Abstract
Methamphetamine (MA) is an illegal stimulant drug of abuse with serious negative health consequences. The neurochemical effects of MA have been partially characterized, with a traditional focus on classical neurotransmitter systems. However, these directions have not yet led to novel drug treatments for MA abuse or toxicity. As an alternative approach, we describe here the first application of metabolomics to investigate the neurochemical consequences of MA exposure in the rodent brain. We examined single exposures at 3 mg/kg and repeated exposures at 3 mg/kg over 5 days in eight common inbred mouse strains. Brain tissue samples were assayed using high-throughput gas and liquid chromatography mass spectrometry, yielding quantitative data on >300 unique metabolites. Association testing and false discovery rate control yielded several metabolome-wide significant associations with acute MA exposure, including compounds such as lactate (p = 4.4 × 10−5, q = 0.013), tryptophan (p = 7.0 × 10−4, q = 0.035) and 2-hydroxyglutarate (p = 1.1 × 10−4, q = 0.022). Secondary analyses of MA-induced increase in locomotor activity showed associations with energy metabolites such as succinate (p = 3.8 × 10−7). Associations specific to repeated (5 day) MA exposure included phosphocholine (p = 4.0 × 10−4, q = 0.087) and ergothioneine (p = 3.0 × 10−4, q = 0.087). Our data appear to confirm and extend existing models of MA action in the brain, whereby an initial increase in energy metabolism, coupled with an increase in behavioral locomotion, gives way to disruption of mitochondria and phospholipid pathways and increased endogenous antioxidant response. Our study demonstrates the power of comprehensive MS-based metabolomics to identify drug-induced changes to brain metabolism and to develop neurochemical models of drug effects.
Keywords: Drugs of abuse, Psychostimulants, Inbred mice, Mass spectrometry, Neurotoxicity
1 Introduction
Methamphetamine (MA) is an illegal stimulant drug with over 500,000 individuals estimated to abuse MA in the United States each month (Substance Abuse and Mental Health Services Administration 2010). The negative health consequences to the individual and the costs to society of MA abuse are substantial. Long term users are at greatly increased risk for several health problems, including cardiovascular disease, psychosis, depression and dental pathology (Curtis 2006; Hendrickson et al. 2008; Kaye et al. 2007; Zweben et al. 2004). Over the last 2 decades, the number of MA users seeking, or being referred to, treatment programs has increased approximately threefold, with almost 40 % of state and local law enforcement agencies citing MA as their greatest drug threat (Gonzales et al. 2010). The total annual cost of MA use to the US was estimated to be $23.4 billion in 2005 (Nicosia et al. 2009).
In common with other psychostimulant drugs, previous research into the effects of MA on the brain has largely involved the monoamine neurotransmitters (Volz et al. 2007). The classical mechanism is that MA causes excess cytoplasmic dopamine, by increasing release and blocking re-uptake, leading to increased formation of reactive oxygen species that damage neurons (Carvalho et al. 2012). Additional research suggests that MA neurotoxicity involves not only oxidative stress, but also glutamate excitotoxicity and mitochondrial dysfunction (Kita et al. 2009; Krasnova and Cadet 2009; Yamamoto et al. 2010). Our lack of comprehensive understanding of how these mechanisms may work in concert, coupled with the fact that other mechanisms such as neuroinflammation are of emerging importance, suggests that our understanding of the neurochemical effects of MA is far from complete (Yamamoto et al. 2010). In addition, there are no approved pharmacotherapies for treating MA abuse currently available, and conventional, neurotransmitter receptor-mediated approaches to have not met with success (Karila et al. 2010). Therefore, novel pharmacological directions will likely hold the greatest potential for future progress. Furthering our knowledge of MA’s effects in the brain could potentially facilitate the design of novel treatments for psychostimulant toxicity and addiction.
A potentially powerful tool for understanding the global biological consequences of drug administration is meta-bolomics (Clayton et al. 2006; Kaddurah-Daouk et al. 2008; Lindon et al. 2006). In recent years, metabolomics has matured, with many applications pertinent to central nervous system (CNS) drug effects now possible (Kaddurah-Daouk and Krishnan 2009). While metabolomics analysis of human samples has been used to investigate drugs of abuse such as cocaine (Patkar et al. 2009), the greater experimental control afforded by model organisms has stimulated a larger body of research. Laboratory rodents have been used in metabolomics investigations of alcohol (Loftus et al. 2011; Shi et al. 2012), MDMA (or “ecstasy”) (Perrine et al. 2009) and, of particular relevance to the current study, MA (Shima et al. 2011). A possible drawback of these studies from a CNS perspective is that they examined biofluids such as blood or urine, or tissues such as liver, and did not examine the brain directly. While several metabolomics studies have probed the rodent brain, these have typically focused on murine models of disease (Constantinou et al. 2011; Nomura et al. 2011), or the effects of genetic knock-outs (Kopp et al. 2010), rather than the effects of drugs. To our knowledge, only two studies to date have used metabolomics to probe the effects of drugs in the rodent brain. The first (McLoughlin et al. 2009) focused on therapeutic psychiatric compounds, while a very recent study (Lee et al. 2012) examined the effects of chronic ethanol administration.
The lack of previous metabolomics studies of MA in the rodent brain suggested that an exploratory neurochemical investigation could yield new insights and potentially inform new treatments. In planning our study, we incorporated several design features to maximize our chances of obtaining a comprehensive overview of MA effects in the brain. First, we used a combination of both gas and liquid mass spectrometry (MS)-based metabolomics, to enable detection of a wide range of metabolites. MS-based metabolomics is typically considered to be more sensitive than nuclear magnetic resonance (Pan and Raftery 2007), the main alternative technology to MS currently in use. Second, we employed a large sample size for a study of this type, examining a total of 150 brains. Third, in addition to larger scale, a further advance over previous studies is our use of focused beam microwave irradiation (FBMI) to instantaneously and permanently denature all enzymes in the brain at time of sacrifice (de Graaf et al. 2009). In a high-intensity microwave pulse lasting only milliseconds, brain metabolism is arrested completely and metabolites are preserved in the pre-sacrifice state (Ikarashi et al. 1985; Login and Dvorak 1994). The effectiveness of this study design is demonstrated by our detection of multiple highly significant MA-induced disruptions to murine neurochemistry.
2 Materials and methods
2.1 Subjects
Eight week old male mice representing eight genetically diverse (Petkov et al. 2004) inbred strains (129S1/SvImJ, A/J, C3H/HeJ, C57BL/6J, CAST/EiJ, DBA/2J, NOD/ShiLtJ, PWD/PhJ) were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were allowed to acclimate to the vivarium (AAALAC-accredited) for 1 week prior to testing and were housed up to four per cage. Food (7012 Teklad LM-485 Sterilizable Diet, Harlan Laboratories, Indianapolis, IN) and water were available ad libitum under a 12-h/12-h light/dark cycle (lights on at 0700–1,900 h) with all testing occurring during the light phase. An overview of the study design is provided in Table 1. All procedures were approved by the Institutional Animal Care and Use Committee of VCU.
