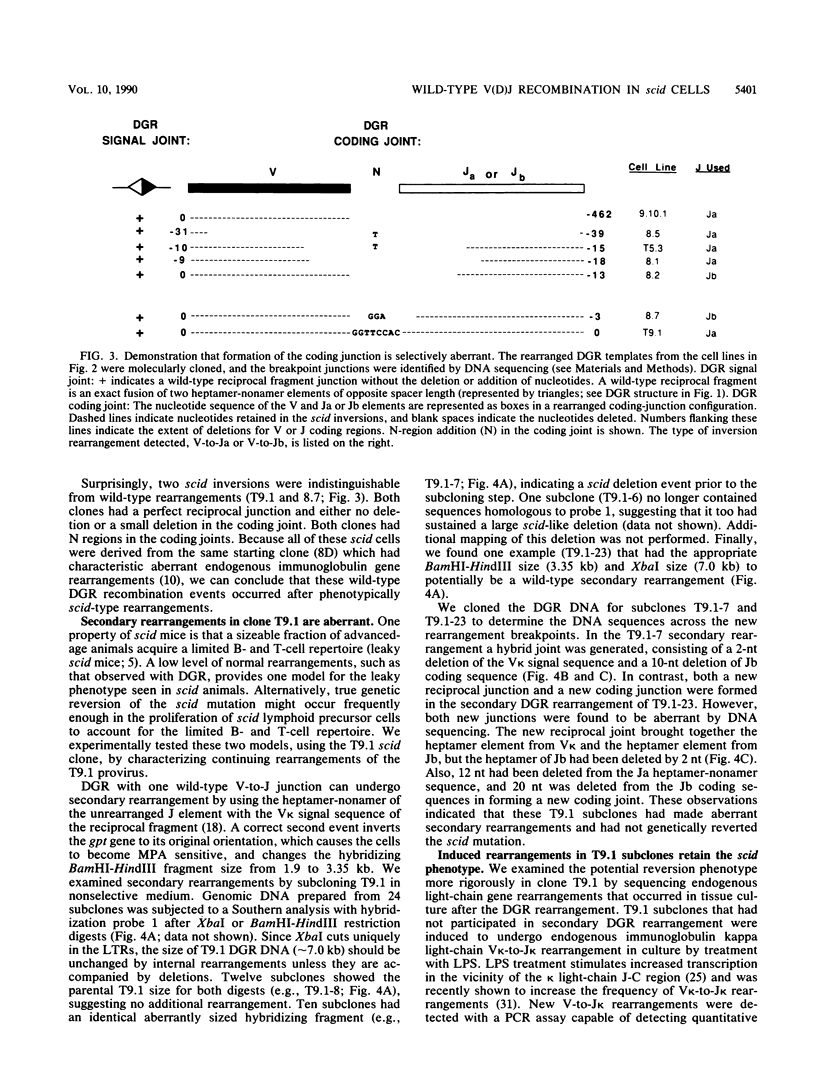
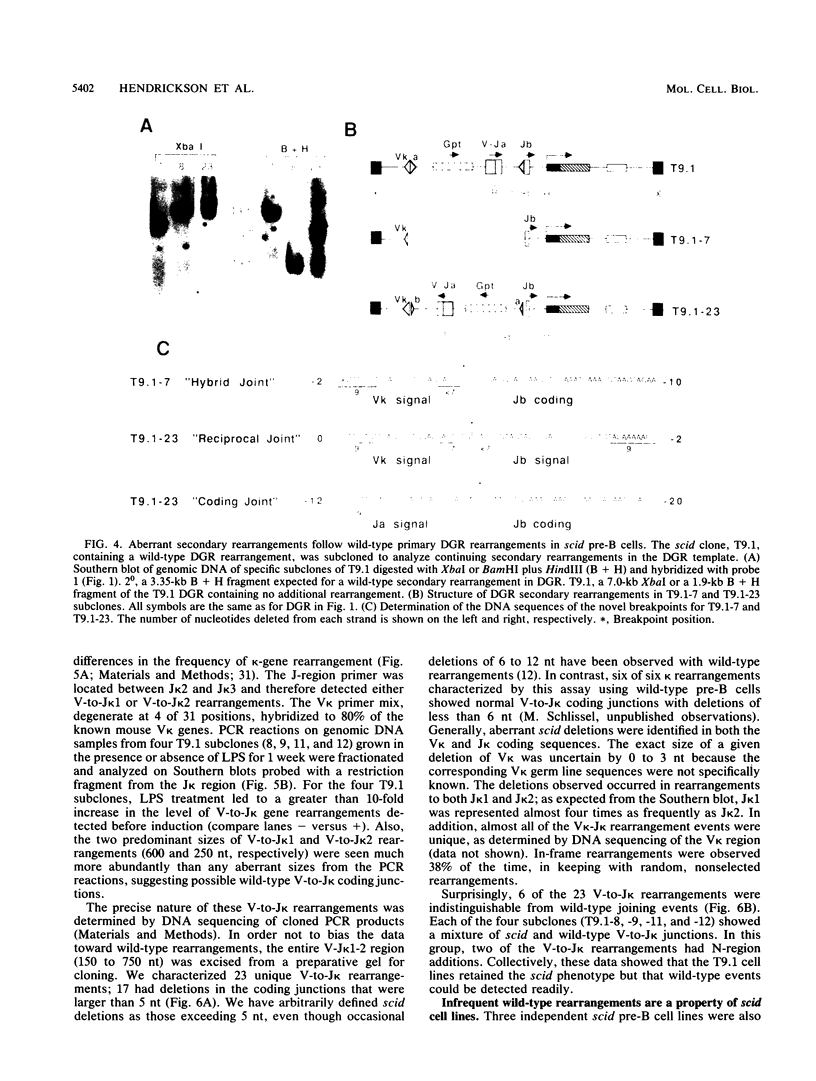
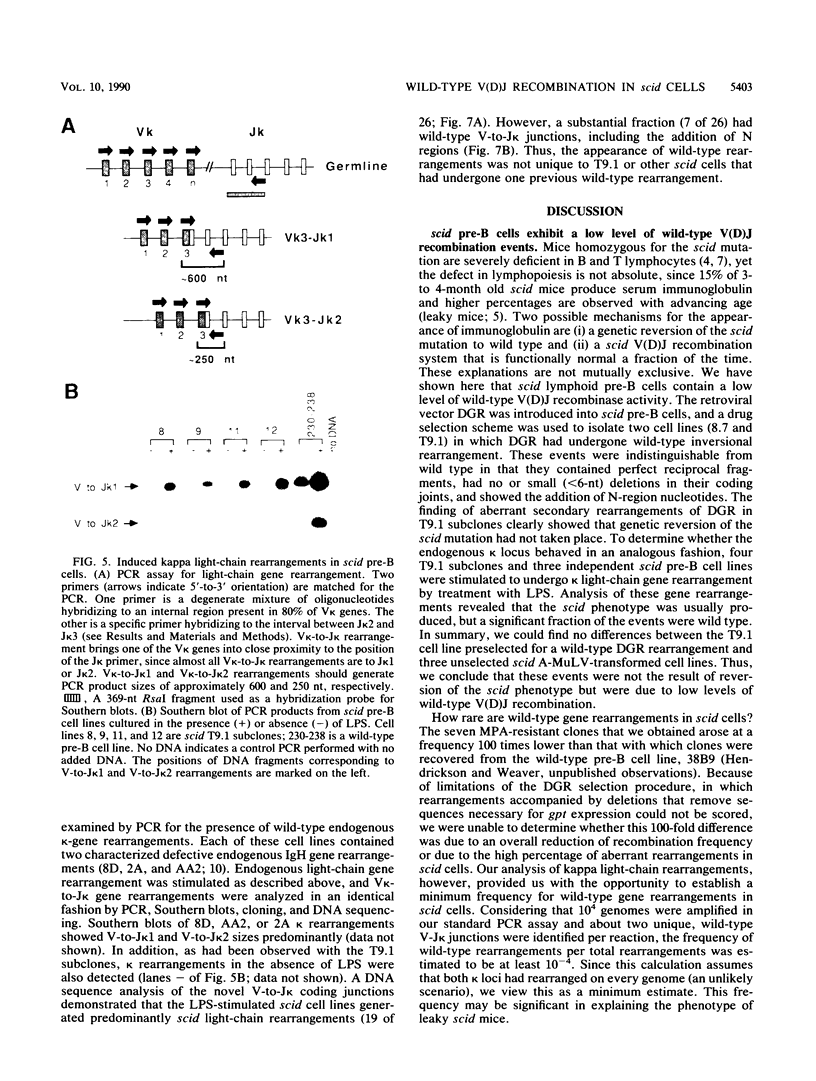
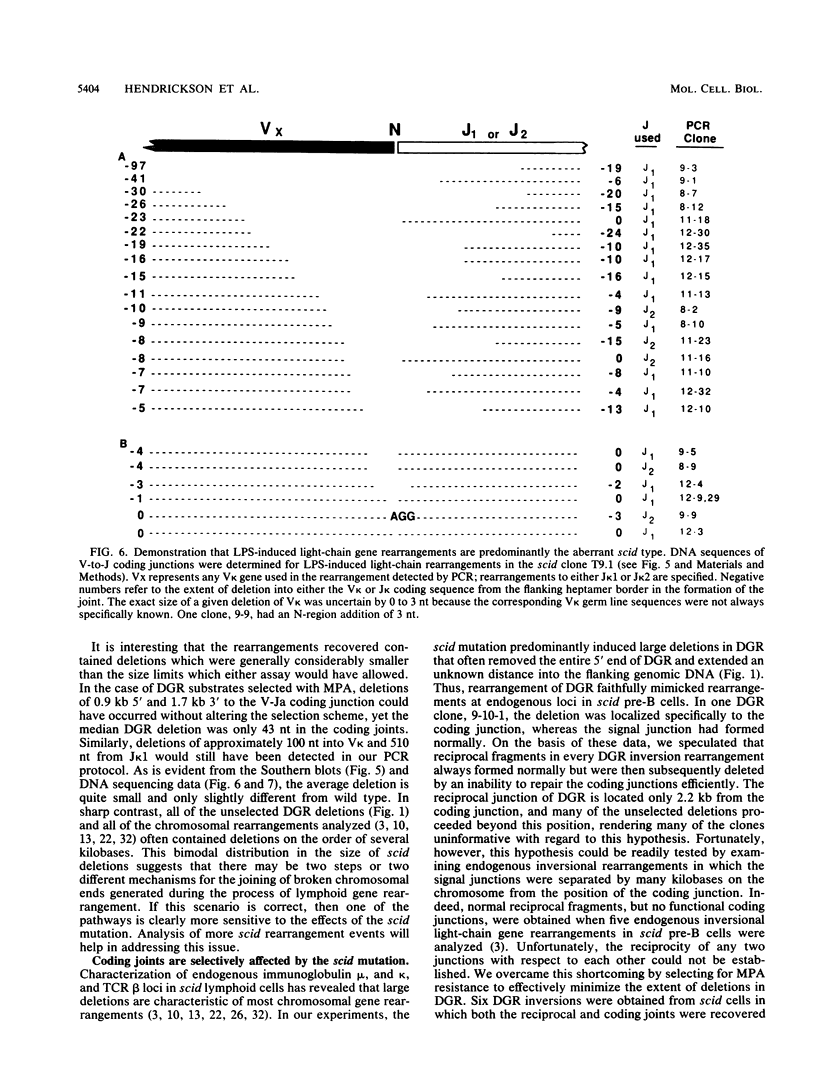
Abstract
Homozygous mutation at the scid locus in the mouse results in the aberrant rearrangement of immunoglobulin and T-cell receptor gene segments. We introduced a retroviral vector containing an inversional immunoglobulin rearrangement cassette into scid pre-B cells. Most rearrangements were accompanied by large deletions, consistent with previously characterized effects of the scid mutation. However, two cell clones were identified which contained perfect reciprocal fragments and wild-type coding joints, documenting, on a molecular level, the ability of scid pre-B cells to generate functional protein-coding domains. Subsequent rearrangement of the DGR cassette in one of these clones was accompanied by a deletion, suggesting that this cell clone had not reverted the scid mutation. Indeed, induced rearrangement of the endogenous kappa loci in these two cell clones resulted in a mixture of scid and wild-type V-J kappa joints, as assayed by a polymerase chain reaction and DNA sequencing. In addition, three immunoglobulin mu- scid pre-B cell lines showed both scid and wild-type V-J kappa joins. These experiments strongly suggest that the V(D)J recombinase activity in scid lymphoid cells is diminished but not absent, consistent