Abstract
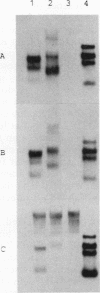
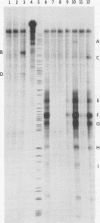
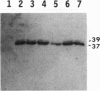
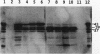
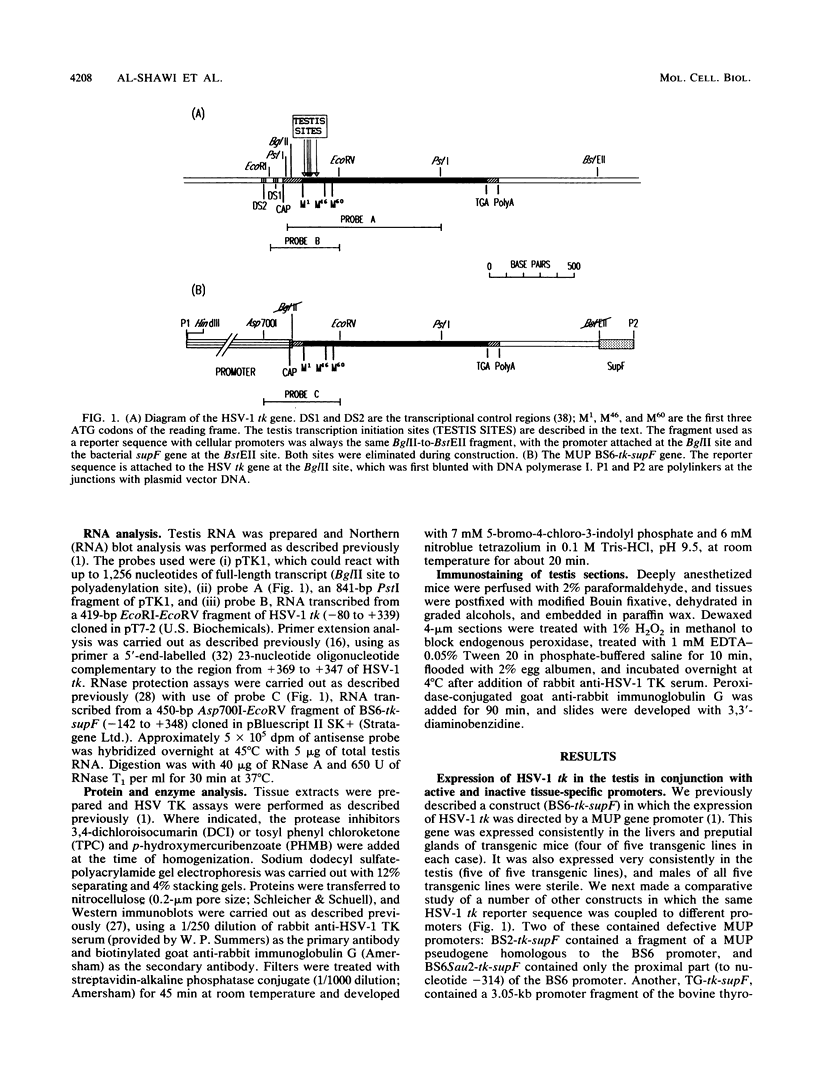
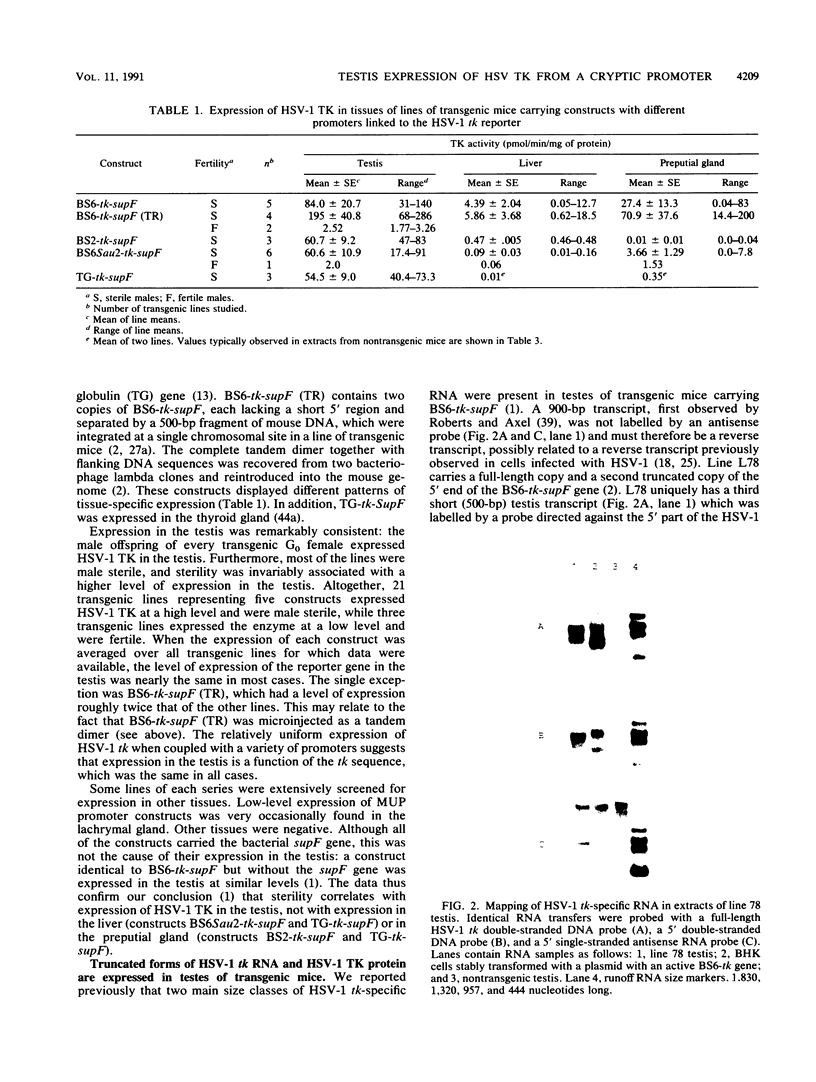
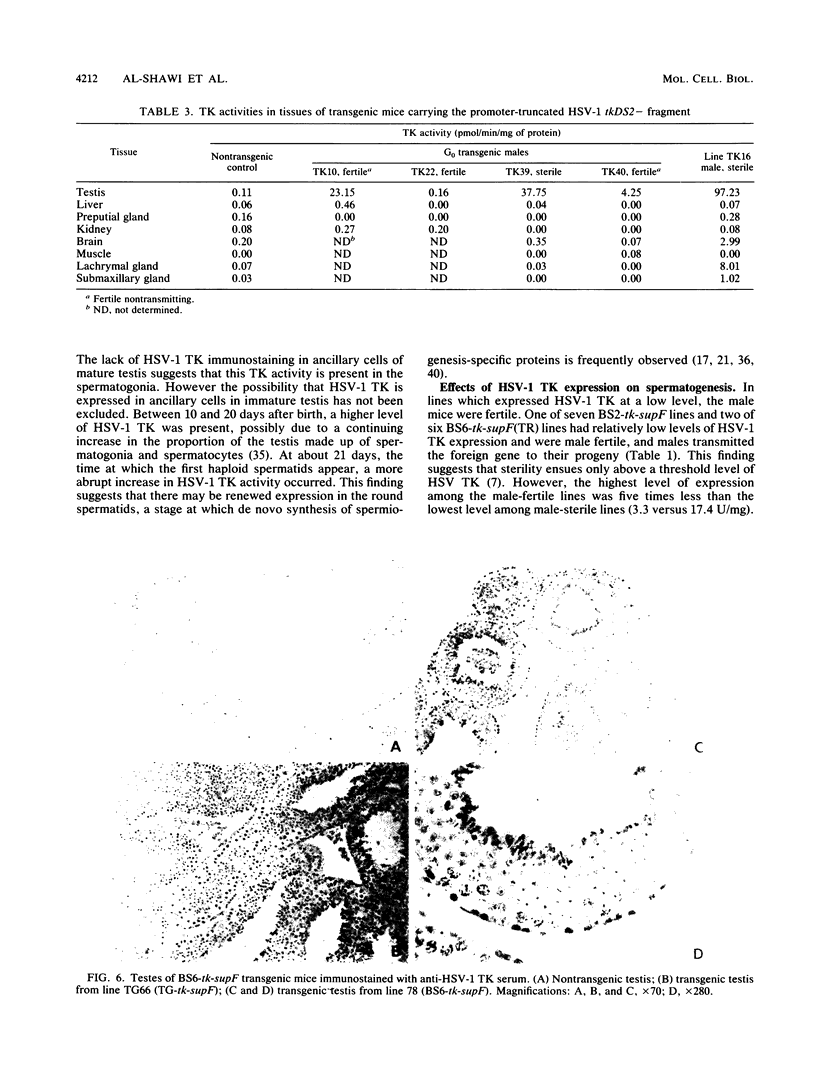
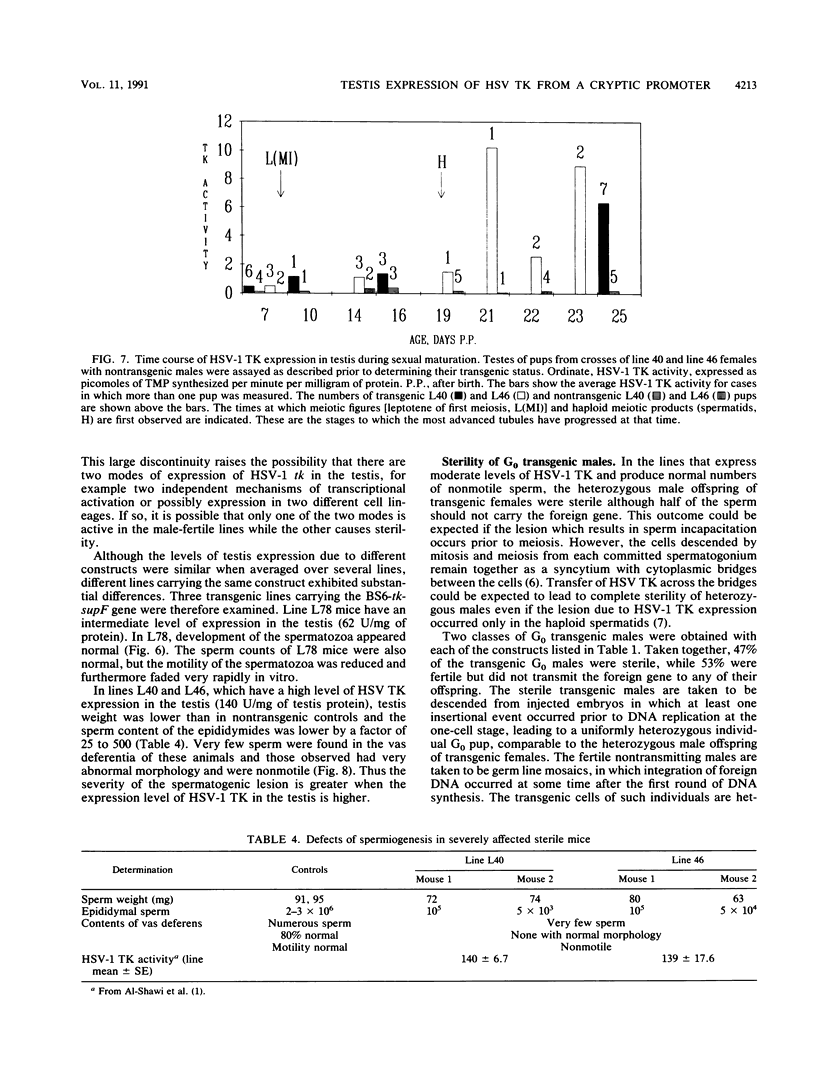
We reported previously that the herpes simplex virus type 1 (HSV-1) thymidine kinase reporter gene (tk) was expressed in the testes of transgenic mice when coupled to the promoter of a liver-specific mouse major urinary protein (MUP) gene. Here we show that HSV-1 tk is also expressed in the testis when coupled to a MUP pseudogene promoter, to a truncated MUP promoter that is not active in the liver, and to the promoter of the bovine thyroglobulin gene. Furthermore, HSV-1 tk itself was expressed in the testis, although its normal expression had been disabled by removing an upstream regulator of transcription. In every case, the same multiple transcripts were observed, with their 5' ends located downstream of the normal HSV-1 tk translation initiation codon. We conclude that the transcription of HSV-1 tk in the testis is directed by a cryptic TATA box-independent promoter located in the coding region of the gene. The longest HSV-1 thymidine kinase (TK) polypeptides synthesized in the testis were shorter than full-length TK and probably result from translational initiation at Met46 and Met60, the second and third ATG codons of the tk reading frame. Male mice of most transgenic lines were sterile, and the severity of the lesion in spermatogenesis was directly related to the level of TK expression. In the most highly expressing lines, sperm counts were low and morphologically defective sperm were common. In other sterile lines, TK was expressed at a lower level and sperm counts were normal but sperm motility was greatly reduced. Lines with the lowest levels of HSV-1 TK expression were fertile. HSV-1 TK was expressed in germ line cells, mainly in