Table 1.
Outline of study design, showing drug doses, locomotor activity test sessions and time points of tissue fixation by microwave irradiation
| Days | Acute vehicle (AV) | Acute 3 mg/kg MA (AM) | Repeated vehicle (RV) | Repeated 3 mg/kg MA (RM) |
|---|---|---|---|---|
| 1 | 1-h baseline 1-h test (vehicle) + Microwave fixation |
1-h baseline 1-h test (3 mg/kg) + Microwave fixation |
1-h baseline 1-h test (vehicle) |
1-h baseline 1-h test 3 mg/kg |
| 2,3,4 | 1-h test (vehicle) | 1-h test 3 mg/kg | ||
| 5 | 1-h baseline 1-h test (3 mg/kg) + Microwave fixation |
1-h baseline 1-h test (3 mg/kg) + Microwave fixation |
||
| Number of animals per strain Number of animals total |
6 48 |
3* 27* |
3* 27* |
6 48 |
Each test group comprised either 3 or 6 animals from each of the 8 strains, with 18 animals tested per strain, * except for C57BL/6 J with 24 (6 animals in all groups), to give 150 subjects in total. A 1 h baseline locomotor activity session, preceded by vehicle injection (i.p. saline), was followed by a 1 h test session where the mouse received either vehicle or 3 mg/kg MA. Locomotor activity was assessed using automated infrared recording equipment in 10 min periods. “Microwave fixation” indicates brain tissue fixation, which occurred immediately after the final test session. Note that the AM and RV groups both received a single 3 mg/kg dose of MA 1 h prior to sacrifice. However, the RV group allows us to estimate the control effect of repeated vehicle injections (see text)
2.2 Drugs
(±)-Methamphetamine (MA), obtained from the National Institute on Drug Abuse (Rockville, MD), was prepared in 0.9 % saline stock solutions sterilized by filtration through 0.2 µm filtration disks. MA solutions were injected intra-peritoneally in a volume equivalent to 10 ml/kg body weight. A dose of 3 mg/kg was used in this study. This dose elicits maximal locomotor activity response, which persists significantly above baseline for over 90 min (Caligiuri and Buitenhuys 2005; Peachey et al. 1976).
2.3 Behavioral measurement
Locomotor activity tests were conducted in eight automated activity monitoring devices, each enclosed in sound-and light-attenuating chambers (AccuScan Instruments, Columbus OH). Sixteen photobeam sensors were spaced 2.5 cm apart along the walls of the chamber to detect movement. Behavioral variables (e.g., distance traveled in cm) were recorded in 10-min bins via computer. The interior of each device was divided into separate 20 × 20 × 30 cm arenas permitting the independent and simultaneous measurement of two mice.
2.4 Sample preparation
One hour after final drug or vehicle injection, mice were immediately sacrificed by microwave fixation (Muromachi 10 kW Microwave Fixation System, TMW-4012C, Tokyo). Brains were collected by dissection, flash frozen in liquid nitrogen and stored at −80 °C prior to overnight shipment on dry ice to the metabolomics facility (Metabolon Inc, Research Triangle Park, NC). Brain tissue was homogenized using a GenoGrinder (OPS Diagnostics, Lebanon, NJ) and extracted, using proprietary methods (Metabolon, Research Triangle Park, NC), into two fractions; one for analysis by liquid chromatography mass spectrometry and one for analysis by gas chromatography mass spectrometry. Samples were placed briefly on a TurboVap (Zymark, Hopkinton, MA) to remove organic solvent and then frozen and vacuum dried before preparation for the appropriate instrument. An aliquot of each experimental sample was pooled and aliquots of these “matrix” samples were injected throughout the platform day run and served as technical replicates. As such, variability in quantitation of the experimental samples was monitored.
2.5 Liquid chromatography/mass spectrometry (LC/MS, LC/MS2)
The LC/MS component of our metabolomics approach has been described previously (Evans et al. 2009). Briefly, the LC/MS platform was based on a Waters (Milford, MA) Acquity ultra performance liquid chromatography (UPLC) and a Thermo-Finnigan (ThermoFisher Scientific, Waltham, MA) LTQ mass spectrometer, which consists of an electrospray ionization (ESI) source and linear ion-trap (LIT) mass analyzer. The sample extract was split into two aliquots, dried, then reconstituted in acidic or basic LC-compatible solvents, each of which contain eleven injection standards at fixed concentrations. One aliquot was analyzed using acidic positive ion optimized conditions and the other using basic negative ion optimized conditions in two independent injections using separate dedicated columns. Extracts reconstituted in acidic conditions were gradient eluted using water and methanol, both containing 0.1 % formic acid, while the basic extracts, which also use water/methanol, contained 6.5 mM ammonium bicarbonate. The MS analysis was alternated between MS and data-dependent MS2scans using dynamic exclusion.
The LC/MS mass accurate portion of the platform was based on a Surveyor high performance liquid chromatography (HPLC) and a Thermo-Finnigan LTQ-FT mass spectrometer, which has a LIT front end and a Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer backend. For ions with counts >2 million, an accurate mass measurement was performed. Accurate mass measurements were made on the parent ion as well as fragments and the typical mass error was <5 ppm. Ions with less than two million counts required a greater amount of effort to characterize. Fragmentation spectra (MS/MS) were typically generated in data dependent manner, but where necessary, targeted MS/MS was employed, such as in the case of lower level signals.
2.6 Gas chromatography/mass spectroscopy (GC/MS)
A previous implementation of the GC/MS platform can be found in (Ohta et al. 2009) The samples destined for GC/MS analysis were re-dried under vacuum desiccation for a minimum of 24 h prior to being derivatized under dried nitrogen using bistrimethyl-silyl-triflouroacetamide (BSTFA). The GC column used was 5 % phenyl and the temperature ramp was from 40 to 300° C in a 16 min period. Samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization.
2.7 Data pre-processing
Proprietary in-house software (Metabolon Inc) was used to perform detection and integration of MS peaks as described previously (Evans et al. 2009). Briefly, extracted ion chromatograms were binned by mass in a given range, baseline noise was determined, peaks areas were calculated, and various peak thresholds including minimum height, signal-to-noise, width, symmetry, and area were applied. MS peaks passing above threshold criteria were grouped based on peak apex retention time for ease of viewing similarly retained ion features. Deconvolution and identification of correlated ion features was performed as described previously (Dehaven et al. 2010), whereby correlated ion features from multiple platforms that belong to a given biochemical are organized into a single ion group that can be used to represent that metabolite in statistical analyses. Signatures for specific biochemicals were identified by comparison to library entries of purified standards (~ 1,500 for the GC and LC platforms at time of assay). Since MA is not an endogenous metabolite, it was added to the compound library so that it could be identified and quantified for validation purposes. The combination of chromatographic properties and mass spectra give an indication of a match to the specific compound or isobaric entity. Automated library matches for each compound were checked manually and corrected if necessary.
