In this issue of SLEEP, Matricciani and colleagues,1 after a careful review, conclude that there is insufficient evidence to support recommendations for optimal sleep durations in children and adolescents. In a previous review,2 they found that despite long standing concerns that children are not getting “sufficient” sleep, recommendations for longer sleep durations (i.e., time in bed) were not demonstrably evidence-based. In this issue, Matricciani et al. conclude that current recommendations of children's sleep durations are not based on “high level, low risk of bias data.”1 This seems to be true even for expert recommendations by such authoritative institutions such as the NHLBI, the National Sleep Foundation, and Harvard University. These groups recommend essentially constant levels for time in bed (8.5-10 h) for youngsters aged 10 to 18 years. Despite a diligent search, Matricciani et al. were unable to find experimental data that justify these recommendations. The authors also note, in another context, the implausibility of assertions that optimal sleep durations do not change across the second decade of life.
Matricciani et al. conclude that there is a need for dose-response studies that measure the effects of various sleep durations on daytime function(s) of adolescents of different ages. They make other important points: that one cannot infer sleep need from sleep durations under ad libitum conditions (any more than one can judge caloric need from intake under unlimited food availability); that optimal sleep durations might differ for different waking functions; and that intra- and inter-subject variability could severely challenge dose-response studies. Nevertheless, well-planned experiments should be able to obtain adequate dose-response data. When such data are in hand, there exist sensible guidelines,3 cited by Matricciani et al.1 that could help develop rational consensus recommendations.
One cannot doubt that the dose-response sleep data called for by Matricciani et al. are much needed. The fact that ex cathedra recommendations for adolescent sleep durations have been issued without adequate supporting data embarrasses our field. This embarrassment is made worse because daytime sleepiness in adolescents is a major public concern. Many pediatric sleep specialists have asserted that the adolescent sleepiness reflects insufficient time in bed. In response to such expert opinion, some school systems have adopted later start times. Although Matricciani et al. mention adolescent sleepiness in their review, they do not focus on this issue. They mention sleepiness only once in their text, and the word appears in the titles of only 7 of their 82 references. Instead, their emphasis is almost entirely on the lack of scientific evidence that supports recommended sleep durations.
At several points in their paper, Matricciani et al. refer to children and adolescents subjects as though they were a homogeneous group. They should have been considered separately because their sleep biology (and brains) are quite different. As discussed elsewhere,4 children do not manifest daytime sleepiness in the absence of illness or significant sleep loss. But daytime sleepiness is a prominent aspect of adolescent behavior. It is daytime sleepiness, rather than sleep duration per se, that is the major focus of public concern.
Are our children getting enough sleep? Does the occurrence of sleepiness in adolescents prove that they are sleep deprived? The answer to this question is complex. There is evidence that adolescent sleepiness has a biological component and is not entirely the result of insufficient time in bed. Thus, Carskadon's pioneering “summer camp” study5 found that more mature adolescents were sleepier (determined by MSLT) than less mature subjects, even though all subjects had been habituated to 10 h in bed and averaged ∼9 h of sleep. Carskadon and colleagues concluded that the increased sleepiness in the older subjects was produced by “a maturational factor.”5
More recently, my colleagues and I measured longitudinally the increase in subjective sleepiness across early adolescence (shown here in Figure 1).4 Increasing sleepiness was strongly related to the steep declines in NREM delta and theta power. These relations to developmental changes in brain biology were independent of sleep schedules and objectively measured sleep durations. These were not chance or tenuous results; they confirmed the study's a priori hypothesis at P < 0.0001. Interestingly, subjective sleepiness was more strongly related to the theta than to the delta decline, though both relations were significant. A recent paper from this study reveals strikingly different maturational curves for NREM theta and delta and proposes that NREM theta specifically reflects the recuperation of brain arousal systems.6
Figure 1.
