Abstract
Whole genome sequencing was used to characterize the resistome of intensive care unit (ICU) outbreak-associated carbapenem-resistant K. pneumoniae isolates. Importantly, and of particular concern, the carbapenem-hydrolyzing β-lactamase gene bla OXA-48 and the extended-spectrum β-lactamase gene bla CTX-M-14, were identified on a single broad host-range conjugative plasmid. This represents the first report of bla OXA-48 in Australia and highlights the importance of resistance gene surveillance, as such plasmids can silently spread amongst enterobacterial populations and have the potential to drastically limit treatment options. Furthermore, the in vivo evolution of these isolates was also examined after 18 months of intra-abdominal carriage in a patient that transited through the ICU during the outbreak period. Reflecting the clonality of K. pneumoniae, only 11 single nucleotide polymorphisms (SNPs) were accumulated during this time-period and many of these were associated with genes involved in tolerance/resistance to antibiotics, metals or organic solvents, and transcriptional regulation. Collectively, these SNPs are likely to be associated with changes in virulence (at least to some extent) that have refined the in vivo colonization capacity of the original outbreak isolate.
Introduction
Klebsiella pneumoniae is a common cause of infections worldwide, both in community and hospital settings [1], [2]. Based on data from the Study for Monitoring Antimicrobial Resistance Trends (SMART), carbapenems remain the most effective treatment option for these infections, especially those caused by strains producing extended-spectrum β-lactamases (ESBLs) [1], [2]. While the occurrence of ESBL-producing K. pneumoniae infections has been variable over the past decade, there has been an overall increase in the number of these strains [1], [3]. The consequence of ESBL-associated infections is a greater reliance on carbapenems as one of the few remaining effective agents. Therefore, the emergence of carbapenem-resistant K. pneumoniae is particularly worrisome, as not only are treatment options limited but these infections are associated with increased morbidity and mortality [4], [5].
In Australia, carbapenem resistance in K. pneumoniae is uncommon and over the past decade has generally been secondary to the expression of metallo-β-lactamase (MBL) genes (specifically bla IMP-4) [6], in combination with changes in outer-membrane porins. Recently, two K. pneumoniae isolates have been reported that produce either the MBL NDM-1 [7] or an Ambler Class A KPC-type carbapenem-hydrolyzing β-lactamase [8]. Furthermore, with respect to Enterobacteriaceae, Ambler class D carbapenem-hydrolyzing β-lactamase (CHDL) genes have also recently emerged in Australia with the report of a clinical K. pneumoniae isolate carrying a plasmid with bla OXA-181 [9]. However, a related gene, bla OXA-48, which was first identified in a K. pneumoniae isolate from Turkey in 2001 [10], and that has spread to Africa, Asia and Europe, has not previously been detected in Australia [11]. The broad dissemination of bla OXA-48, which has largely been due to an association with plasmid-borne Tn1999 or related transposons [11], is of major concern given the ease at which transmission and spread occurs and the subsequent consequence for therapy.
In this study we used whole genome sequencing to characterize the resistome of the first known OXA-48 producing carbapenem-resistant K. pneumoniae isolates following an introduction resulting in an outbreak in a metropolitan Sydney Intensive Care Unit (ICU). In addition, we examine the in vivo evolution of this strain based on recovery of the same isolate from an “outbreak” patient following 18 months of carriage.
Methods
Bacterial Strains, Growth Conditions and Antibiotic Resistance Profiles
Isolates: In 2010, a multi-drug carbapenem-resistant K. pneumoniae (Kp001) was introduced into the ICU of a Sydney Metropolitan Hospital by a patient recently returned from Egypt. Three additional patients acquired the organism over several months before termination of the outbreak. All four patients who developed an infection with this organism died. However, 18 months later, a similar K. pneumoniae isolate (Kp002) was recovered from the abdominal fluid of a patient (post-hernia repair) who had transited through the ICU at the time of the initial outbreak, despite negative rectal screening swabs at the time of the outbreak. Upon referral to a reference laboratory, both isolates were indistinguishable by either antibiotic resistance profiling or molecular diagnostics (pulsed-field gel electrophoresis and enterobacterial repetitive intergenic consensus sequence PCR; data not shown).
