Abstract
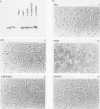
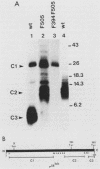
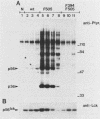
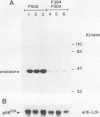
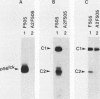
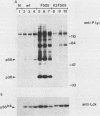
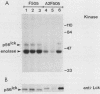
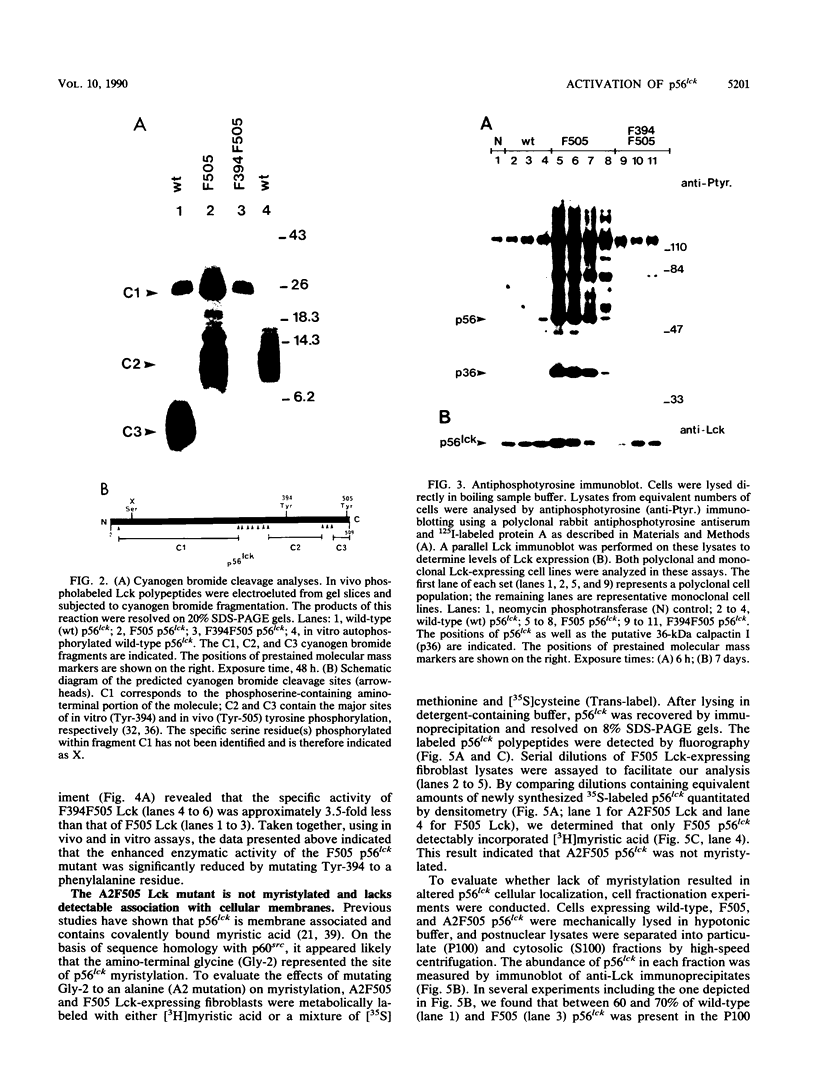
Mutation of the major site of in vivo tyrosine phosphorylation of p56lck (tyrosine 505) to a phenylalanine constitutively enhances the p56lck-associated tyrosine-specific protein kinase activity. The mutant polypeptide is extensively phosphorylated in vivo at the site of in vitro Lck autophosphorylation (tyrosine 394) and is capable of oncogenic transformation of rodent fibroblasts. These observations have suggested that phosphorylation at Tyr-505 down regulates the tyrosine protein kinase activity of p56lck. Herein we have attempted to examine whether other posttranslational modifications may be involved in regulation of the enzymatic function of p56lck. The results indicated that activation of p56lck by mutation of Tyr-505 was prevented by a tyrosine-to-phenylalanine substitution at position 394. Furthermore, activation of p56lck by mutation of the carboxy-terminal tyrosine residue was rendered less efficient by substituting an alanine residue for the amino-terminal glycine. This second mutation prevented p56lck myristylation and stable membrane association and was associated with decreased in vivo phosphorylation at Tyr-394. Taken together, these findings imply that lack of phosphorylation at Tyr-505 may be insufficient for enhancement of the p56lck-associated tyrosine protein kinase activity. Our data suggest that activation of p56lck may be dependent on phosphorylation at Tyr-394 and that this process may be facilitated by myristylation, membrane association, or both.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A. A function for the lck proto-oncogene. Trends Biochem Sci. 1989 Oct;14(10):404–407. doi: 10.1016/0968-0004(89)90288-0. [DOI] [PubMed] [Google Scholar]
- Cartier M., Chang M. W., Stanners C. P. Use of the Escherichia coli gene for asparagine synthetase as a selective marker in a shuttle vector capable of dominant transfection and amplification in animal cells. Mol Cell Biol. 1987 May;7(5):1623–1628. doi: 10.1128/mcb.7.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymecki S. M., Niederhuber J. E., Desiderio S. V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990 Jan 19;247(4940):332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Harvey R., Hehir K. M., Smith A. E., Cheng S. H. pp60c-src variants containing lesions that affect phosphorylation at tyrosines 416 and 527. Mol Cell Biol. 1989 Sep;9(9):3647–3656. doi: 10.1128/mcb.9.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jove R., Kornbluth S., Hanafusa H. Enzymatically inactive p60c-src mutant with altered ATP-binding site is fully phosphorylated in its carboxy-terminal regulatory region. Cell. 1987 Sep 11;50(6):937–943. doi: 10.1016/0092-8674(87)90520-4. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Moran M., Sadowski I., Pawson T. The common src homology region 2 domain of cytoplasmic signaling proteins is a positive effector of v-fps tyrosine kinase function. Mol Cell Biol. 1989 Oct;9(10):4131–4140. doi: 10.1128/mcb.9.10.