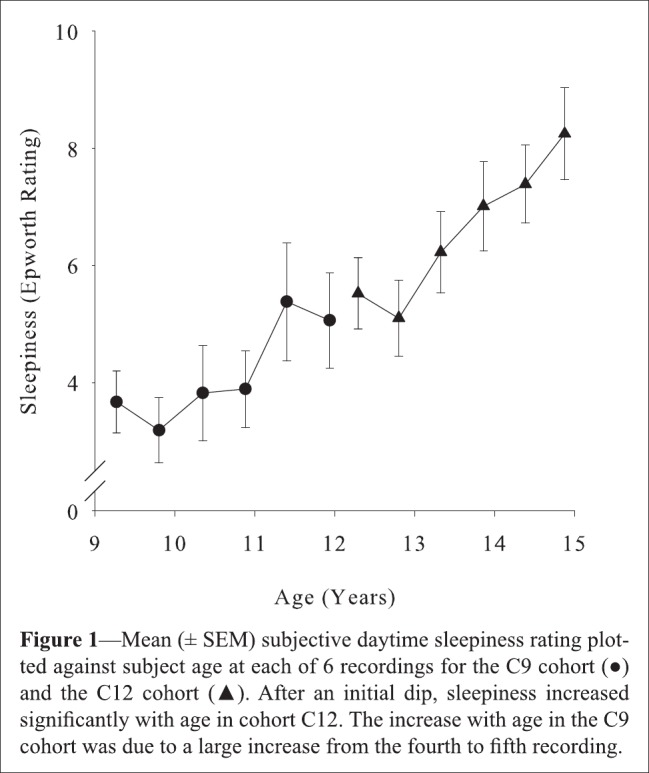
From Campbell et al.4
While it is now widely recognized that the human brain undergoes a major reorganization during adolescence,7 the fact that this hypothesis was partly stimulated by adolescent changes in sleep EEG is less well known. The initial proposal for widespread adolescent brain reorganization was subsequently bolstered by evidence that the maturational decline of NREM delta EEG parallels the declines in synaptic density and cerebral metabolic rate.8 These parallel patterns suggest that the sleep EEG can provide an inexpensive, noninvasive index of adolescent brain reorganization—one that may be more sensitive and reliable (c.f., Campbell9) than the typically more expensive methods now in wider use. Moreover, the possibility that errors might occur in adolescent brain reorganization and give rise to mental illnesses such as schizophrenia expands the potential impact of studies of late (i.e., adolescent) brain development.
In summary, the sleep changes of adolescence bear on major themes in basic and clinical neuroscience. Matricciani et al.1 have identified a major gap in our knowledge of adolescent sleep. Addressing this gap with the dose-response data they seek will advance sleep medicine.
CITATION
Feinberg I. Recommended sleep durations for children and adolescents: the dearth of empirical evidence. SLEEP 2013;36(4):461-462.
DISCLOSURE STATEMENT
Dr. Feinberg has indicated no financial conflicts of interest.
REFERENCES
- 1.Matricciani L, Blunden S, Rigney G, Williams MT, Olds TS. Children's sleep needs: Is there sufficient evidence to recommend optimal sleep for children? Sleep. 2013;36:527–34. doi: 10.5665/sleep.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 2012;129:548–56. doi: 10.1542/peds.2011-2039. [DOI] [PubMed] [Google Scholar]
- 3.Turner T, Misso M, Harris C, Green S. Development of evidence-based clinical practice guidelines (CPGs): comparing approaches. Implement Sci. 2008;3:45. doi: 10.1186/1748-5908-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell IG, Higgins LM, Trinidad JM, Richardson P, Feinberg I. The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. Sleep. 2007;30:1677–87. doi: 10.1093/sleep/30.12.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–60. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. In Press: Am J Physiol Regul Integr Comp Physiol. 2012 Nov 28; doi: 10.1152/ajpregu.00422.2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982/1983;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg I, Thode HC, Jr., Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–61. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 9.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]