with the known leakiness of the scid mutation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Malynn B. A., Pollock R. R., Ferrier P., Covey L. R., Fulop G. M., Phillips R. A., Yancopoulos G. D., Alt F. W. Isolation of scid pre-B cells that rearrange kappa light chain genes: formation of normal signal and abnormal coding joins. EMBO J. 1989 Mar;8(3):735–742. doi: 10.1002/j.1460-2075.1989.tb03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. M., Bosma M. J. Detection and characterization of functional T cells in mice with severe combined immune deficiency. Eur J Immunol. 1988 Dec;18(12):1965–1971. doi: 10.1002/eji.1830181215. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Ferrier P., Covey L. R., Li S. C., Suh H., Malynn B. A., Blackwell T. K., Morrow M. A., Alt F. W. Normal recombination substrate VH to DJH rearrangements in pre-B cell lines from scid mice. J Exp Med. 1990 Jun 1;171(6):1909–1918. doi: 10.1084/jem.171.6.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Schatz D. G., Weaver D. T. The scid gene encodes a trans-acting factor that mediates the rejoining event of Ig gene rearrangement. Genes Dev. 1988 Jul;2(7):817–829. doi: 10.1101/gad.2.7.817. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Kim M. G., Schuler W., Bosma M. J., Marcu K. B. Abnormal recombination of Igh D and J gene segments in transformed pre-B cells of scid mice. J Immunol. 