Bacterial strains used in this study are listed in Table 1. Bacterial strains were grown at 37°C in LB medium (Sigma-Aldrich; St. Louis, USA) or on plates containing LB medium and 1.5% w/v agar (Amresco; Solon, USA), unless otherwise stated. When required, media was supplemented with 100 µg mL−1 ampicillin (Amresco; Solon, USA) and/or 100 µg mL−1 rifampicin (Sigma-Aldrich; St. Louis, USA). Antibiotic resistance profiles were determined on a VITEK 2 AST-N149 card using the global and natural resistance interpretive criteria (bioMérieux; Marcy L’Étoile, FRA).
Table 1. Bacterial strains, plasmids and primers.
| Strain, plasmid or primer | Genotype, relevant characteristics or sequence | Source |
| Strains E. coli | ||
| Ec002 | RifampicinR derivative of DH5α: F- endA hsdR17 supE44 thi-1 λ- recA1 gyrA96 relA1 φ80 dLacZΔM15 | This study |
| Ec003 | Ec002 carrying pJEG011 and pJEG012 | This study |
| K. pneumoniae Kp001 | Clinical outbreak-associated isolate carrying pJEG011 and pJEG012 | This study |
| Kp002 | Clinical outbreak-associated isolate phenotypically and genotypically indistinguishable from Kp001: isolated after 18 months of intra-abdominal carriage | This study |
| Plasmids | ||
| pJEG011 | IncL/M, Tn1999, ISEcp1-bla CTX-M-14, Tn5393jΔ | This study |
| pJEG012 | pir, Tn1331 | This study |
| Primers | ||
| aacA4-F | 5′- gaagagtatgagtattcaaacatttcc -3′ | This study |
| aacA4-R | 5′- ggaagggttaggcaacac -3′ | This study |
| aacC2-F | 5′- gtttagaggagatatcgcgatg-3′ | This study |
| aacC2-R | 5′- ttgtcgacggcctctaac-3′ | This study |
| CTX-M-14-F | 5′- gagagtgcaacggatgatg -3′ | This study |
| CTX-M-14-R | 5′- tgcggctgggtaaaatag -3′ | This study |
| OXA-48-F | 5′- gcttccaccctaatttgatg -3′ | This study |
| OXA-48-R | 5′- cgagcatcagcattttgtc -3′ | This study |
DNA Manipulations
DNA was extracted from K. pneumoniae and Escherichia coli cells using the ISOLATE Genomic DNA and Plasmid Mini Kits (Bioline; London, UK), respectively. DNA fragments were PCR-amplified using BioTaq (Bioline; London, UK) and primer pairs described in Table 1. Capillary sequencing of PCR products was performed by Macrogen Inc (Seoul, KOR).
Whole Genome Sequencing
Kp001 DNA was sent to The Ramaciotti Centre (University of New South Wales; Sydney, AUS) for sequencing on an Illumina HiSeq 2000 system (Illumina Inc; San Diego, USA). A fragment library of Kp002 DNA was generated and sequenced on an Ion Torrent PGM (Life Technologies; Carlsbad, USA) according to the manufacturer’s instructions. Analysis of Kp001 and Kp002 genomic data was performed using CLC Genomics Workbench 5.5 (CLC bio; Katrinebjerg, DEN). Reference mapping of reads to the genome of the non-multi-drug resistant K. pneumoniae strain NTUH-K2044 (GenBank accession no. AP006725) facilitated variant analysis using a quality-based algorithm (as implemented in CLC Genomics Workbench) applying an 80% genotype frequency cutoff with a minimum coverage of 10 reads. Homopolymer variants as well as variants present in both Kp001 and Kp002 were excluded. The remaining variants were curated manually to ensure accurate identification. Raw data for this project has been uploaded to the Sequencing Read Archive under accession number SRA062913. A de novo assembly of reads that did not map to NTUH-K2044 was used to query an in-house database (constructed within CLC Genomics Workbench) of clinically relevant antibiotic resistance genes (see Table S1). BLASTn analyses of resultant contigs using the NCBI non-redundant nucleotide database were also performed in order to examine the genetic context of identified resistance determinants. Gaps in plasmid read mappings were closed via PCR amplification and capillary sequencing.