Following deconvolution and library matching, each identified compound was represented by a single, uncorrected quantitative variable. Metabolites with >50 % missing values were dropped from further analysis. Missing values for remaining metabolites were imputed as half the observed minimum value on the assumption that they were below the limits of detection. A data normalization step was also performed to correct variation resulting from instrument inter-day tuning differences to avoid confounding experimental variation with instrument sensitivity. Each compound was corrected in run-day blocks by registering the medians to equal one (1.00) and normalizing each data point proportionately (Dehaven et al. 2010; Evans et al. 2009).
2.8 Statistical analysis
There were four experimental groups: acute vehicle (AV), single acute dose of 3 mg/kg MA (AM), repeated vehicle (RV) and repeated 3 mg/kg MA (RM). Both acute groups had only a single test day, while the latter two groups received injections for 5 consecutive days. It should be noted that the RV group received vehicle injections for 5 days, but also received a single injection of 3 mg/kg MA on the final day 1 h before sacrifice (see Table 1).
In our main metabolomics analyses, linear mixed models (Searle 1971; Searle et al. 1992) were used to assess associations with the above experimental conditions by analyzing data from all conditions simultaneously. This method is superior to standard group-wise comparisons for two reasons. First, mice from the same strain will be more similar to each other than mice from different strains. Mixed models allow us to effectively handle the resulting dependent observations and perform accurate significance tests. Second, it allows us to obtain maximum likelihood parameter estimates by fitting models to data from all conditions simultaneously. This means that our estimates have desirable properties such as being asymptotically unbiased with the highest possible precision. Specifically, the following model was estimated for each metabolite separately:
yij = b0 + b1D1 + b2D2 + b3D3 + u0j + eij
where y is metabolite concentration of mouse i from strain j, b0 is the intercept, u0j is the random effect of strain; and eij is the model residual. Parameters b1, b2 and b3 are the effects of dummy variables D1, D2 and D3 that represent the experimental conditions that are coded as:
AV Group: D1 = 0, D2 = 0, D3 = 0;
AM Group: D1 = 1, D2 = 0, D3 = 0;
RV Group: D1 = 1, D2 = 1, D3 = 0;
RM Group: D1 = 1, D2 = 1, D3 = 1.
Thus, with this dummy coding:
-
–
Parameter b1 captures the difference: mean (AM Group)—mean (AV Group), which can be interpreted as the effects of being treated with acute MA at 3 mg/kg, compared to vehicle.
-
–
Parameter b2 captures the difference: mean (RV Group)—mean (AM Group), which can be interpreted as the effect of repeated injections, recalling that the RV group received a single dose of MA on the final test day.
-
–
Parameter b3 captures the difference: mean (RM Group)—mean (RV Group), which can be interpreted as effects of repeated MA treatment, net of the effects all other groups (acute vehicle, acute MA and repeated vehicle injections).
The p values of these parameters were then analyzed using false discovery rate (FDR) control (Benjamini and Hochberg 1995; Storey 2003; van den Oord and Sullivan 2003). An FDR threshold <0.1 was used for declaring metabolome-wide significance (van den Oord and Sullivan 2003). This ensures that, on average, only 10 % of associations declared significant are expected to be false discoveries. Operationally, these FDR levels were controlled using q values, which are FDRs calculated using the p value of the metabolite associations as thresholds for declaring significance (Storey 2003). Q values were estimated as outlined by (Bukszar et al. 2009).
Behavioral metabolomics analyses, in which we tested metabolites for association with locomotor activity, were also conducted using linear mixed models. Here, however, we examined only animals receiving a single MA exposure and our dependent variable was acute MA-induced change in total distance traveled. This was calculated by subtracting the mouse-specific mean baseline total distance traveled in the 1 h baseline session from total distance traveled in the final 10 min period in the test session, i.e. the period most proximal to sacrifice (see Table 1). MA-induced change in total distance traveled was regressed on metabolite levels, with mouse strain once again included as a random effect to account for within-strain similarity. FDR control and q values were calculated as described above. All data management, plots and analysis were conducted using R (www.r-project.org) or Stata (www.stata.com).
Metabolite set enrichment analysis (Xia and Wishart 2010), implemented using the MetaboAnalyst package (Xia and Wishart 2011), was used to test metabolites for enrichment within known biological pathways. Enrichment was calculated using overrepresentation analysis (ORA), where the p value from ORA indicates the probability of seeing at least a particular number of metabolites from a known pathway in the selected compound list. In our analysis, we tested three lists of selected compounds, the first being all metabolites showing nominal association (p values <0.05) with the D1 variable (acute MA), the second was all metabolites showing nominal significance with the D3 variable (repeated MA-specific), while the third was all metabolites showing nominal significance with MA-induced change in locomotor activity.
3 Results and discussion
Animals receiving acute 3 mg/kg MA demonstrated greatly elevated locomotor activity compared to baseline over the entire 1 h period following the injection (see Supplementary Material, Figure S1), consistent with long-standing observations (Caligiuri and Buitenhuys 2005; Peachey et al. 1976; Wise and Bozarth 1987). Specifically, for the 10 min period immediately preceding sacrifice, locomotor activity remained elevated (p = 5.76 × 10−5), and highly significant levels of MA were detectable in brain material of MA-treated animals compared to controls (p = 6.9 × 10−15). These observations indicate that the mice were under the influence of MA at time of biomaterial collection. Besides MA, which was added specifically to our compound library for this study, we were able to identify 347 unique compounds in our mass spectra of the homogenized brain tissue samples. Following quality control, 301 metabolite variables remained with which to perform association testing. While the complete murine metabolome is not yet fully elucidated, the Human Metabolome Database version 2.5 (www.hmdb.ca) has data on ~ 700–1,000 compounds per biofluid or tissue type, on average. We anticipate the murine brain metabolome to comprise of a similar number, so our assessment of over 300 in this study likely represents a sizeable portion of all relevant metabolites.
