Abstract
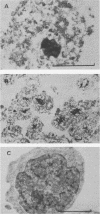
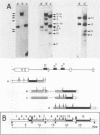
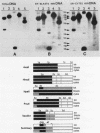
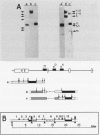
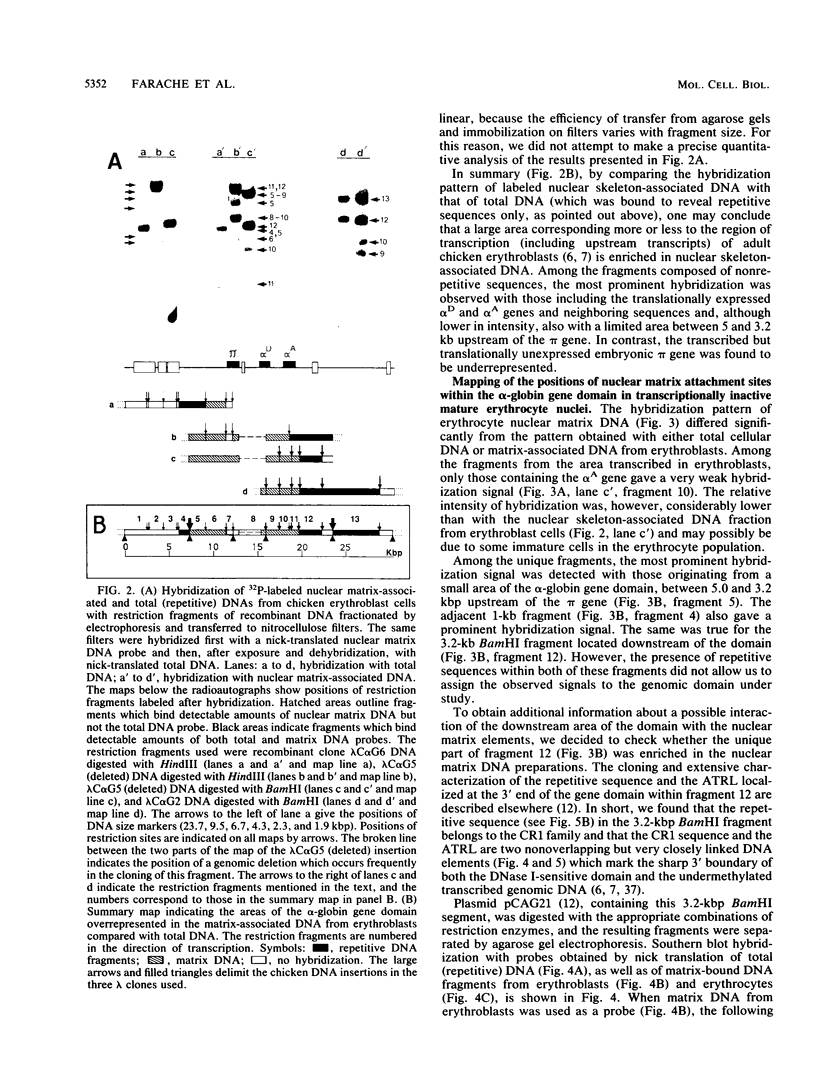
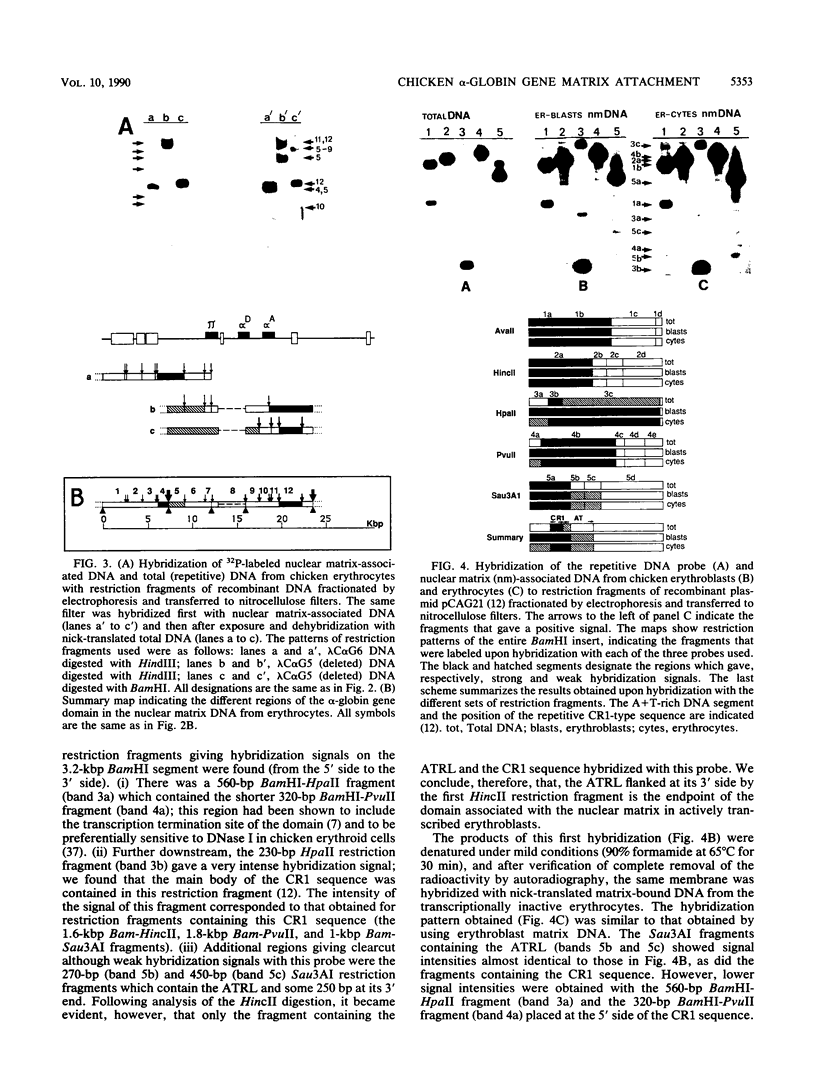
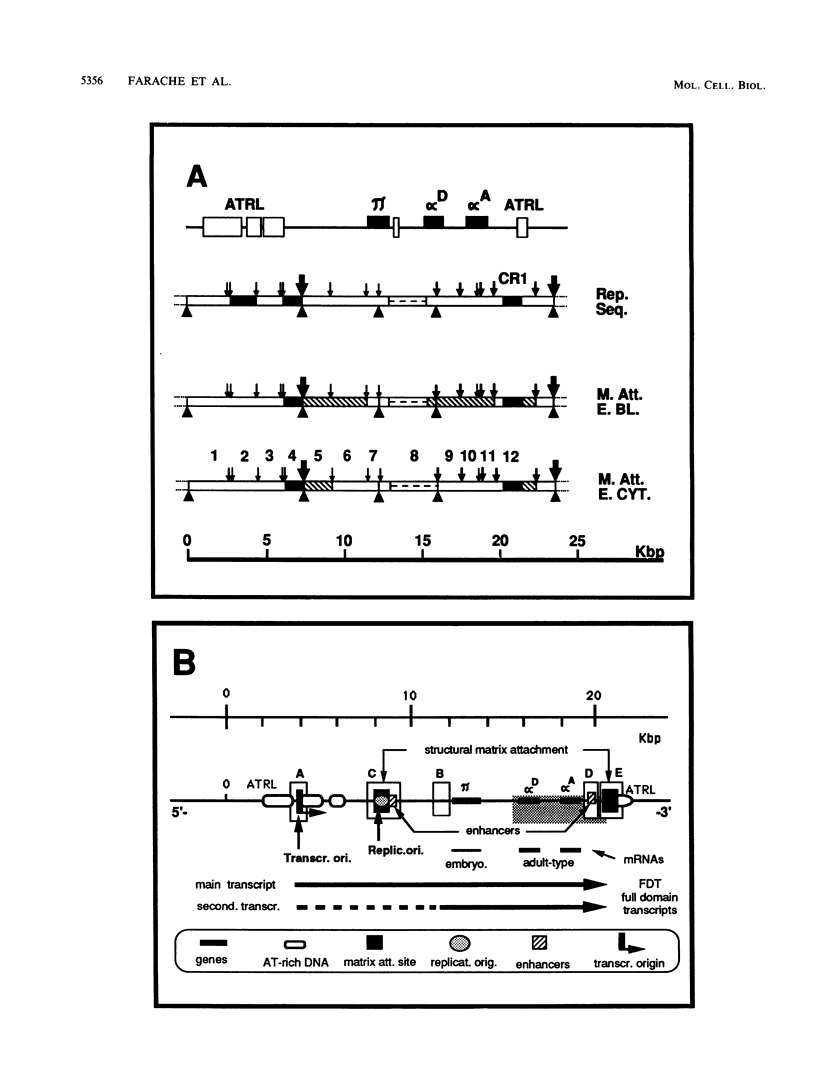
The positions of preferential DNA interaction with the nuclear matrix were mapped within the domain of the chicken alpha-globin genes in transcriptionally active erythroblast nuclei and inactive nuclei of mature erythrocytes. In the latter, only two major distinct attachment sites were observed, close to the A + T-rich sequences previously found at the boundaries of the domain. Sequencing of these structural matrix attachment points revealed several known DNA motifs; some of them were present on both sides of the domain. In actively transcribing erythroblast nuclei of adult animals, a large fraction of the transcribed area was represented in nuclear matrix DNA, including upstream and downstream elements. In particular, adult alpha A- and alpha D-globin genes were found in matrix DNA, while the transcribed but translationally unexpressed embryonic pi gene was underrepresented. The data are discussed in terms of the existence of stable or structural and expression-related matrix attachment sites; correlations to the origin of replication and the units of transcription of the domain are shown.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph K. W., Cheng S. M., Laemmli U. K. Role of nonhistone proteins in metaphase chromosome structure. Cell. 1977 Nov;12(3):805–816. doi: 10.1016/0092-8674(77)90279-3. [DOI] [PubMed] [Google Scholar]