Conjugation Experiments
Filter-based conjugation experiments were performed as previously described [12] using a rifampicin-resistant mutant Escherichia coli DH5α strain (Ec002), which was obtained via growth on an LB agar plate containing 100 µg mL−1 rifampicin.
Results and Discussion
Defining the Common Resistome
The mobile resistome
Both Kp001 and Kp002 were shown to be resistant to aminoglycosides and most β-lactams including, and of particular concern, meropenem. In order to fully define the resistome for both isolates, WGS reads that did not map to NTUH-K2044 were de novo assembled and examined via BLASTn analysis using an in-house database (created within CLC Genomics Workbench 5.5) of known antibiotic resistance determinants in Gram-negative bacteria (see Methods). Included in the local resistance gene database (RGD) were representative alleles of common aminoglycoside resistance genes [13], [14], quinolone resistance genes [15] and β-lactam resistance genes, including those that encode extended-spectrum and carbapenem-hydrolyzing β-lactamases [16]–[20]. Further BLASTn analysis and reference mapping was then performed in order to determine the genetic context of the identified determinants.
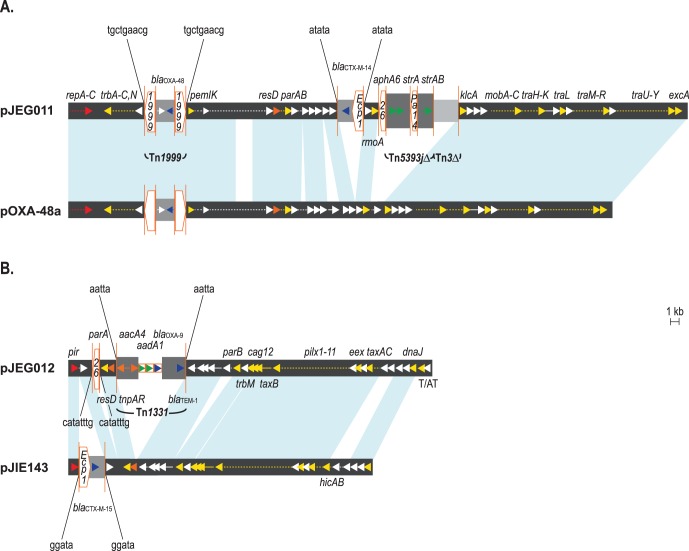
Based on interrogation of the RGD, four β-lactamase genes were identified in both Kp001 and Kp002: bla SHV-1 (which is ubiquitous in K. pneumoniae), bla CTX-M-14 (which differs from bla CTX-M-9 by 4 nucleotides), bla OXA-9 and, of particular importance, the bla OXA-48 CHDL gene. Further analysis revealed bla CTX-M-14 was present as part of an ISEcp1 transposition unit which had inserted into a plasmid designated pJEG011 (GenBank accession no. KC354801). pJEG011 shares >95% nucleotide sequence similarity with the backbone structure of the multi-resistance IncL/M conjugative plasmid pOXA-48a (GenBank accession no. JN626286) (11), including Tn1999 containing the bla OXA-48 gene [21] (Figure 1A).
Figure 1. Structural features of plasmids pJEG011 and pJEG012.