the haploid spermatids. However, low-level HSV-1 TK activity was found in the testis before the first germ cells entered meiosis, showing that if expression is confined to the germ cells, it also occurs in spermatogonia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Shawi R., Burke J., Jones C. T., Simons J. P., Bishop J. O. A Mup promoter-thymidine kinase reporter gene shows relaxed tissue-specific expression and confers male sterility upon transgenic mice. Mol Cell Biol. 1988 Nov;8(11):4821–4828. doi: 10.1128/mcb.8.11.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Heyman R. A., Arias C., Sawchenko P. E., Evans R. M. Transgenic mice with inducible dwarfism. Nature. 1989 Jun 15;339(6225):538–541. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Heyman R., Hsi M., Evans R. M. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7572–7576. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. E., Behringer R. R., Peschon J. J., Brinster R. L., Palmiter R. D. Genetically haploid spermatids are phenotypically diploid. Nature. 1989 Jan 26;337(6205):373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Braun R. E., Lo D., Pinkert C. A., Widera G., Flavell R. A., Palmiter R. D., Brinster R. L. Infertility in male transgenic mice: disruption of sperm development by HSV-tk expression in postmeiotic germ cells. Biol Reprod. 1990 Oct;43(4):684–693. doi: 10.1095/biolreprod43.4.684. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M., Senear A. W., Warren R., Palmiter R. D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981 Nov;27(1 Pt 2):223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J. E., Olds-Clarke P., Storey B. T. Oxidative metabolism of spermatozoa from inbred and random bred mice. J Exp Zool. 1981 May;216(2):285–292. doi: 10.1002/jez.1402160209. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Association of thymidylate kinase activity with pyrimidine deoxyribonucleoside kinase induced by herpes simplex virus. J Biol Chem. 1978 Mar 10;253(5):1325–1327. [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Coen D. M., Irmiere A. F., Jacobson J. G., Kerns K. M. Low levels of herpes simplex virus thymidine- thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989 Feb;168(2):221–231. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Coen D. M., McKnight S. L. Promoter domains required for expression of plasmid-borne copies of the herpes simplex virus thymidine kinase gene in virus-infected mouse fibroblasts and microinjected frog oocytes. Mol Cell Biol. 1985 Aug;5(8):1940–1947. doi: 10.1128/mcb.5.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity P. A., Wold B. J. Tissue-specific expression from a compound TATA-dependent and TATA-independent promoter. Mol Cell Biol. 1990 Nov;10(11):5646–5654. doi: 10.1128/mcb.10.11.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Goldman D. S., Kiessling A. A., Millette C. F., Cooper G. M. Expression of c-mos RNA in germ cells of male and female mice. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4509–4513. doi: 10.1073/pnas.84.13.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Haarr L., Marsden H. S., Preston C. M., Smiley J. R., Summers W. C., Summers W. P. Utilization of internal AUG codons for initiation of protein synthesis directed by mRNAs from normal and mutant genes encoding herpes simplex virus-specified thymidine kinase. J Virol. 1985 Nov;56(2):512–519. doi: 10.1128/jvi.56.2.512-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. E., Smiley J. R. Effects of deletions on expression of the herpes simplex virus thymidine kinase gene from the intact viral genome: the amino terminus of the enzyme is dispensable for catalytic activity. J Virol. 1984 Jun;50(3):733–738. doi: 10.1128/jvi.50.3.733-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht N. B., Bower P. A., Waters S. H., Yelick P. C., Distel R. J. Evidence for haploid expression of mouse testicular genes. Exp Cell Res. 1986 May;164(1):183–190. doi: 10.1016/0014-4827(86)90465-9. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Borrelli E., Lesley J., Anderson D., Richman D. D., Baird S. M., Hyman R., Evans R. M. Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2698–2702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y., Asano M., Nishimune Y., Kawade Y. Male sterility of transgenic mice carrying exogenous mouse interferon-beta gene under the control of the metallothionein enhancer-promoter. EMBO J. 1988 Dec 1;7(12):3757–3762. doi: 10.1002/j.1460-2075.1988.tb03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J. G., Martin S. L., Coen D. M. A conserved open reading frame that overlaps the herpes simplex virus thymidine kinase gene is important for viral growth in cell culture. J Virol. 1989 Apr;63(4):1839–1843. doi: 10.1128/jvi.63.4.1839-1843.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Summers W. C. Site-directed mutagenesis of a nucleotide-binding domain in HSV-1 thymidine kinase: effects on catalytic activity. Virology. 1988 Apr;163(2):638–642. doi: 10.1016/0042-6822(88)90308-x. [DOI] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Haarr L., Preston C. M. Processing of herpes simplex virus proteins and evidence that translation of thymidine kinase mRNA is initiated at three separate AUG codons. J Virol. 1983 May;46(2):434–445. doi: 10.1128/jvi.46.2.434-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. C., Spence A., Smith M. The distal transcription signals of the herpesvirus tk gene share a common hexanucleotide control sequence. Cell. 1984 May;37(1):253–262. doi: 10.1016/0092-8674(84)90321-0. [DOI] [PubMed] [Google Scholar]
- NEBEL B. R., AMAROSE A. P., HACKET E. M. Calendar of gametogenic development in the prepuberal male mouse. Science. 1961 Sep 22;134(3482):832–833. doi: 10.1126/science.134.3482.832. [DOI] [PubMed] [Google Scholar]