- Basler J., Hastie N. D., Pietras D., Matsui S. I., Sandberg A. A., Berezney R. Hybridization of nuclear matrix attached deoxyribonucleic acid fragments. Biochemistry. 1981 Nov 24;20(24):6921–6929. doi: 10.1021/bi00527a027. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios M., Osheroff N., Fisher P. A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broders F., Razin S., Farache G., Moreau J., Scherrer K. Correlations of repetitive and AT-rich DNA segments within the chicken globin gene domains. Mol Biol Rep. 1986;11(3):177–187. doi: 10.1007/BF00419739. [DOI] [PubMed] [Google Scholar]
- Broders F., Scherrer K. Transcription of the alpha globin gene domain in normal and AEV-transformed chicken erythroblasts: mapping of giant globin-specific RNA including embryonic and adult genes. Mol Gen Genet. 1987 Sep;209(2):210–220. doi: 10.1007/BF00329645. [DOI] [PubMed] [Google Scholar]
- Broders F., Zahraoui A., Scherrer K. The chicken alpha-globin gene domain is transcribed into a 17-kilobase polycistronic RNA. Proc Natl Acad Sci U S A. 1990 Jan;87(2):503–507. doi: 10.1073/pnas.87.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Lang J., Hayday A., Lania L., Fried M., Chiswell D. J., Wyke J. A. Active viral genes in transformed cells lie close to the nuclear cage. EMBO J. 1982;1(4):447–452. doi: 10.1002/j.1460-2075.1982.tb01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Analysis of the closely linked adult chicken alpha-globin genes in recombinant DNAs. Proc Natl Acad Sci U S A. 1980 May;77(5):2596–2600. doi: 10.1073/pnas.77.5.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farache G., Razin S. V., Targa F. R., Scherrer K. Organization of the 3'-boundary of the chicken alpha globin gene domain and characterization of a CR 1-specific protein binding site. Nucleic Acids Res. 1990 Feb 11;18(3):401–409. doi: 10.1093/nar/18.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. doi: 10.1016/0092-8674(86)90877-9. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., McCready S. J., Cook P. R. RNA is synthesized at the nuclear cage. Nature. 1981 Aug 6;292(5823):552–555. doi: 10.1038/292552a0. [DOI] [PubMed] [Google Scholar]