Comparisons to related plasmids pOXA-48a (A) and pJIE143 (B) are shown, respectively; plasmid backbones are represented by thick gray lines and areas of ≥99% sequence identity between plasmids are indicated by the light blue areas. Only the following selected genes are annotated and represented by colored arrows: plasmid replication genes, red; transposon-related genes, orange; plasmid partitioning, maintenance (e.g., toxin/antitoxin systems (T/AT)), mobilization and conjugation genes, yellow; aminoglycoside resistance genes, green; β-lactam resistance genes, blue. Dashed arrows represent more than one gene or open reading frame. Insertion sequences (IS) are represented by orange pentagons with the IS number indicated within; the direction of the IS with respect to the transposase gene is indicated by the point of the pentagon. Inverted repeats associated with IS and transposons are indicated by vertical orange lines; the nucleotide sequences of the direct repeats resulting from IS and transposon insertion are indicated above or below the plasmid figures. Integron gene cassettes are represented by orange rectangles.
The aminoglycoside resistances genes aphA6 and strAB were also present as part of pJEG011, and were located within a novel Tn5393 module (Figure 1A). The other aminoglycoside resistance genes detected (aacC2, aacA4 and aadA1) were not part of pJEG011. Further analysis revealed that the aacC2 gene was located on a contig that is flanked by two copies of IS26 in the same orientation as previously described for certain IncFII plasmids [22], however the genetic context of this determinant remained unclear. In contrast, both the aacA4 and aadA1 genes were located on the same contig along with bla OXA-9, and these were all present as part of Tn1331. Further examination of this contig revealed that Tn1331 had inserted into a pir-type plasmid designated pJEG012 (GenBank accession no. KC354802), which shares >85% nucleotide sequence similarity with the backbone structure of another plasmid, pJIE143 (GenBank accession no. JN194214) [23]; pJEG012 also contained a putative toxin/anti-toxin system and a single copy of IS26 (Figure 1B).
Contribution of single nucleotide variants
Both isolates had SNPs in gyrA and parC, which encode subunits of DNA gyrase and topoisomerase IV, respectively, and are involved in DNA replication and segregation. These SNPs result in amino acid changes within the quinolone resistance determining regions of GyrA (S80I) and ParC (S83Y and D87G), and collectively are known to confer high-level resistance to ciprofloxacin and nalidixic acid [24].
Transfer of the Mobile Resistome
Conjugation experiments revealed that pJEG011 and pJEG012 could be readily transferred from Kp001 to Ec002. The presence of both plasmids in a single E. coli transconjugant, Ec003, was determined by PCR amplification of bla CTX-M-14, bla OXA-48 and aacA4 (Table 2). The aacC2 gene was not detected by PCR in the transconjugant (in agreement with the above analysis), suggesting that it is most likely located on the chromosome. Despite increased β-lactam MICs, Ec003 was fully susceptible to meropenem (Table 2). This was not unexpected as OXA-48 only hydrolyzes carbapenems at low levels [10].
Table 2. β-lactam MICs and PCR results for Kp001 and Ec003.
| Antibiotica | MICs (mg/L)b | ||
| Kp001 | Ec003 (Ec002 transconjugant) | Ec002 | |
| Ampicillin | ≥32 | ≥32 | ≤2 |
| Amoxicillin/CLA | ≥32 | 16 | ≤2 |
| Ticarcillin/CLA | ≥128 | ≥128 | ≤8 |
| Piperacillin/TZB | ≥128 | ≥128 | ≤4 |
| Cefazolin | ≥64 | ≥64 | ≤4 |
| Cefoxitin | ≥64 | ≤4 | ≤4 |
| Ceftazidime | 4 | ≤1 | ≤1 |
| Ceftriaxone | ≥64 | 32 | ≤1 |
| Cefepime | ≥64 | ≤1 | ≤1 |
| Meropenem | ≥16 | ≤0.25 | ≤0.25 |
| Gene Target | PCR Results | ||
| aacA4 | + | + | − |
| bla CTX-M-14 | + | + | − |
| bla OXA-48 | + | + | − |
| aacC2 | + | − | − |
Antibiotic abbreviations: CLA, clavulanic acid, TZB, tazobactam.
MICs were determined using a VITEK 2 AST-N149 card.