3.1 Main analysis
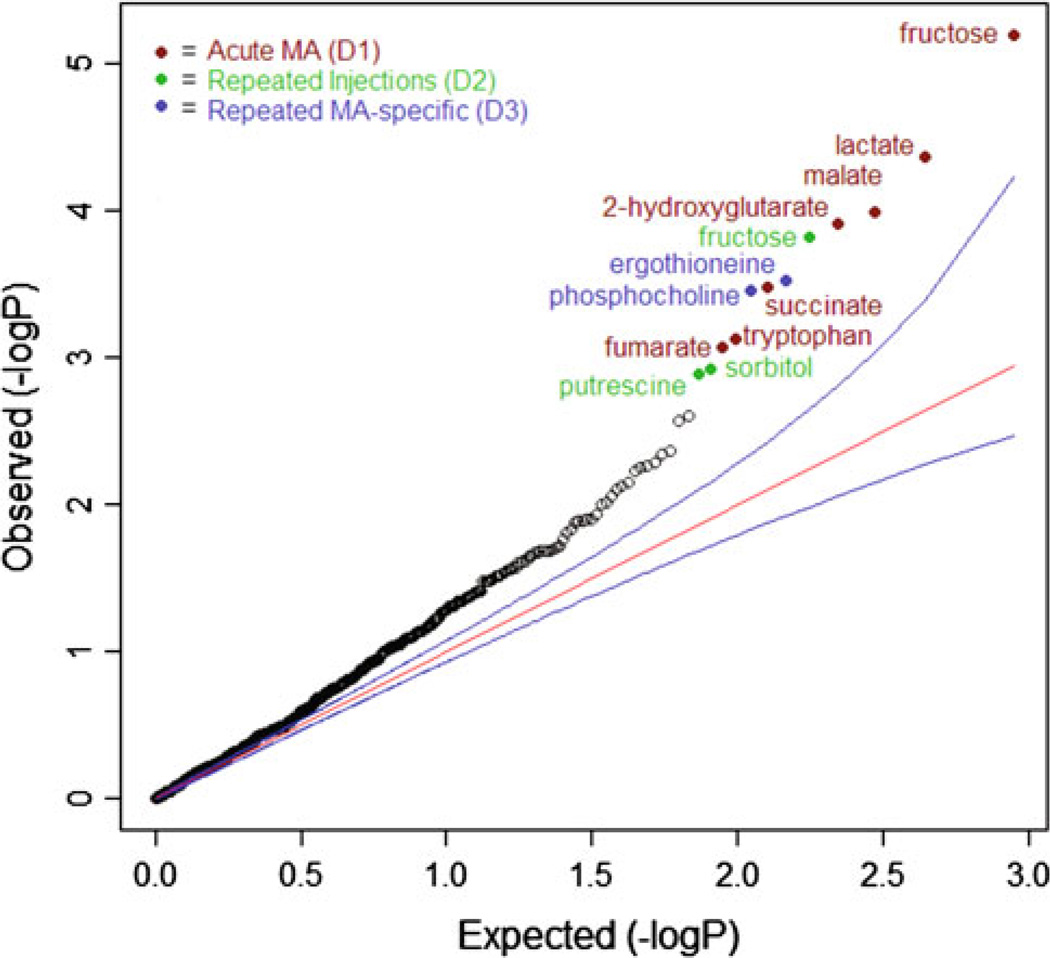
Our primary metabolomics analysis tested for metabolite associations with the different treatment conditions. Results are displayed in a quantile–quantile (QQ) plot (Fig. 1). In a QQ plot, the ordered observed p values, on a −log10 (p values) scale, are plotted against those expected under the null hypothesis represented by the straight line and assuming that none of null hypotheses can be rejected (no “true” effects). The QQ plot shows that many of the observed p values deviated systematically from this straight line and were outside the 95 % confidence intervals. The implication is that many of the metabolites differed between experimental conditions.
Fig. 1.
Quantile-quantile (QQ) plot of all p values in primary analysis. The plot compares observed p values to expected p values under the null distribution. Each point represents a neurochemical association p value and points falling above the null expectation, represented by the red line, are higher than expected by chance. The blue line represents the 95 % confidence interval. A total of 301 metabolites were tested for association with each of the three experimental conditions. The fact that many p values fall above the red (and upper blue) line indicates many small effects. Twelve findings were significant after false discovery rate control at the 10 % level (q < 0.1) and these are identified in the figure (Color figure online)
We observed 12 metabolome-wide significant associations using our pre-defined FDR significance criterion (q < 0.1) (Table 2). Of these, seven were with the D1 variable, which captures the effects of being treated with a single acute dose of MA at 3 mg/kg. Many of these compounds are associated with energy metabolism (e.g. lactate, malate and succinate). A further three compounds were associated with the D2 variable, which captures the effects of repeated injections. Associations with D2 can be considered the effects of simply being in the 5 day arm of the study, over and above the effects of acute vehicle or acute MA. Finally, two compounds were associated with the D3 variable, which can be considered to be specific to repeated MA only.
Table 2.
Metabolome-wide significant neurochemical associations with the different MA treatment conditions
| Compound | ChEBI ID | Beta | StdErr | p value | q value | Variable |
|---|---|---|---|---|---|---|
| Fructose | 28,757 | −0.282 | 0.063 | 6.30E-06 | 0.003 | D1 (acute MA) |
| Lactate | 24,996 | 0.305 | 0.075 | 4.36E-05 | 0.013 | D1 (acute MA) |
| Malate | 15,595 | 0.223 | 0.057 | 0.0001 | 0.022 | D1 (acute MA) |
| 2-hydroxyglutarate | 11,596 | 0.129 | 0.034 | 0.0001 | 0.022 | D1 (acute MA) |
| Succinate | 30,031 | 0.191 | 0.053 | 0.0003 | 0.035 | D1 (acute MA) |
| Tryptophan | 27,897 | 0.156 | 0.046 | 0.0007 | 0.066 | D1 (acute MA) |
| Fumarate | 29,806 | 0.118 | 0.035 | 0.0008 | 0.068 | D1 (acute MA) |
| Fructose | 28,757 | 0.273 | 0.072 | 0.0002 | 0.022 | D2 (repeated injections) |
| Sorbitol | 30,911 | 0.289 | 0.089 | 0.0012 | 0.087 | D2 (repeated injections) |
| Putrescine | 17,148 | 0.313 | 0.097 | 0.0013 | 0.087 | D2 (repeated injections) |
| Ergothioneine | 4,828 | 0.191 | 0.053 | 0.0003 | 0.035 | D3 (repeated MA-specific) |
| Phosphocholine | 18,132 | 0.118 | 0.033 | 0.0004 | 0.035 | D3 (repeated MA-specific) |
Samples were collected at 1 h post-treatment on the final test day (see Table 1) and subjected to large scale metabolomics analysis (301 compounds assayed following QC). Compounds are identified by their entry in the chemical entities of biological interest (ChEBI) database (http://www.ebi.ac.uk/chebi/). A multi-level mixed modeling approach was used to analyze the data to account for the fact that observations are nested within inbred strains. The parameterization of the model tested for the acute effects of 3 mg/kg MA (D1), then the marginal effects of receiving repeated injections (D2), then the marginal effects of being subjected to repeated MA (D3), over and above all other effects. The sign of the regression coefficient (Beta) indicates the effect direction, with negative indicating a decrease following MA administration and vice versa
The three compounds associated with D2 (repeated injections) are not of substantive interest in relation to the biology of MA. However, by identifying these compounds we can drop them from further analyses (see below). Furthermore, while we observed 12 significant associations overall, these were accounted for by only 11 unique compounds. This is because fructose was associated with both D1 (acute 3 mg/kg MA) and D2 (repeated injections). Our analysis was designed such that metabolite associations were with the marginal (i.e. net) effects of each treatment condition. The association of a single compound with two conditions can be explained because the effect of fructose is opposite in each group. That is, it was lower in concentration following acute MA, whereas it was increased following repeated injections. Nevertheless, because of its association with repeated injections, we do not give further consideration to fructose in this study.
