Abstract
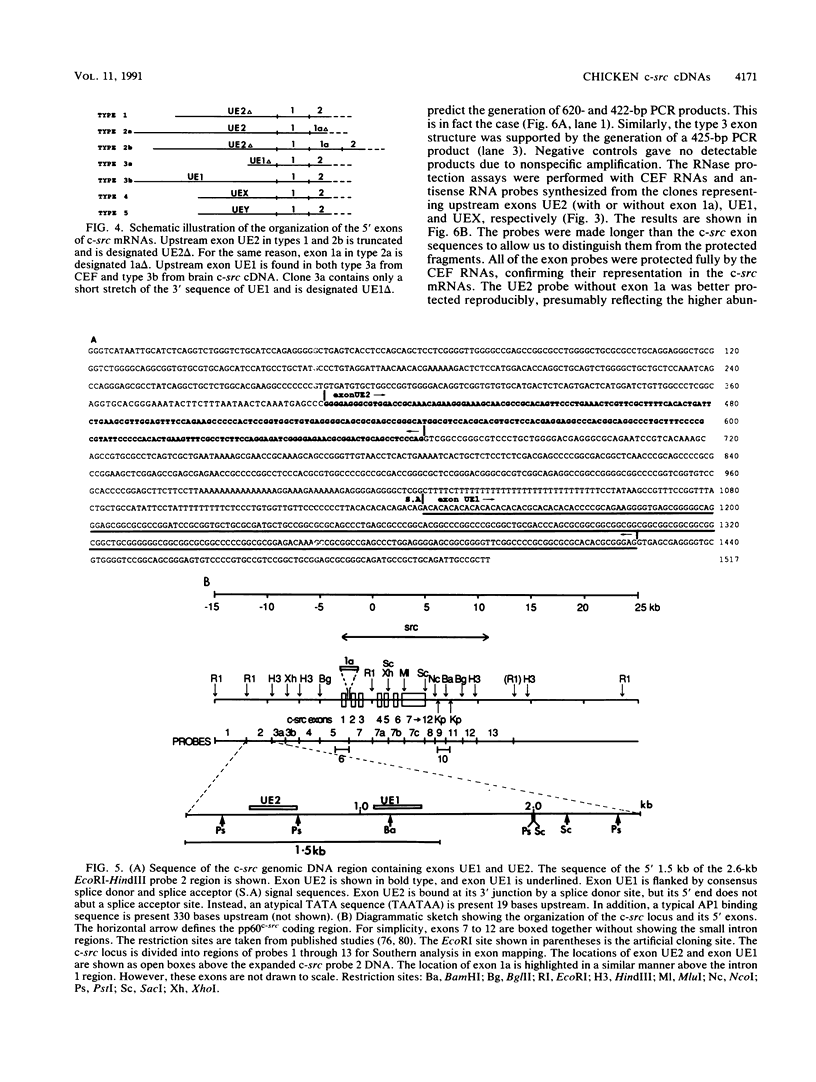
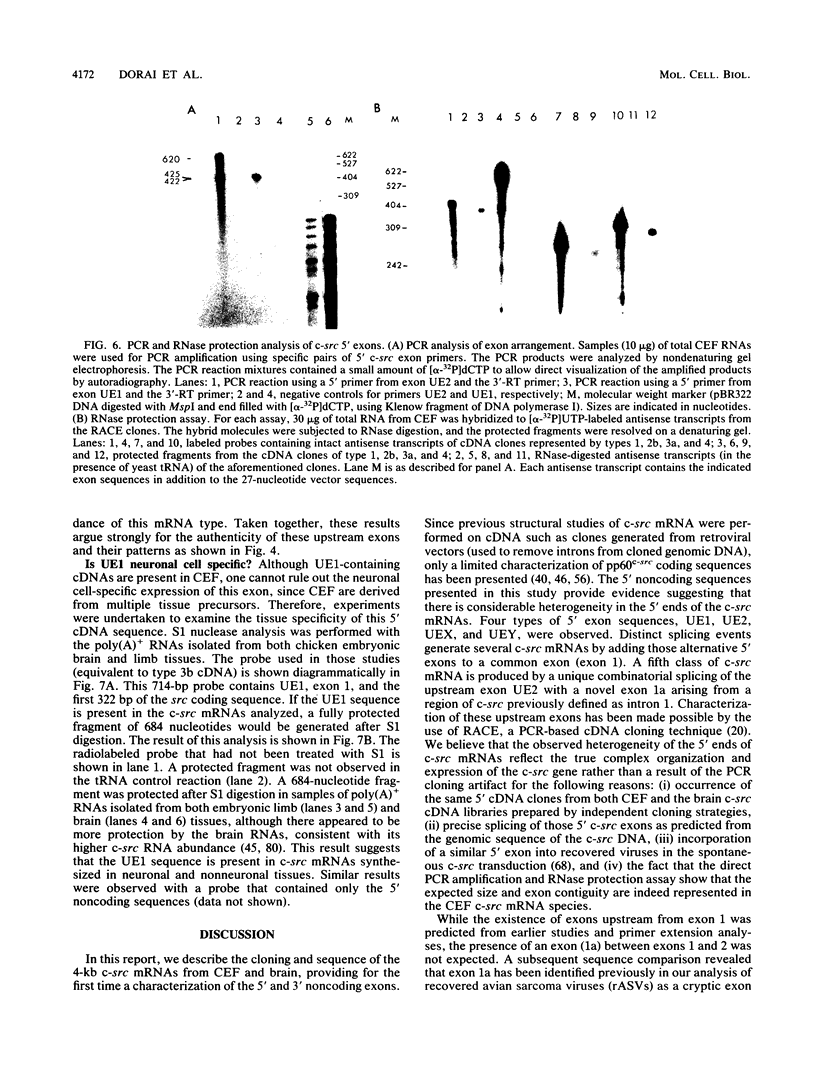
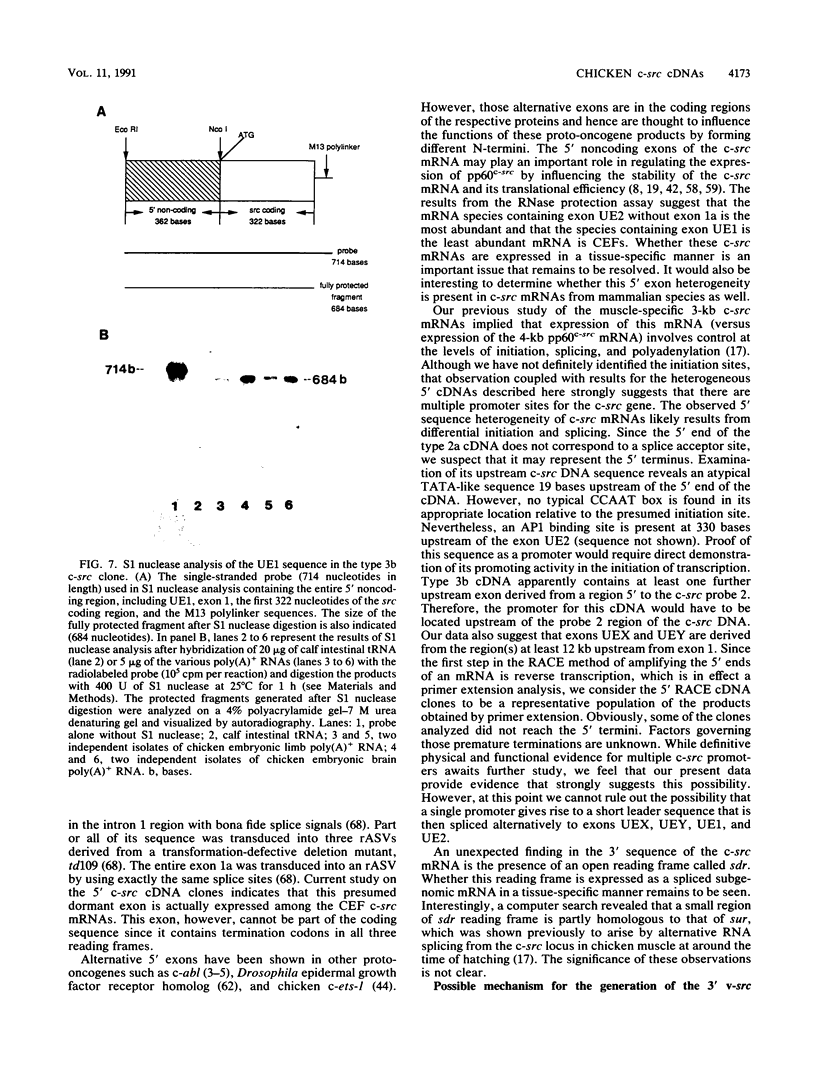
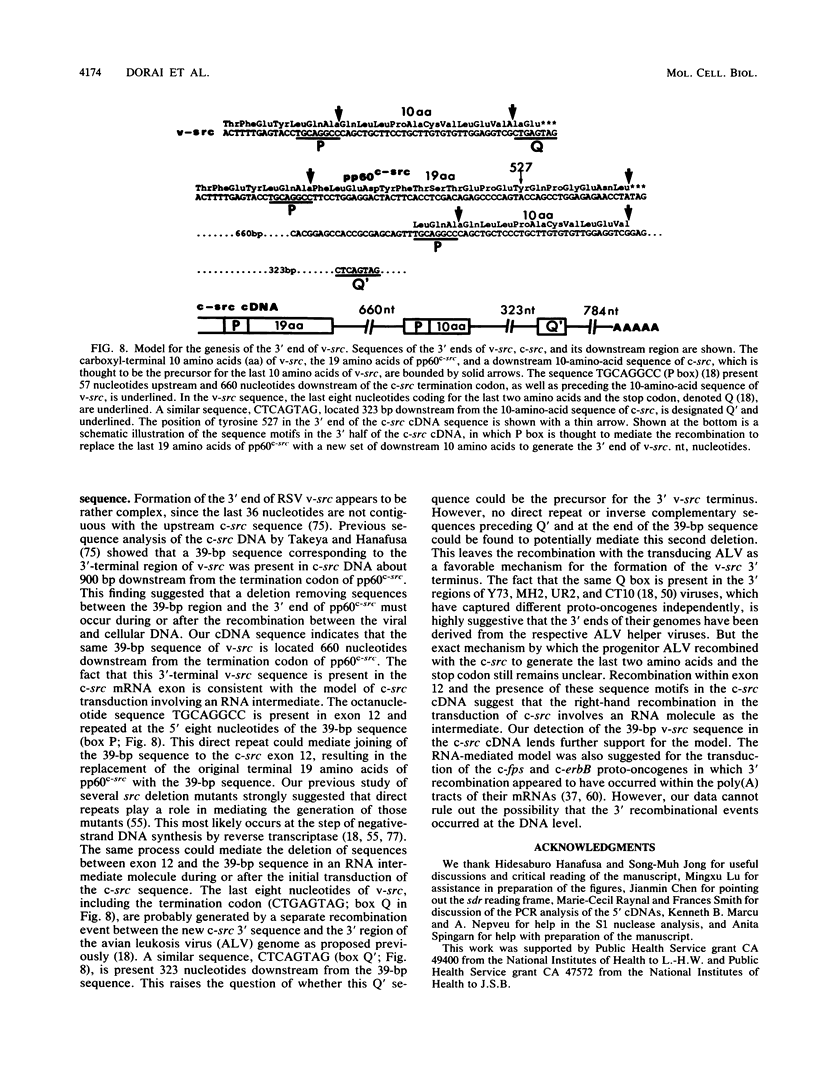
To further characterize the gene structure of the proto-oncogene c-src and the mechanism for the genesis of the v-src sequence in Rous sarcoma virus, we have analyzed genomic and cDNA copies of the chicken c-src gene. From a cDNA library of chicken embryo fibroblasts, we isolated and sequenced several overlapping cDNA clones covering the full length of the 4-kb c-src mRNA. The cDNA sequence contains a 1.84-kb sequence downstream from the 1.6-kb pp60c-src coding region. An open reading frame of 217 amino acids, called sdr (src downstream region), was found 105 nucleotides from the termination codon for pp60c-src. Within the 3' noncoding region, a 39-bp sequence corresponding to the 3' end of the RSV v-src was detected 660 bases downstream of the pp60c-src termination codon. The presence of this sequence in the c-src mRNA exon supports a model involving an RNA intermediate during transduction of the c-src sequence. The 5' region of the c-src cDNA was determined by analyzing several cDNA clones generated by conventional cloning methods and by polymerase chain reaction. Sequences of these chicken embryo fibroblast clones plus two c-src cDNA clones isolated from a brain cDNA library show that there is considerable heterogeneity in sequences upstream from the c-src coding sequence. Within this region, which contains at least 300 nucleotides upstream of the translational initiation site in exon 2, there exist at least two exons in each cDNA which fall into five cDNA classes. Four unique 5' exon sequences, designated exons UE1, UE2, UEX, and UEY, were observed. All of them are spliced to the previously characterized c-src exons 1 and 2 with the exception of type 2 cDNA. In type 2, the exon 1 is spliced to a novel downstream exon, designated exon 1a, which maps in the region of the c-src DNA defined previously as intron 1. Exon UE1 is rich in G+C content and is mapped at 7.8 kb upstream from exon 1. This exon is also present in the two cDNA clones from the brain cDNA library. Exon UE2 is located at 8.5 kb upstream from exon 1. The precise locations of exons UEX and UEY have not been determined, but both are more than 12 kb upstream from exon 1. The existence and exon arrangements of these 5' cDNAs were further