In K. pneumoniae, carbapenem resistance in the setting of OXA-48 production is usually co-dependent upon the presence of additional mechanisms of resistance, such as outer membrane porin defects. These mutations generally occur within the OmpK35 and OmpK36 porins, which allow carbapenem entry into the cell. Analysis of the isolates revealed that ompK35 was truncated via a 485 bp chromosomal deletion (nt 1,880,269–1,880,753) while ompK36 contained a duplication of the sequence GGCGAC (nt 1,879,495–1,879,500). This duplication would most likely result in partial occlusion of the OmpK36 channel as a result of insertion of two additional amino acids into loop 3 [25].
In vivo Evolution
Kp001 and Kp002 were considered identical based on their antibiotic resistance profiles (Table 3), molecular (see methods; data not shown) and in silico multi-locus sequence typing [26] which revealed that both isolates belonged to ST101. Nucleotide variant analysis revealed that Kp001 and Kp002 differed by 11 single nucleotide polymorphisms (SNPs; Table 4), many of which are associated with proteins involved in tolerance/resistance to antibiotics, metals or organic solvents, and transcriptional regulation.
Table 3. Antibiotic MICs for Kp001 and Kp002.
| Antibiotica | MICs (mg/L)b | ||
| Kp001 | Kp002 | ||
| Ampicillin | ≥32 | ≥32 | |
| Amoxicillin/CLA | ≥32 | ≥32 | |
| Ticarcillin/CLA | ≥128 | ≥128 | |
| Piperacillin/TZB | ≥128 | ≥128 | |
| Cefazolin | ≥64 | ≥64 | |
| Cefoxitin | ≥64 | ≥64 | |
| Ceftazidime | 4 | 4 | |
| Ceftriaxone | ≥64 | ≥64 | |
| Cefepime | ≥64 | ≥64 | |
| Meropenem | ≥16 | ≥16 | |
| Amikacin | ≥64 | ≥64 | |
| Gentamicin | ≥16 | ≥16 | |
| Tobramycin | ≥16 | ≥16 | |
| Nalidixic acid | ≥32 | ≥32 | |
| Ciprofloxacin | ≥4 | ≥4 | |
| Norfloxacin | ≥16 | ≥16 | |
| Trimethoprim | 1 | 1 | |
| TMP/SXT | ≤20 | ≤20 | |
Antibiotic abbreviations: CLA, clavulanic acid, TZB, tazobactam; TMP/SXT, trimethoprim/sulfamethoxazole.
MICs were determined using a VITEK 2 AST-N149 card.
Table 4. SNPs present in Kp002.
| Mutationa | Gene or Locusb | Function | Amino Acid Change |
| C→A (98,127) | KP1_0101 | putative LysR-type transcriptional regulator | T131N |
| A→G (228,794) | rpoB | RNA polymerase, β subunit | D527G |
| A→T (678,116) | cusS | copper-sensing two-component system sensor kinase | S446C |
| C→T (1,767,550) | yliC | ABC transport system periplasmic binding component | P256S |
| A→C (2,910,808) | slyA | Transcriptional regulator | V120G |
| C→T (2,960,076) | Upstream of KP1_3109 | putative LysR-type transcriptional regulator | – |
| G→A (3,490,471) | gyrI | DNA gyrase inhibitor | A99V |
| C→A (3,721,779) | glpC | sn-glycerol-3-phosphate dehydrogenase K, small subunit | P383T |
| A→G (4,360,381) | Intergenic | – | |
| G→A (4,380,149) | Intergenic | – | |
| G→A (5,084,490) | KP1_5543 | putative acetyltransferase | G120Stop |
Nucleotide change (genetic location in K. pneumoniae strain NTUH-K2044).
Locus name as annotated in K. pneumoniae strain NTUH-K2044.
Compared to Kp001, a SNP in Kp002 was observed in a region of rpoB known to contribute to rifampicin resistance [27]. Subsequently, rifampicin resistance was demonstrated in vitro for Kp002, but not Kp001 (wild-type rpoB), as it could be cultured on LB agar containing 100 µg mL−1 rifampicin. In Australia, it is common practice to soak the surgical mesh in a solution of rifampicin prior to surgery as an infection prevention measure. This exposure most likely contributed to the in vivo selection of the rpoB mutation as Kp002 was isolated from the patients’ intra-abdominal mesh associated collection post hernia repair.