1988 Aug 15;141(4):1341–1347. [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Cooper M. D., Carroll A. J., Burrows P. D. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2356–2360. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau N. R., Schatz D. G., Rosa M., Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol Cell Biol. 1987 Sep;7(9):3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. M., Hesse J. E., Mizuuchi K., Gellert M. Novel strand exchanges in V(D)J recombination. Cell. 1988 Dec 23;55(6):1099–1107. doi: 10.1016/0092-8674(88)90254-1. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. DNA elements are asymmetrically joined during the site-specific recombination of kappa immunoglobulin genes. Science. 1985 May 10;228(4700):677–685. doi: 10.1126/science.3158075. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. Joining of V kappa to J kappa gene segments in a retroviral vector introduced into lymphoid cells. 1984 Mar 29-Apr 4Nature. 308(5958):425–428. doi: 10.1038/308425a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Lymphoid V(D)J recombination: nucleotide insertion at signal joints as well as coding joints. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8588–8592. doi: 10.1073/pnas.85.22.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Morzycka-Wroblewska E., Lee F. E., Desiderio S. V. Unusual immunoglobulin gene rearrangement leads to replacement of recombinational signal sequences. Science. 1988 Oct 14;242(4876):261–263. doi: 10.1126/science.3140378. [DOI] [PubMed] [Google Scholar]
- Nelson K. J., Kelley D. E., Perry R. P. Inducible transcription of the unrearranged kappa constant region locus is a common feature of pre-B cells and does not require DNA or protein synthesis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5305–5309. doi: 10.1073/pnas.82.16.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Nishikawa S., Sakano H. Aberrant immunoglobulin gene rearrangement in scid mouse bone marrow cells. J Immunol. 1988 Aug 15;141(4):1348–1352. [PubMed] [Google Scholar]
- Petrini J. H., Carroll A. M., Bosma M. J. T-cell receptor gene rearrangements in functional T-cell clones from severe combined immune deficient (scid) mice: reversion of the scid phenotype in individual lymphocyte progenitors. Proc Natl Acad Sci U S A. 1990 May;87(9):3450–3453. doi: 10.1073/pnas.87.9.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Petrac E., Wiese P., Lobel L., Alt F. W. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO J. 1987 Nov;6(11):3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D. G., Baltimore D. Stable expression of immunoglobulin gene V(D)J recombinase activity by gene transfer into 3T3 fibroblasts. Cell. 1988 Apr 8;53(1):107–115. doi: 10.1016/0092-8674(88)90492-8. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]