confirmed by RNase protection assays and polymerase chain reactions using specific primers. Our findings indicate that the heterogeneity in the 5' sequences of the c-src mRNAs results from differential splicing and perhaps use of distinct initiation sites. All of these RNAs have the potential of coding for pp60c-src, since their 5' exons are all eventually joined to exon 2.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnekow A., Gessler M. Activation of the pp60c-src kinase during differentiation of monomyelocytic cells in vitro. EMBO J. 1986 Apr;5(4):701–705. doi: 10.1002/j.1460-2075.1986.tb04270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnekow A., Schartl M. Cellular src gene product detected in the freshwater sponge Spongilla lacustris. Mol Cell Biol. 1984 Jun;4(6):1179–1181. doi: 10.1128/mcb.4.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y., Bernards A., Paskind M., Daley G. Q., Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986 Feb 28;44(4):577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- Bernards A., Paskind M., Baltimore D. Four murine c-abl mRNAs arise by usage of two transcriptional promoters and alternative splicing. Oncogene. 1988 Apr;2(4):297–304. [PubMed] [Google Scholar]
- Bernards A., Rubin C. M., Westbrook C. A., Paskind M., Baltimore D. The first intron in the human c-abl gene is at least 200 kilobases long and is a target for translocations in chronic myelogenous leukemia. Mol Cell Biol. 1987 Sep;7(9):3231–3236. doi: 10.1128/mcb.7.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Rosen N., Israel M. A. Increased pp60c-src tyrosyl kinase activity in human neuroblastomas is associated with amino-terminal tyrosine phosphorylation of the src gene product. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7275–7279. doi: 10.1073/pnas.82.21.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Cotton P., Lustig A., Yonemoto W., Lipsich L., Coussens P., Barrett J. N., Nonner D., Keane R. W. Characterization of the altered form of the c-src gene product in neuronal cells. Genes Dev. 1987 May;1(3):287–296. doi: 10.1101/gad.1.3.287. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Cowan W. M., Hunter T., Eckhart W. pp60c-src expression in the developing rat brain. Proc Natl Acad Sci U S A. 1988 May;85(10):3348–3352. doi: 10.1073/pnas.85.10.