In Kp002, a SNP in gyrI resulted in an amino acid change that may affect protein activity and play a role in decreased quinolone susceptibility. Although overexpression of gyrI (aka sbmC), has been shown to confer protection against quinolones and toxin/anti-toxin plasmid maintenance systems in E. coli [28], it is unlikely to have had much additional effect in our isolate given the high level quinolone resistance mutations already present.
In the context of regulation, two SNPs were associated with LysR-type transcriptional regulators (LTTRs), which represent the largest group of transcriptional regulators regulating genes/pathways associated with metabolism, motility, quorum sensing and virulence [29]. The amino acid change in the gene product of locus KP1_0101 is flanked by amino acids involved in dimerization, based on the conserved domain database [30], suggesting that this mutation is likely to have functional significance. Furthermore, the mutation upstream of the other putative LTTR gene (locus KP1_3109; Table 4) may have a bearing on promoter activity, as it is located 35 bp upstream of the start codon. In addition, there is also a SNP present in slyA, which encodes a known transcriptional regulator of virulence genes. SlyA is involved in conferring resistance to antimicrobial peptides and oxidative stress in salmonellae [31], [32] as well as regulation of fimbriae in E. coli, which have an important role in colonization and pathogenesis [33]; based on the crystal structure of SlyA, the resulting amino acid change (V120G) is located between two α-helices involved in dimerization [34]. Although the functional consequences of these mutations are not directly known, it is interesting that they occurred during 18 months of intra-abdominal carriage after an initial outbreak event that resulted in patient deaths. As such, it is likely that they are collectively associated with changes in virulence (at least to some extent) that have refined the in vivo colonization capacity of Kp002. In this context it is relevant to note that Young et al., [35] recently suggested that truncation of a Staphylococcus aureus transcriptional regulator (implicated in pathogenicity) after 13 months of carriage, was a key factor driving changes in virulence capacity.
Concluding Remarks
To the best of our knowledge, this study represents the first report of the bla OXA-48 CHDL gene in Australia. This study also illustrates the in vivo evolution of a multidrug-resistant K. pneumoniae isolate during 18 months of carriage. Of note, some of the SNPs identified, particularly those associated with transcriptional regulators, may be involved in modulation of Kp002 virulence capacity. In a global context, this is also the first report of bla CTX-M-14 and bla OXA-48 co-residing on a single broad host-range conjugative plasmid (i.e., pJEG011). While international travel has facilitated the clonal spread of bla OXA-48-containing K. pneumoniae ST101 isolates [36]–[38], especially from countries along the Mediterranean Sea, the presence of bla OXA-48 within Tn1999 (and related transposons) on different Inc group plasmids [21], [39], [40], has played a crucial role in its dissemination. The emergence of plasmids such as pJEG011, and the one recently described by Potron et al. [41], is of great clinical concern as they have the potential to more broadly disseminate resistance associated with these determinants. In this respect, it is also concerning that these determinants have the potential to go undetected based on antibiotic susceptibility profiles. Therefore, this study highlights the importance of surveillance based on resistance screening, especially in environments where antibiotic selection pressure is prevalent.
Supporting Information
List of antibiotic resistance genes used in the in-house database for resistome determination.