3.2 Secondary analyses—behavioral associations, PLS-DA and pathways
In a secondary analysis, we examined metabolite associations with individual differences in acute MA-induced change in locomotor activity. Our hypothesis was that many of the metabolites that were associated with receiving acute MA would also be associated with increased locomotor activity. The outcome of this analysis is shown in Table 3. Here we also see a highly significant association with the energy-related metabolite succinate (p = 3.9 × 10−7) and a nominally significant association (p < 0.05) with malate. Since hyperlocomotion is a primary consequence of MA exposure, these associations indicate a consistency of findings across domains.
Table 3.
Metabolite associations p < 0.05 with acute MA-induced change in locomotor activity
| Metabolite | ChEBI ID | Beta | Std Err | p value |
|---|---|---|---|---|
| Succinate | 30,031 | 1,606.11 | 270.44 | 3.85E-07 |
| Malate | 15,595 | 907.07 | 370.19 | 0.0182 |
| Tyrosine | 18,186 | 1,101.14 | 475.81 | 0.0253 |
| Serotonin | 28,790 | −665.32 | 298.97 | 0.0311 |
| 3-dehydrocarnitine | 57,885 | 362.82 | 166.22 | 0.0343 |
Results of a mixed model regression analysis, whereby metabolite levels were tested for association with MA-induced change in total distance traveled, for animals receiving a single 3 mg/kg MA injection. The most proximal 10 min period of locomotor activity to sacrifice and collection of brain material was used as the outcome variable, minus the mean baseline activity for that animal. The sign of the regression coefficient (Beta) indicates the effect direction, with positive value indicating a positive correlation between metabolite levels and locomotor activity and vice versa
Multivariate statistical tools are frequently employed to examine metabolomics data and a typical method of this type is partial least squares-discriminant analysis (PLS-DA) (Wishart 2007). This supervised classification method provides an alternative, although theoretically less optimal, approach to our primary mixed model analysis. We first applied PLS-DA to distinguish acute MA from acute vehicle (for a complete description of methods, see Supplementary Material Sect. 2). The most important variables separating acute MA versus acute vehicle were 2-hydroxyglutarate, tryptophan, lactate and succinate (see Supplementary Figure S6). These were among the top findings in our primary analysis (Table 2), suggesting that differences in the levels of these metabolites are robust and not dependent on the analytical strategy used. We also performed PLS-DA between acute and repeated MA. However, in this analysis, the model was indicated non-significant in permutation testing (p = 0.404). Hence, our PLS-DA analysis of acute versus repeated MA administration was not informative.
In Fig. 1, we observe a general inflation of small p values, suggesting that there are multiple small effects that are insufficiently large to be detected individually, even in a sample of this size. Nevertheless, if we can aggregate these effects into cohesive, higher order units, we may be able to detect their combined effects. Metabolites are ordered into discrete pathways that are interconnected and display conservation of mass. It therefore makes theoretical sense to look for disruptions of multiple metabolites within pathways. To carry this out, we performed metabolite set enrichment analysis (MSEA) (Xia and Wishart 2010) on all metabolites showing association with the D1 (acute 3 mg/kg MA) or D3 (repeated 3 mg/kg MA) variables with p values < 0.05 in our primary analysis (see Supplementary materials for complete lists), in addition to all metabolites nominally associated (p < 0.05) with MA-induced change in locomotor activity (Table 3). However, we dropped all compounds significantly associated with repeated injections (fructose, sorbitol, and putrescine). The MSEA method tests for enrichment, or over-representation, of multiple compounds in the same pathway, beyond what would be expected by chance. All pathways showing significant enrichment in the MSEA are shown in Table 3. For D1 (acute 3 mg/kg MA), we find three significant pathways: alpha linolenic and linoleic acid metabolism, the citric acid cycle and mitochondrial electron transport. For D3 (repeated 3 mg/kg MA), we detect enrichment among metabolites involved in the oxidation of branched chain fatty acids. Our MSEA on metabolites associated with MA-induced change in locomotor activity was not significant because no reference pathway contained more than one behaviorally-associated metabolite.
3.3 Acute MA associations—disturbances to energy metabolism
In this study, we identified several specific metabolites in the murine brain that were altered following MA administration. Primarily, the effects of acute MA were observed in changes to energy metabolism. Compounds related to the citric acid cycle that we found to be associated with acute MA administration include succinate, fumarate and malate. Fumarate is formed by the oxidation of succinate by succinate dehydrogenase and fumarate is then converted by fumarase to malate. In addition to these compounds, citrate was nominally significant (p < 0.05) but not metabolome-wide significant (q < 0.1). Citrate was, however, one of the most important metabolites in distinguishing acute MA treated animals versus vehicle controls in our PLS-DA analysis (Supplementary Material Figure S6). Together, these findings provide strong evidence for disruption of the citric acid cycle by MA, confirming long-standing observations in this area (Carvalho et al. 2012; Krasnova and Cadet 2009; Sylvia et al. 1977).
Associations with fumarate, malate and succinate were also found in a previous metabolomics study of the urine of MA-treated rats (Shima et al. 2011). However, levels of these compounds were reduced compared to controls, suggestive of a reduction in energy metabolism, whereas in our study we found levels of these metabolites to be increased. The explanation for this discrepancy is in the timing, dose and duration of administration in the two studies. Here, we observed increased levels of citric acid cycle metabolites 1 h after a single dose of MA at 3 mg/kg. At this time point, locomotor activity was still highly elevated in our test animals (Supplementary material Figure S1). Indeed, the increase in locomotor activity was directly associated with higher levels of energy metabolites (Table 3). Furthermore, our association with the alpha linolenic acid and linoleic acid metabolism pathway (Table 4), whereby levels of long chain metabolites such as docosahexaenoic acid and dihomo-linolenic acid were elevated (see Supplementary material), suggests a concomitant increase in fatty acid metabolism. These findings are consistent with in vivo imaging observations of rats administered a single dose of MA at 5 mg/kg, which resulted in an increase in brain metabolism and mitochondrial overactivation (Shiba et al. 2011). In the previous metabolomics study mentioned above, rats received MA at 10 mg/kg once per hour for 24 h. After this time, energy stores are likely depleted. Furthermore, after the initial increase in energy consumption, MA subsequently disrupts energy metabolism by inhibiting mitochondria (Yamamoto and Bankson 2005).