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Kaplan P. L., Hunter T., Eckhart W. Alterations in pp60c-src accompany differentiation of neurons from rat embryo striatum. Mol Cell Biol. 1987 May;7(5):1830–1840. doi: 10.1128/mcb.7.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dorai T., Wang L. H. An alternative non-tyrosine protein kinase product of the c-src gene in chicken skeletal muscle. Mol Cell Biol. 1990 Aug;10(8):4068–4079. doi: 10.1128/mcb.10.8.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., Wang L. H., Hanafusa T., Hanafusa H. Partial nucleotide sequence of Rous sarcoma virus-29 provides evidence that the original Rous sarcoma virus was replication defective. J Virol. 1985 Sep;55(3):728–735. doi: 10.1128/jvi.55.3.728-735.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Piechaczyk M., Henglein B., Blanchard J. M., Traub B., Kofler E., Wiest S., Lenoir G. M., Bornkamm G. W. Aberrant c-myc RNAs of Burkitt's lymphoma cells have longer half-lives. EMBO J. 1985 Dec 30;4(13B):3717–3725. doi: 10.1002/j.1460-2075.1985.tb04140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol Cell Biol. 1985 Jan;5(1):27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Hanafusa H. NH2-terminal sequences of two src proteins that cause aberrant transformation. Proc Natl Acad Sci U S A. 1987 Jan;84(1):80–84. doi: 10.1073/pnas.84.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee C. E., Griffin J., Sastre L., Miller L. J., Springer T. A., Piwnica-Worms H., Roberts T. M. Differentiation of myeloid cells is accompanied by increased levels of pp60c-src protein and kinase activity. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5131–5135. doi: 10.1073/pnas.83.14.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler M., Barnekow A. Differential expression of the cellular oncogenes c-src and c-yes in embryonal and adult chicken tissues. Biosci Rep. 1984 Sep;4(9):757–770. doi: 10.1007/BF01128817. [DOI] [PubMed] [Google Scholar]
- Gibbs C. P., Tanaka A., Anderson S. K., Radul J., Baar J., Ridgway A., Kung H. J., Fujita D. J. Isolation and structural mapping of a human c-src gene homologous to the transforming gene (v-src) of Rous sarcoma virus. J Virol. 1985 Jan;53(1):19–24. doi: 10.1128/jvi.53.1.19-24.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandori C., Hanafusa H. p60c-src is complexed with a cellular protein in subcellular compartments involved in exocytosis. J Cell Biol. 1988 Dec;107(6 Pt 1):2125–2135. doi: 10.1083/jcb.107.