(XLSX)
Funding Statement
MAC acknowledges the support of a National Health and Medical Research Council (NHMRC) Australia Fellowship (AF511105). SMG is an NHMRC Senior Research Fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Cantón R, et al. (2010) Incidence and antimicrobial susceptibility of Escherichia coli and Klebsiella pneumoniae with extended-spectrum β-lactamases in community- and hospital-associated intra-abdominal infections in Europe: Results of the 2008 Study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob Agents Chemother 54: 3043–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsueh P-R, Badal RE, Hawser SP, Hoban DJ, Bouchillon SK, et al. (2010) Epidemiology and antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia–Pacific region: 2008 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). Int J Antimicrob Agents 36: 408–414. [DOI] [PubMed] [Google Scholar]
- 3. Huang C-C, Chen Y-S, Toh H-S, Lee Y-L, Liu Y-M, et al. (2012) Impact of revised CLSI breakpoints for susceptibility to third-generation cephalosporins and carbapenems among Enterobacteriaceae isolates in the Asia-Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2002–2010. Int J Antimicrob Agents 40 Supplement 1S4–S10. [DOI] [PubMed] [Google Scholar]
- 4. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP (2008) Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 5. Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, et al. (2008) Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and fffect of acquisition on mortality. Antimicrob Agents Chemother 52: 1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Espedido BA, Partridge SR, Iredell JR (2008) bla IMP-4 in different genetic contexts in Enterobacteriaceae isolates from Australia. Antimicrob Agents Chemother 52: 2984–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sidjabat H, Nimmo GR, Walsh TR, Binotto E, Htin A, et al. (2011) Carbapenem resistance in Klebsiella pneumoniae due to the New Delhi metallo-β-lactamase. Clin Infect Dis 52: 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coatsworth NR, Huntington PG, Hardiman RP, Hudson BJ, Fernandes CJ (2012) A case of carbapenemase-producing Klebsiella pneumoniae in Australia. Pathology 44: 42–44. [DOI] [PubMed] [Google Scholar]
- 9. Sidjabat HE, Kennedy K, Silvey A, Collignon P, Paterson DL (2013) Emergence of bla OXA-181-carrying ColE plasmid in Klebsiella pneumoniae in Australia. Int J Antimicrob Agents 41: 294–296. [DOI] [PubMed] [Google Scholar]
- 10. Poirel L, Héritier C, Tolün V, Nordmann P (2004) Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae . Antimicrob Agents Chemother 48: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poirel L, Potron A, Nordmann P (2012) OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67: 1597–1606. [DOI] [PubMed] [Google Scholar]
- 12. Valenzuela JK, Thomas L, Partridge SR, van der Reijden T, Dijkshoorn L, et al. (2007) Horizontal gene transfer in a polyclonal outbreak of carbapenem-resistant Acinetobacter baumannii . J Clin Microbiol 45: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho P-L, Wong RC, Lo SW, Chow K-H, Wong SS, et al. (2010) Genetic identity of aminoglycoside-resistance genes in Escherichia coli isolates from human and animal sources. J Med Microbiol 59: 702–707. [DOI] [PubMed] [Google Scholar]
- 14. Fritsche TR, Castanheira M, Miller GH, Jones RN, Armstrong ES (2008) Detection of methyltransferases conferring high-level resistance to aminoglycosides in Enterobacteriaceae from Europe, North America, and Latin America. Antimicrob Agents Chemother 52: 1843–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang M, Guo Q, Xu X, Wang X, Ye X, et al. (2009) New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis . Antimicrob Agents Chemother 53: 1892–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanschagrin F, Couture F, Levesque RC (1995) Primary structure of OXA-3 and phylogeny of oxacillin-hydrolyzing class D beta-lactamases. Antimicrob Agents Chemother 39: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Philippon LN, Naas T, Bouthors AT, Barakett V, Nordmann P (1997) OXA-18, a class D clavulanic acid-inhibited extended-spectrum beta-lactamase from Pseudomonas aeruginosa . Antimicrob Agents Chemother 41: 2188–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cornaglia G, Giamarellou H, Rossolini GM (2011) Metallo-β-lactamases: a last frontier for β-lactams? The Lancet Infectious Diseases 11: 381–393. [DOI] [PubMed] [Google Scholar]
- 19. Walther-Rasmussen J, Høiby N (2006) OXA-type carbapenemases. J Antimicrob Chemother 57: 373–383. [DOI] [PubMed] [Google Scholar]