Table 4.
Pathways showing significant enrichment for metabolites affected by MA
| Pathway | Total | Hits | Expected | p value | |
|---|---|---|---|---|---|
| Acute MA | Alpha linolenic acid and linoleic acid metabolism | 9 | 4 | 0.316 | 0.00014 |
| Citric acid cycle | 23 | 4 | 0.808 | 0.007 | |
| Mitochondrial electron transport chain | 15 | 3 | 0.527 | 0.013 | |
| Repeated MA | Oxidation of branched chain fatty acids | 14 | 2 | 0.255 | 0.025 |
For each analysis, those metabolites showing association in the main analysis with p values <0.05 were examined using metabolite set enrichment analysis (Xia and Wishart 2010), except that fructose, putrescine and sorbitol (associated with repeated injections—see Table 2) were dropped. “Unknown” metabolites, or those without matching library entries, were also not included since these cannot be assigned to specific pathways. Complete lists of the compounds entered into each analysis are provided in the Supplementary Material
Total total number of metabolite entries for reference pathway, Hits number of metabolites in data matching to pathway, Expected number of hits by chance
Energy consumption and supply in the brain are tightly linked to neuronal activity. Neuronal depolarization causes opening of voltage-gated sodium channels and activation of the Na+/K+ ATPases, leading to an increased demand for energy (ATP). In response, the mitochondrial respiratory chain is rapidly activated (Kasischke et al. 2004). We observed metabolites in the mitochondrial electron transport chain pathway to be significantly associated with MA in our study (Table 4) and the effect of MA on mitochondria unifies many of our observations. The pathway by which excess 2-hydroxyglutarate builds in the mammalian brain following MA exposure is probably as a by-product of disrupted mitochondrial activity. The formation of 2-hydroxyglutarate results from a side-activity of mitochondrial malate dehydrogenase, the enzyme that inter-converts oxaloacetate and malate, but which also catalyses, usually very slowly, the NADH-dependent conversion of alpha-ketoglutarate to 2-hydroxyglutarate (Van Schaftingen et al. 2009). However, under conditions of excess mitochondrial activity, 2-hydroxyglutarate may accumulate via this mechanism. Excessive accumulation of this compound in the human brain causes 2-hydroxyglutaric aciduria, a rare inherited neurological disorder (Van Schaftingen et al. 2009). In addition, this compound has been shown to cause oxidative apoptosis in the zebrafish brain (Parng et al. 2006). It is therefore conceivable that increased 2-hydroxyglutarate following MA administration may be damaging.
3.4 Associations with repeated MA
The initial mitochondrial overactivation following MA administration is soon accompanied by disruption of the mitochondrial electron transport chain by inhibition of complexes II and IV (Yamamoto and Bankson 2005). This disruption involves increased production of reactive oxygen and nitrogen species (Yamamoto et al. 2010). Via this mechanism, MA can cause nerve terminal degeneration and may lead to apoptosis (Krasnova and Cadet 2009). This disruption to mitochondrial activity may also lead to decreased levels of N-acetylaspartate (NAA), a marker of neuronal health and viability that is synthesized in mitochondria (Krasnova and Cadet 2009; Moffett et al. 2007). Decreased NAA has been repeatedly recorded in MA abusers (Ariyannur et al. 2010; Salo et al. 2011) and we also found NAA to be decreased at a nominal level of significance with repeated MA administration (D3 variable—see Supplementary Material Table S2).
In addition to lower levels of NAA, a consistent observation in the brains of MA abusers is an increase in levels of choline compounds (Chang et al. 2005; Ernst et al. 2000). In the current study, we found increased phosphocholine to be associated with repeated MA administration (D3 variable) at a metabolome-wide level of significance, consistent with observations in humans. Notably, phosphocholine levels have also been shown to be increased following chronic ethanol administration in rats (Lee et al. 2012). Phosphocholine is a common phospholipid in the brain and is an intermediate in the synthesis of biological membranes. It is formed by the action of choline kinase on choline and the fate of most cellular phosphocholine is conversion to CDP choline by phosphocholine cytidylyltransferase (PCCT) (Li and Vance 2008). While reduced PCCT has been observed in the brains of human cocaine abusers, no difference in PCCT activity could be detected between MA abusers and controls (Ross et al. 2002). However, the sample size in this study was small (10 MA abusers), so the lack of association with MA may be a Type II error. Notably, administration of CDP choline to MA addicts was associated with abstinence from the drug and an increase NAA levels in the brain, indicating improved neuronal viability (Yoon et al. 2010). The potential efficacy of CDP-choline in treating MA addicts, plus the increase in phosphocholine levels seen with MA administration, suggest that PCCT may indeed be inhibited by MA in some way. Further exploration of this pathway in the context of developing treatments for MA abuse seems warranted.
Ergothioneine was also associated with repeated MA administration in our study, yet the function of ergothioineine has remained unclear since its discovery in the early twentieth century (Grundemann et al. 2005). It is sourced from the diet, present in most tissues and is notable for its strong antioxidant activity (Akanmu et al. 1991). Ergothioneine has been demonstrated to protect against glutamatergic excitotoxicity (Moncaster et al. 2002) and its dynamic response to oxidative stress has been measured in the intact erythrocyte (Reglinski et al. 1988). In our study, ergothioneine levels increased following repeated MA treatment. Given the lack of known endogenous synthesis pathways for this compound, but the presence of a specific transporter (Grundemann et al. 2005), a plausible hypothesis is that ergothioneine uptake from the diet increases in response to oxidative stress, in our case from MA exposure. While requiring more research, this presents the possibility of administering this dietary compound to MA abusers, to increase endogenous levels and perhaps to attenuate MA-induced neurotoxicity.
3.5 Limitations
Classical neurotransmitters such as dopamine, serotonin, etc. were identified in our samples, but were not associated with MA at metabolome-wide levels of significance. This would appear contrary to established findings, given the well-characterized increase in dopamine release associated with MA exposure. However, metabolomics can only detect changes affecting the overall metabolite pool (Goodacre et al. 2004). Highly localized effects, such as synaptic release of neurotransmitters, will not be detectable in the tissue homogenate because it is impossible to distinguish between, for example, vesicular and synaptic neurotransmitter molecules. Furthermore, low relative abundance of many neurotransmitters may mean changes in their levels are often insufficient in magnitude to be detected reliably. Refinement of our method, for example by incorporating additional fractionation steps that enrich for important, low abundance compounds, could improve this situation. However, as a screening tool to detect the largest changes in metabolite levels following drug administration, our method already appears to be successful.