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Einat P., Shilo B. Z., Hoffmann F. M. Drosophila melanogaster DNA clones homologous to vertebrate oncogenes: evidence for a common ancestor to the src and abl cellular genes. Cell. 1983 Feb;32(2):589–598. doi: 10.1016/0092-8674(83)90478-6. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Hay N., Bishop J. M. The role of RNA molecules in transduction of the proto-oncogene c-fps. Cell. 1986 Mar 28;44(6):935–940. doi: 10.1016/0092-8674(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kaplan P. L., Simon S., Cartwright C. A., Eckhart W. cDNA cloning with a retrovirus expression vector: generation of a pp60c-src cDNA clone. J Virol. 1987 May;61(5):1731–1734. doi: 10.1128/jvi.61.5.1731-1734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beau J. M., Wiestler O. D., Walter G. An altered form of pp60c-src is expressed primarily in the central nervous system. Mol Cell Biol. 1987 Nov;7(11):4115–4117. doi: 10.1128/mcb.7.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Dorai T., Wang L. H., Brugge J. S. The structurally distinct form of pp60c-src detected in neuronal cells is encoded by a unique c-src mRNA. Mol Cell Biol. 1987 Nov;7(11):4142–4145. doi: 10.1128/mcb.7.11.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F., Aubry M., Shores C. G., Frame L., Pfenninger K. H. c-src gene product in developing rat brain is enriched in nerve growth cone membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5001–5005. doi: 10.1073/pnas.85.14.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F. pp60c-src encoded by the proto-oncogene c-src is a product of sensory neurons. J Neurosci Res. 1986;16(1):127–139. doi: 10.1002/jnr.490160113. [DOI] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Jove R., Krane J. F., Poirier F., Calothy G., Hanafusa H. Genetic lesions involved in temperature sensitivity of the src gene products of four Rous sarcoma virus mutants. J Virol. 1986 Dec;60(3):858–867. doi: 10.1128/jvi.60.3.858-867.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Shibuya M., Hsu M. T., Wang L. H. Proto-oncogene c-ros codes for a molecule with structural features common to those of growth factor receptors and displays tissue specific and developmentally regulated expression. Mol Cell Biol. 1986 May;6(5):1478–1486. doi: 10.1128/mcb.6.