- 20. Bonnet R (2004) Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother 48: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poirel L, Bonnin RA, Nordmann P (2012) Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother 56: 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonnin RA, Poirel L, Carattoli A, Nordmann P (2012) Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS ONE 7: e34752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Partridge SR, Ellem JA, Tetu SG, Zong Z, Paulsen IT, et al. (2011) Complete sequence of pJIE143, a pir-type plasmid carrying ISEcp1-bla CTX-M-15 from an Escherichia coli ST131 isolate. Antimicrob Agents Chemother 55: 5933–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heisig P (1996) Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli . Antimicrob Agents Chemother 40: 879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dutzler R, Rummel G, Albertí S, Hernández-Allés S, Phale PS, et al. (1999) Crystal structure and functional characterization of OmpK36, the osmoporin of Klebsiella pneumoniae . Structure 7: 425–434. [DOI] [PubMed] [Google Scholar]
- 26. Diancourt L, Passet V, Verhoef J, Grimont PAD, Brisse S (2005) Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43: 4178–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin DJ, Gross CA (1988) Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol 202: 45–58. [DOI] [PubMed] [Google Scholar]
- 28. Chatterji M, Sengupta S, Nagaraja V (2003) Chromosomally encoded gyrase inhibitor GyrI protects Escherichia coli against DNA-damaging agents. Arch Microbiol 180: 339–346. [DOI] [PubMed] [Google Scholar]
- 29. Maddocks SE, Oyston PCF (2008) Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154: 3609–3623. [DOI] [PubMed] [Google Scholar]
- 30. Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, et al. (2011) CDD: a Conserved Domain Database for the functional annotation of proteins. Nucl Acids Res 39: D225–D229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi Y, Latifi T, Cromie MJ, Groisman EA (2004) Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J Biol Chem 279: 38618–38625. [DOI] [PubMed] [Google Scholar]
- 32. Buchmeier N, Bossie S, Chen CY, Fang FC, Guiney DG, et al. (1997) SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect Immun 65: 3725–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McVicker G, Sun L, Sohanpal BK, Gashi K, Williamson RA, et al. (2011) SlyA protein activates fimB gene expression and type 1 fimbriation in Escherichia coli K-12. J Biol Chem 286: 32026–32035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dolan KT, Duguid EM, He C (2011) Crystal structures of SlyA Protein, a master virulence regulator of Salmonella, in free and DNA-bound states. J Biol Chem 286: 22178–22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young BC, Golubchik T, Batty EM, Fung R, Larner-Svensson H, et al. (2012) Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc Natl Acad Sci USA 109: 4550–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitart C, Solé M, Roca I, Fàbrega A, Vila J, et al. (2011) First outbreak of a plasmid-mediated carbapenem-hydrolyzing OXA-48 β-lactamase in Klebsiella pneumoniae in Spain. Antimicrob Agents Chemother 55: 4398–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hammerum AM, Larsen AR, Hansen F, Justesen US, Friis-Møller A, et al. (2012) Patients transferred from Libya to Denmark carried OXA-48-producing Klebsiella pneumoniae, NDM-1-producing Acinetobacter baumannii and meticillin-resistant Staphylococcus aureus . Int J Antimicrob Agents 40: 191–192. [DOI] [PubMed] [Google Scholar]
- 38. Adler A, Shklyar M, Schwaber MJ, Navon-Venezia S, Dhaher Y, et al. (2011) Introduction of OXA-48-producing Enterobacteriaceae to Israeli hospitals by medical tourism. J Antimicrob Chemother 66: 2763–2766. [DOI] [PubMed] [Google Scholar]
- 39. Carrër A, Poirel L, Yilmaz M, Akan ÖA, Feriha C, et al. (2010) Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob Agents Chemother 54: 1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ktari S, Mnif B, Louati F, Rekik S, Mezghani S, et al. (2011) Spread of Klebsiella pneumoniae isolates producing OXA-48 β-lactamase in a Tunisian university hospital. J Antimicrob Chemother 66: 1644–1646. [DOI] [PubMed] [Google Scholar]
- 41.Potron A, Nordmann P, Rondinaud E, Jaureguy F, Poirel L (2012) A mosaic transposon encoding OXA-48 and CTX-M-15: towards pan-resistance. J Antimicrob Chemother. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of antibiotic resistance genes used in the in-house database for resistome determination.
(XLSX)