- Knezetic J. A., Felsenfeld G. Identification and characterization of a chicken alpha-globin enhancer. Mol Cell Biol. 1989 Mar;9(3):893–901. doi: 10.1128/mcb.9.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsovali A., Huesca M., Marcaud L. Mise en évidence d'une activité "enhancer" dans un fragment d'ADN situé en 3' du gène alpha globine adulte de canard. C R Acad Sci III. 1988;307(10):563–568. [PubMed] [Google Scholar]
- Kretsovali A., Marcaud L., Moreau J., Scherrer K. Conservation and variation in the large scale organisation of the globin gene domains of duck and chicken. Mol Gen Genet. 1986 May;203(2):193–201. doi: 10.1007/BF00333954. [DOI] [PubMed] [Google Scholar]
- Lawson G. M., Knoll B. J., March C. J., Woo S. L., Tsai M. J., O'Malley B. W. Definition of 5' and 3' structural boundaries of the chromatin domain containing the ovalbumin multigene family. J Biol Chem. 1982 Feb 10;257(3):1501–1507. [PubMed] [Google Scholar]
- Maundrell K., Maxwell E. S., Puvion E., Scherrer K. The nuclear matrix of duck erythroblasts is associated with globin mRNA coding sequences but not with the major proteins of 40S nuclear RNP. Exp Cell Res. 1981 Dec;136(2):435–445. doi: 10.1016/0014-4827(81)90023-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Post-replicative nonribosomal transcription units in D. melanogaster embryos. Cell. 1979 Jul;17(3):551–563. doi: 10.1016/0092-8674(79)90263-0. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Moreau J., Marcaud L., Maschat F., Kejzlarova-Lepesant J., Lepesant J. A., Scherrer K. A + T-rich linkers define functional domains in eukaryotic DNA. Nature. 1982 Jan 21;295(5846):260–262. doi: 10.1038/295260a0. [DOI] [PubMed] [Google Scholar]