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAuley A., Cooper J. A. The carboxy-terminal sequence of p56lck can regulate p60c-src. Mol Cell Biol. 1988 Aug;8(8):3560–3564. doi: 10.1128/mcb.8.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchildon G. A., Casnellie J. E., Walsh K. A., Krebs E. G. Covalently bound myristate in a lymphoma tyrosine protein kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7679–7682. doi: 10.1073/pnas.81.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Disteche C., Pravtcheva D., Ruddle F., Krebs E. G., Perlmutter R. M. Localization of a lymphocyte-specific protein tyrosine kinase gene (lck) at a site of frequent chromosomal abnormalities in human lymphomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7400–7404. doi: 10.1073/pnas.83.19.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H. L., Shackelford D. A., Hurley T. R., Johnson P., Hyman R., Sefton B. M., Trowbridge I. S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Reynolds A. B., Roesel D. J., Kanner S. B., Parsons J. T. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989 Feb;9(2):629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. S., Chalupny J., Whitney J. A., Hammond C., Amrein K. E., Kavathas P., Sefton B. M., Rose J. K. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol Cell Biol. 1990 May;10(5):1853–1862. doi: 10.1128/mcb.10.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bolen J. B., Bookman M. A. Alterations in tyrosine protein phosphorylation induced by antibody-mediated cross-linking of the CD4 receptor of T lymphocytes. Mol Cell Biol. 1989 Oct;9(10):4441–4446. doi: 10.1128/mcb.9.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bolen J. B. src-related protein tyrosine kinases. Cancer Treat Res. 1989;47:121–142. doi: 10.1007/978-1-4613-1599-5_5. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Foss F. M., Sausville E. A., Bolen J. B., Rosen N. Expression of the lck tyrosine kinase gene in human colon carcinoma and other non-lymphoid human tumor cell lines. Oncogene Res. 1987 Sep-Oct;1(4):357–374. [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Bolen J. B. Post-translational alterations of the tyrosine kinase p56lck in response to activators of protein kinase C. Oncogene Res. 1988 May;2(4):385–401. [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Horak E. M., Bookman M. A., Bolen J. B. Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T-cell activation. Mol Cell Biol. 1988 Oct;8(10):4353–4361. doi: 10.1128/mcb.8.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Zúiga-Pflücker J. C., Bolen J. B., Kruisbeek A. M. Engagement of CD4 and CD8 expressed on immature thymocytes induces activation of intracellular tyrosine phosphorylation pathways. J Exp Med. 1989 Nov 1;170(5):1671–1680. doi: 10.1084/jem.170.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Buss J. E., Patschinsky T., Hunter T., Sefton B. M. Characterization of the protein apparently responsible for the elevated tyrosine protein kinase activity in LSTRA cells. Mol Cell Biol. 1984 Dec;4(12):2705–2713. doi: 10.1128/mcb.4.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]