4 Concluding remarks
Overall, our study has taken some known aspects of MA action, such as increased energy metabolism, but has linked these with very specific metabolites. In addition, we observed novel metabolite associations, such as 2-hydroxyglutarate, that are likely due to disrupted mitochondrial activity. Finally, with sustained MA exposure, we see altered phosphocholine levels which, in conjunction with lowered N-acetyl-aspartate, has been associated with neuronal damage (Salo et al. 2007; Yoon et al. 2010). We also see increases in ergothioneine, a product with strong antioxidant properties. Thus, we are able to synthesize several aspects of the neurochemical effects of MA in a single experiment. Future studies could extend to different classes of psychoactive drugs, in addition to examining multiple doses and time points, in order to further our understanding of neurochemical processes.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Danny Alexander at Metabolon, Inc, Research Triangle Park, North Carolina. Financial support: This work was supported by grants from the US National Institute on Drug Abuse to E. J. C. G. van den Oord (R21DA021411) and the US National Institute of Mental Health (K01MH093731) to D. E. Adkins.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11306-012-0456-y) contains supplementary material, which is available to authorized users.
Contributor Information
Joseph L. McClay, Email: jlmcclay@vcu.edu, Center for Biomarker Research and Personalized Medicine, School of Pharmacy, Medical College of Virginia Campus, Virginia Commonwealth University, McGuire Hall, 1112 East Clay Street, Richmond, VA 23298-0533, USA.
Daniel E. Adkins, Center for Biomarker Research and Personalized Medicine, School of Pharmacy, Medical College of Virginia Campus, Virginia Commonwealth University, McGuire Hall, 1112 East Clay Street, Richmond, VA 23298-0533, USA
Sarah A. Vunck, Department of Psychology, Virginia Commonwealth University, Richmond, VA, USA
Angela M. Batman, Department of Pharmacology and Toxicology, School of Medicine, Medical College of Virginia Campus, Virginia Commonwealth University, Richmond, VA, USA
Robert E. Vann, Department of Pharmacology and Toxicology, School of Medicine, Medical College of Virginia Campus, Virginia Commonwealth University, Richmond, VA, USA
Shaunna L. Clark, Center for Biomarker Research and Personalized Medicine, School of Pharmacy, Medical College of Virginia Campus, Virginia Commonwealth University, McGuire Hall, 1112 East Clay Street, Richmond, VA 23298-0533, USA
Patrick M. Beardsley, Center for Biomarker Research and Personalized Medicine, School of Pharmacy, Medical College of Virginia Campus, Virginia Commonwealth University, McGuire Hall, 1112 East Clay Street, Richmond, VA 23298-0533, USA Department of Pharmacology and Toxicology, School of Medicine, Medical College of Virginia Campus, Virginia Commonwealth University, Richmond, VA, USA.
Edwin J. C. G. van den Oord, Center for Biomarker Research and Personalized Medicine, School of Pharmacy, Medical College of Virginia Campus, Virginia Commonwealth University, McGuire Hall, 1112 East Clay Street, Richmond, VA 23298-0533, USA
References
- Akanmu D, Cecchini R, Aruoma OI, Halliwell B. The antioxidant action of ergothioneine. Archives of Biochemistry and Biophysics. 1991;288:10–16. doi: 10.1016/0003-9861(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Ariyannur PS, Moffett JR, Manickam P, et al. Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: Implications for specialized acetyl coenzyme A metabolism in the CNS. Brain Research. 2010;1335:1–13. doi: 10.1016/j.brainres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57:289–300. [Google Scholar]
- Bukszar J, McClay JL, van den Oord EJ. Estimating the posterior probability that genome-wide association findings are true or false. Bioinformatics. 2009;25:1807–1813. doi: 10.1093/bioinformatics/btp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Buitenhuys C. Do preclinical findings of methamphetamine-induced motor abnormalities translate to an observable clinical phenotype? Neuropsychopharmacology. 2005;30:2125–2134. doi: 10.1038/sj.npp.1300859. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carmo H, Costa VM, et al. Toxicity of amphetamines: An update. Archives of Toxicology. 2012;86(8):1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. American Journal of Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton TA, Lindon JC, Cloarec O, et al. Pharmacometabonomic phenotyping and personalized drug treatment. Nature. 2006;440:1073–1077. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- Constantinou C, Chrysanthopoulos PK, Margarity M, Klapa MI. GC-MS metabolomic analysis reveals significant alterations in cerebellar metabolic physiology in a mouse model of adult onset hypothyroidism. Journal of Proteome Research. 2011;10:869–879. doi: 10.1021/pr100699m. [DOI] [PubMed] [Google Scholar]
- Curtis EK. Meth mouth: a review of methamphetamine abuse and its oral manifestations. General Dentistry. 2006;54:125–129. quiz 130. [PubMed] [Google Scholar]
- de Graaf RA, Chowdhury GM, Brown PB, Rothman DL, Behar KL. In situ 3D magnetic resonance metabolic imaging of microwave-irradiated rodent brain: A new tool for metabolomics research. Journal of Neurochemistry. 2009;109:494–501. doi: 10.1111/j.1471-4159.2009.05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. Journal of Cheminformatics. 2010;2:9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical Chemistry. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annual Review of Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre R, Vaidyanathan S, Dunn WB, Harrigan GG, Kell DB. Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends in Biotechnology. 2004;22:245–252. doi: 10.1016/j.tibtech.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Grundemann D, Harlfinger S, Golz S, et al. Discovery of the ergothioneine transporter. Proceedings of National Academy of Sciences of the United States of America. 2005;102:5256–5261. doi: 10.1073/pnas.0408624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson RG, Cloutier R, McConnell KJ. Methamphetamine-related emergency department utilization and cost. Academic Emergency Medicine. 2008;15:23–31. doi: 10.1111/j.1553-2712.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- Ikarashi Y, Sasahara T, Maruyama Y. Postmortem changes in catecholamines, indoleamines, and their metabolites in rat brain regions: Prevention with 10-kW microwave irradiation. Journal of Neurochemistry. 1985;45:935–939. doi: 10.1111/j.1471-4159.1985.tb04083.x. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Krishnan KR. Metabolomics: A global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology. 2009;34:173–186. doi: 10.1038/npp.2008.174. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Kristal BS, Weinshilboum RM. Metabolomics: A global biochemical approach to drug response and disease. Annual Review of Pharmacology and Toxicology. 2008;48:653–683. doi: 10.1146/annurev.pharmtox.48.113006.094715. [DOI] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: A focused review. British Journal of Clinical Pharmacology. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Kaye S, McKetin R, Duflou J, Darke S. Methamphetamine and cardiovascular pathology: A review of the evidence. Addiction. 2007;102:1204–1211. doi: 10.1111/j.1360-0443.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- Kita T, Miyazaki I, Asanuma M, Takeshima M, Wagner GC. Dopamine-induced behavioral changes and oxidative stress in methamphetamine-induced neurotoxicity. International Review of Neurobiology. 2009;88:43–64. doi: 10.1016/S0074-7742(09)88003-3. [DOI] [PubMed] [Google Scholar]