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Cellular homologue (c-src) of the transforming gene of Rous sarcoma virus: isolation, mapping, and transcriptional analysis of c-src and flanking regions. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5842–5846. doi: 10.1073/pnas.78.9.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. J., Creutz C. E. p60c-src activity detected in the chromaffin granule membrane. Biochem Biophys Res Commun. 1986 Jan 29;134(2):736–742. doi: 10.1016/s0006-291x(86)80482-x. [DOI] [PubMed] [Google Scholar]
- Parvin J. D., Wang L. H. Mechanisms for the generation of src-deletion mutants and recovered sarcoma viruses: identification of viral sequences involved in src deletions and in recombination with c-src sequences. Virology. 1984 Oct 30;138(2):236–245. doi: 10.1016/0042-6822(84)90348-9. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Kaplan D. R., Whitman M., Roberts T. M. Retrovirus shuttle vector for study of kinase activities of pp60c-src synthesized in vitro and overproduced in vivo. Mol Cell Biol. 1986 Jun;6(6):2033–2040. doi: 10.1128/mcb.6.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper J. M., Bolen J. B. Identification of a novel neuronal C-SRC exon expressed in human brain. Mol Cell Biol. 1990 May;10(5):2035–2040. doi: 10.1128/mcb.10.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts P. H., Forster A., Stinson M. A., Rabbitts T. H. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNa stability. EMBO J. 1985 Dec 30;4(13B):3727–3733. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Maihle N. J., Moscovici C., Crittenden L., Kung H. J. Mechanism of c-erbB transduction: newly released transducing viruses retain poly(A) tracts of erbB transcripts and encode C-terminally intact erbB proteins. J Virol. 1988 Jul;62(7):2437–2443. doi: 10.1128/jvi.62.7.2437-2443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartl M., Barnekow A. Differential expression of the cellular src gene during vertebrate development. Dev Biol. 1984 Oct;105(2):415–422. doi: 10.1016/0012-1606(84)90298-7. [DOI] [PubMed] [Google Scholar]
- Schejter E. D., Segal D., Glazer L., Shilo B. Z. Alternative 5' exons and tissue-specific expression of the Drosophila EGF receptor homolog transcripts. Cell. 1986 Sep 26;46(7):1091–1101. doi: 10.1016/0092-8674(86)90709-9. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Relationship of polypeptide products of the transforming gene of Rous sarcoma virus and the homologous gene of vertebrates. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2059–2063. doi: 10.1073/pnas.77.4.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Zelenetz A. D., Cooper G. M. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981 May;24(2):531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Drees B., Kornberg T., Bishop J. M. The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell. 1985 Oct;42(3):831–840. doi: 10.1016/0092-8674(85)90279-x. [DOI] [PubMed] [Google Scholar]
- Soong M. M., Iijima S., Wang L. H. Transduction of c-src coding and intron sequences by a transformation-defective deletion mutant of Rous sarcoma virus. J Virol. 1986 Sep;59(3):556–563. doi: 10.1128/jvi.59.3.556-563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baker B., Varmus H. E., Bishop J. M. Characteristics of cellular RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):381–386. doi: 10.1016/0092-8674(78)90206-4. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Sudol M., Alvarez-Buylla A., Hanafusa H. Differential developmental expression of cellular yes and cellular src proteins in cerebellum. Oncogene Res. 1988 May;2(4):345–355. [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H., Junghans R. P., Ju G., Skalka A. M. Comparison between the viral transforming gene (src) of recovered avian sarcoma virus and its cellular homolog. Mol Cell Biol. 1981 Nov;1(11):1024–1037. doi: 10.1128/mcb.1.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Edelstein B., Mayer B. J. Induction of tumors and generation of recovered sarcoma viruses by, and mapping of deletions in, two molecularly cloned src deletion mutants. J Virol. 1984 Jun;50(3):904–913. doi: 10.1128/jvi.50.3.904-913.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Iijima S., Dorai T., Lin B. Regulation of the expression of proto-oncogene c-src by alternative RNA splicing in chicken skeletal muscle. Oncogene Res. 1987 Jun;1(1):43–59. [PubMed] [Google Scholar]
- Wang L. H. The mechanism of transduction of proto-oncogene c-src by avian retroviruses. Mutat Res. 1987 Sep;186(2):135–147. doi: 10.1016/0165-1110(87)90027-3. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Hayward W. S., Hanafusa H. Genetic variation in the RNA transcripts of endogenous virus genes in uninfected chicken cells. J Virol. 1977 Oct;24(1):64–73. doi: 10.1128/jvi.24.1.64-73.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]