- Peschon J. J., Behringer R. R., Brinster R. L., Palmiter R. D. Spermatid-specific expression of protamine 1 in transgenic mice. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5316–5319. doi: 10.1073/pnas.84.15.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert C. A., Widera G., Cowing C., Heber-Katz E., Palmiter R. D., Flavell R. A., Brinster R. L. Tissue-specific, inducible and functional expression of the E alpha d MHC class II gene in transgenic mice. EMBO J. 1985 Sep;4(9):2225–2230. doi: 10.1002/j.1460-2075.1985.tb03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Axel R. Gene amplification and gene correction in somatic cells. Cell. 1982 May;29(1):109–119. doi: 10.1016/0092-8674(82)90095-2. [DOI] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987 Jul 3;50(1):89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- Veerisetty V., Gentry G. A. HSV1-specific thymidylate kinase activity in infected cells. Intervirology. 1985;24(1):42–49. doi: 10.1159/000149617. [DOI] [PubMed] [Google Scholar]
- Virbasius J. V., Scarpulla R. C. The rat cytochrome c oxidase subunit IV gene family: tissue-specific and hormonal differences in subunit IV and cytochrome c mRNA expression. Nucleic Acids Res. 1990 Nov 25;18(22):6581–6586. doi: 10.1093/nar/18.22.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Stewart T. A., Mintz B. The human beta-globin gene and a functional viral thymidine kinase gene in developing mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5016–5020. doi: 10.1073/pnas.78.8.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T. E., Hoppe P. C., Jollick J. D., Scholl D. R., Hodinka R. L., Gault J. B. Microinjection of a rabbit beta-globin gene into zygotes and its subsequent expression in adult mice and their offspring. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6376–6380. doi: 10.1073/pnas.78.10.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martynoff G., Pohl V., Mercken L., van Ommen G. J., Vassart G. Structural organization of the bovine thyroglobulin gene and of its 5'-flanking region. Eur J Biochem. 1987 May 4;164(3):591–599. doi: 10.1111/j.1432-1033.1987.tb11168.x. [DOI] [PubMed] [Google Scholar]