;284(12):7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. Journal of Molecular and Cellular Cardiology. 2004;37(2):449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Lankat-Buttgereit B, Göke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biology of the Cell. 2009;101(6):309–317. doi: 10.1042/BC20080191. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. Journal of Molecular and Cellular Cardiology. 2009;47(1):5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto T, Hwang PM. Resizing the genomic regulation of restenosis. Circulation Research. 2007;100(11):1537–1539. doi: 10.1161/CIRCRESAHA.107.101103. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C. MicroRNAs: role in cardiovascular biology and disease. Clinical Science. 2008;114(12):699–706. doi: 10.1042/CS20070211. [DOI] [PubMed] [Google Scholar]
- 25.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circulation Research. 2007;100(3):416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 26.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(48):18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nature Medicine. 2007;13(5):613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 28.Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. Journal of Molecular and Cellular Cardiology. 2007;42(6):1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 30.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116(3):258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 31.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. Journal of Biological Chemistry. 2009;284(43):29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, et al. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Molecular Biology of the Cell. 2008;19(8):3272–3282. doi: 10.1091/mbc.E08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circulation Research. 2009;104(5):572–575. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin C, Wang X, Kukreja RC. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia–reperfusion in mice. FEBS Letters. 2008;582(30):4137–4142. doi: 10.1016/j.febslet.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annual Review of Pharmacology and Toxicology. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 38.Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, León H, et al. Activation and modulation of 72 kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochemical Pharmacology. 2009;77(5):826–834. doi: 10.1016/j.bcp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Guo H, Shi Y, Liu L, Sun A, Xu F, Chi J. Rosuvastatin inhibits MMP-2 expression and limits the progression of atherosclerosis in LDLR-deficient mice. Archives of Medical Research. 2009;40(5):345–351. doi: 10.1016/j.arcmed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Thompson M, Cockerill G. Matrix metalloproteinase-2: the forgotten enzyme in aneurysm pathogenesis. Annals of the New York Academy of Sciences. 2006;1085:170–174. doi: 10.1196/annals.1383.034. [DOI] [PubMed] [Google Scholar]
- 41.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;7:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, et al. Altered microRNA expression in human heart disease. Physiological Genomics. 2007;31(3):367–373. doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- 43.Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovascular Research. 2008;79(4):562–570. doi: 10.1093/cvr/cvn137. [DOI] [PubMed] [Google Scholar]
- 44.Krutzfeldt J, Poy MN, Stoffel M. Strategies to determine the biological function of microRNAs. Nature Genetics. 2006;38:S14–19. doi: 10.1038/ng1799. [DOI] [PubMed] [Google Scholar]
- 45.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Research. 2005;33(4):1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroen B, Heymans S. MicroRNAs and beyond: the heart reveals its treasures. Hypertension. 2009;54(6):1189–1194. doi: 10.1161/HYPERTENSIONAHA.109.133942. [DOI] [PubMed] [Google Scholar]