- Moreau J., Matyash-Smirniaguina L., Scherrer K. Systematic punctuation of eukaryotic DNA by A+T-rich sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1341–1345. doi: 10.1073/pnas.78.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Kekelidze M. G., Lukanidin E. M., Scherrer K., Georgiev G. P. Replication origins are attached to the nuclear skeleton. Nucleic Acids Res. 1986 Oct 24;14(20):8189–8207. doi: 10.1093/nar/14.20.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. V., Mantieva V. L., Georgiev G. P. The similarity of DNA sequences remaining bound to scaffold upon nuclease treatment of interphase nuclei and metaphase chromosomes. Nucleic Acids Res. 1979 Nov 24;7(6):1713–1735. doi: 10.1093/nar/7.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. V., Yarovaya O. V., Georgiev G. P. Low ionic strength extraction of nuclease-treated nuclei destroys the attachment of transcriptionally active DNA to the nuclear skeleton. Nucleic Acids Res. 1985 Oct 25;13(20):7427–7444. doi: 10.1093/nar/13.20.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzeszowska-Wolny J., Razin S., Puvion E., Moreau J., Scherrer K. Isolation and characterization of stable nuclear matrix preparations and associated DNA from avian erythroblasts. Biol Cell. 1988;64(1):13–22. doi: 10.1016/0248-4900(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Serfling E. Autoregulation--a common property of eukaryotic transcription factors? Trends Genet. 1989 May;5(5):131–133. doi: 10.1016/0168-9525(89)90049-8. [DOI] [PubMed] [Google Scholar]
- Small D., Nelkin B., Vogelstein B. The association of transcribed genes with the nuclear matrix of Drosophila cells during heat shock. Nucleic Acids Res. 1985 Apr 11;13(7):2413–2431. doi: 10.1093/nar/13.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]