- Kopp F, Komatsu T, Nomura DK, et al. The glycerophospho metabolome and its influence on amino acid homeostasis revealed by brain metabolomics of GDE1(−/−) mice. Chemistry and Biology. 2010;17:831–840. doi: 10.1016/j.chembiol.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Research Reviews. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Kim SY, Lee T, et al. Ex vivo detection for chronic ethanol consumption-induced neurochemical changes in rats. Brain Research. 2012;1429:134–144. doi: 10.1016/j.brainres.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. Journal of Lipid Research. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- Lindon JC, Holmes E, Nicholson JK. Metabonomics techniques and applications to pharmaceutical research and development. Pharmaceutical Research. 2006;23:1075–1088. doi: 10.1007/s11095-006-0025-z. [DOI] [PubMed] [Google Scholar]
- Loftus N, Barnes A, Ashton S, et al. Metabonomic investigation of liver profiles of nonpolar metabolites obtained from alcohol-dosed rats and mice using high mass accuracy MSn analysis. Journal of Proteome Research. 2011;10:705–713. doi: 10.1021/pr100885w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Login GR, Dvorak AM. Application of microwave fixation techniques in pathology to neuroscience studies: A review. Journal of Neuroscience Methods. 1994;55:173–182. doi: 10.1016/0165-0270(94)90209-7. [DOI] [PubMed] [Google Scholar]
- McLoughlin GA, Ma D, Tsang TM, et al. Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. Journal of Proteome Research. 2009;8:1943–1952. doi: 10.1021/pr800892u. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Progress in Neurobiology. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncaster JA, Walsh DT, Gentleman SM, Jen LS, Aruoma OI. Ergothioneine treatment protects neurons against N-methyl-d-aspartate excitotoxicity in an in vivo rat retinal model. Neuroscience Letters. 2002;328:55–59. doi: 10.1016/s0304-3940(02)00427-5. [DOI] [PubMed] [Google Scholar]
- Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States 2005. 2009 [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Masutomi N, Tsutsui N, et al. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicologic Pathology. 2009;37:521–535. doi: 10.1177/0192623309336152. [DOI] [PubMed] [Google Scholar]
- Pan Z, Raftery D. Comparing and combining NMR spectroscopy and mass spectrometry in metabolomics. Analytical and Bioanalytical Chemistry. 2007;387:525–527. doi: 10.1007/s00216-006-0687-8. [DOI] [PubMed] [Google Scholar]
- Parng C, Ton C, Lin YX, Roy NM, McGrath P. A zebrafish assay for identifying neuroprotectants in vivo. Neurotoxicology and Teratology. 2006;28:509–516. doi: 10.1016/j.ntt.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Rozen S, Mannelli P, et al. Alterations in tryptophan and purine metabolism in cocaine addiction: A metabolomic study. Psychopharmacology (Berl) 2009;206:479–489. doi: 10.1007/s00213-009-1625-1. [DOI] [PubMed] [Google Scholar]
- Peachey E, Rogers B, Brien JF, Maclean A, Rogers D. Measurement of acute and chronic behavioural effects of methamphetamine in the mouse. Psychopharmacology (Berl) 1976;48:271–275. doi: 10.1007/BF00496860. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Michaels MS, Ghoddoussi F, Hyde EM, Tancer ME, Galloway MP. Cardiac effects of MDMA on the metabolic profile determined with 1H-magnetic resonance spectroscopy in the rat. NMR in Biomedicine. 2009;22:419–425. doi: 10.1002/nbm.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Research. 2004;14:1806–1811. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglinski J, Smith WE, Sturrock RD. Spin-echo 1H NMR detected response of ergothioneine to oxidative stress in the intact human erythrocyte. Magnetic Resonance in Medicine. 1988;6:217–223. doi: 10.1002/mrm.1910060210. [DOI] [PubMed] [Google Scholar]
- Ross BM, Moszczynska A, Peretti FJ, et al. Decreased activity of brain phospholipid metabolic enzymes in human users of cocaine and methamphetamine. Drug and Alcohol Dependence. 2002;67:73–79. doi: 10.1016/s0376-8716(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Salo R, Buonocore MH, Leamon M, et al. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: A proton MRS study. Drug and Alcohol Dependence. 2011;113:133–138. doi: 10.1016/j.drugalcdep.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biological Psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Searle SR. Linear models. New York: Wiley; 1971. [Google Scholar]
- Searle SR, Casella G, McCulloch CE. Variance components. New York: Wiley; 1992. [Google Scholar]
- Shi X, Yao D, Chen C. Identification of N-acetyltaurine as a novel metabolite of ethanol through metabolomics-guided biochemical analysis. Journal of Biological Chemistry. 2012;287:6336–6349. doi: 10.1074/jbc.M111.312199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T, Yamato M, Kudo W, Watanabe T, Utsumi H, Yamada K. In vivo imaging of mitochondrial function in methamphetamine-treated rats. Neuroimage. 2011;57:866–872. doi: 10.1016/j.neuroimage.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Shima N, Miyawaki I, Bando K, et al. Influences of methamphetamine-induced acute intoxication on urinary and plasma metabolic profiles in the rat. Toxicology. 2011;287:29–37. doi: 10.1016/j.tox.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Storey J. The positive false discovery rate: A Bayesian interpretation and the q value. Annals of Statistics. 2003;31:2013–2035. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of Applied Studies; 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings NSDUH Series H-38A. [Google Scholar]
- Sylvia AL, LaManna JC, Rosenthal M, Jobbis FF. Metabolite studies of methamphetamine effects based upon mitochondrial respiratory state in rat brain. Journal of Pharmacology and Experimental Therapeutics. 1977;201:117–125. [PubMed] [Google Scholar]
- van den Oord E, Sullivan PF. False discoveries and models for gene discovery. Trends in Genetics. 2003;19:537–542. doi: 10.1016/j.tig.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E, Rzem R, Veiga-da-Cunha M. L:-2-Hydroxyglutaric aciduria, a disorder of metabolite repair. Journal of Inherited Metabolic Disease. 2009;32:135–142. doi: 10.1007/s10545-008-1042-3. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102(Suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]
- Wishart DS. Current progress in computational metabolomics. Brief Bioinformatics. 2007;8:279–293. doi: 10.1093/bib/bbm030. [DOI] [PubMed] [Google Scholar]
- Xia J, Wishart DS. MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Research. 2010;38:W71–W77. doi: 10.1093/nar/gkq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Wishart DS. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nature Protocols. 2011;6:743–760. doi: 10.1038/nprot.2011.319. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: Cause and consequence of oxidative stress. Critical Reviews in Neurobiology. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: Classical and emerging mechanisms. Annals of the New York Academy of Sciences. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SJ, Lyoo IK, Kim HJ, et al. Neurochemical alterations in methamphetamine-dependent patients treated with cytidine-5′-diphosphate choline: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2010;35:1165–1173. doi: 10.1038/npp.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. American Journal of Addictions. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.