Abstract
Objective
In adults, one of the major determinants of leukocyte telomere length (LTL), a predictor of age-related diseases and mortality, is cumulative psychosocial stress exposure. More recently we reported that exposure to maternal psychosocial stress during intrauterine life is associated with LTL in young adulthood. The objective of the present study was to determine how early in life this effect of stress on LTL is apparent by quantifying the association of maternal psychosocial stress during pregnancy with newborn telomere length.
Study Design
In a prospective study of N = 27 mother-newborn dyads maternal pregnancy-specific stress was assessed in early gestation and cord blood peripheral blood mononuclear cells were subsequently collected and analyzed for LTL measurement.
Results
After accounting for the effects of potential determinants of newborn LTL (gestational age at birth, weight, sex, and exposure to antepartum obstetric complications), there was a significant, independent, linear effect of pregnancy-specific stress on newborn LTL that accounted for 25% of the variance in adjusted LTL (β = −0.099; P = .04).
Conclusion
Our finding provides the first preliminary evidence in human beings that maternal psychological stress during pregnancy may exert a “programming” effect on the developing telomere biology system that is already apparent at birth, as reflected by the setting of newborn LTL.
Keywords: fetal/developmental programming of health and disease risk, maternal psychosocial stress, newborn telomere biology
The contribution of various forms of stress including psychological stress to the origin and progression of many complex, common aging-related health disorders that represent the major global burden of disease is well established.1 The effects of exposure to (maternal) psychological stress during intrauterine life appear to be particularly salient, with important consequences for not only adverse birth and neonatal outcomes, but also for subsequent health and disease risk-related outcomes over the lifespan, including metabolic, endocrine, immune, and cognitive processes (ie, the concept of fetal or developmental programming of health).2 The elucidation of the molecular mechanism(s) underlying the link between stress and disease risk is an area of considerable interest and effort. In recent years telomere biology has emerged as a particularly attractive candidate mechanism in this context. Telomeres, noncoding double-stranded repeats of guanine-rich tandem DNA sequences, and shelterin protein structures that cap the ends of linear chromosomes3,4 play a central, critical role in maintaining the integrity (stability) of the genome and cell. They protect chromosomes from recognition by the DNA damage-repair system as DNA breaks. Because DNA polymerase is unable to fully replicate the 3′ end of the DNA strand (the so-called “end-replication problem”) telomeres shorten with each cell division. Eventually telomeres reach a critical short length, which, in turn, leads to cellular senescence or apoptosis.5 Telomerase, a cellular enzyme, provides maintenance of telomeres and can counteract shortening and its functional consequences by adding telomeric DNA to shortened telomeres. In just the past few years, telomere biology has emerged as a phenomenon of exceptional interest, moving well beyond its previously recognized role as a biomarker of cellular aging and senescence to one that appears to play a causal and far more pervasive role in regeneration of cells and tissues, physiological function, and aging.6-8
Telomere maintenance has relevance for long-term health. Shortened leukocyte telomere length (LTL) in human beings and/or reduced telomerase activity has been consistently associated with earlier mortality,9-11 and age-associated disease risk (eg, cardiovascular disease, hypertension, atherosclerosis, heart failure, type 2 diabetes).12-16 Interindividual variation in adult LTL is a function of the initial (newborn) setting of telomere length (TL) and subsequent attrition rate.17 With respect to the determinants of the initial setting of TL, despite the relatively high heritability of TL known genetic variants (from candidate gene as well as genome wide association studies approaches) account for only a small proportion of the variance in LTL,18-20 highlighting the potential importance of intrauterine effects in the setting of TL at birth.
The initial observation linking psychological stress in adults with the activity of the telomere biology system21 has subsequently been replicated in several independent studies.22-24 More recently we made the first observation linking maternal psychological stress exposure during intrauterine life with shorter LTL in young adulthood.25 At the present time it is unknown whether the effect of maternal stress during pregnancy on off-spring TL is already evident at birth. Based on observed stress-related alterations in maternal-placental-fetal immune and endocrine processes during gestation26,27 and on the putative effects of these processes on telomere biology28 we hypothesized that exposure to excess maternal stress during pregnancy may impact the fetal telomere biology system in utero, and this effect may already be apparent at birth. Hence, the objective of the present study was to examine the magnitude of the association between maternal psychosocial stress during pregnancy and that proportion of the variance in newborn LTL not accounted for by other potential determinants, ie, newborn gestational age at birth, weight, sex, and exposure to antepartum obstetric complications.29-35
Materials and Methods
Study participants
The study sample was a subsample of women from a larger cohort of women attending prenatal care at a university-based clinic in Pittsburgh, PA, and participating in a prospective, longitudinal research study from early gestation through birth. This subsample comprised subjects in whom additional measures of maternal stress were administered, including the measure of pregnancy-specific stress used in the current report. As depicted in the Appendix (Supplemental Table), this subsample is representative of the larger cohort. The study population was a predominantly Medicaid-insured, low-income population that was approximately 55% black and 44% white. Eligible women had singleton pregnancies and had no known prepregnancy major medical conditions or fetal anomalies. Enrollment took place at < 16 wk of gestation (mean gestational age: 9.5 wk). The study was approved by the University of Pittsburgh Institutional Review Board and informed, written consent was obtained from all participants. At enrollment, women completed a structured interview to provide data on sociodemographic characteristics, medical history, and maternal behaviors. The subsample of N = 27 mother-infant pairs in whom data were also obtained about psychosocial stress during pregnancy comprises the study sample for the current report. The sociodemographic, pregnancy, and newborn birth outcome characteristics of this population are presented in Table 1.
Table 1. Maternal and newborn characteristics.
| Maternal characteristics | Mother-newborn dyads, n = 27 |
|---|---|
| Sociodemographic | |
| Age (y), mean ± SD | 24.0 ± 3.5 |
| Race/ethnicity | |
| Non-Hispanic white | 37% (n = 10) |
| African American | 63% (n = 17) |
| Annual family income | |
| <$10,000 | 44% (n = 12) |
| $10,000-25,000 | 30% (n = 8) |
| >$25,000 | 26% (n = 7) |
| Pregnancy | |
| Presence of obstetric risk condition | 33% (n = 9) |
| Multiparous | 100% (n = 27) |
| Newborn characteristics | |
| Sex (female) | 52% (n = 14) |
| Gestational age at birth (wk), mean ± SD | 38.8 ±1.4 |
| Birthweight (g), mean ± SD | 3290 ± 460 |
Measures
Pregnancy-specific stress
At enrollment (on average at 9.2 weeks' gestation), a 4-item pregnancy-specific stress scale was administered. This scale was adapted from a 10-item version that was specifically developed for use in pregnancy research.36 It assesses the major constructs that encompass pregnancy-specific stress, ie, feelings about being pregnant and concerns about the health of the unborn baby and about labor and delivery. Responses were provided on a 3-point scale (0-2); thus the total score could range from 0–8. This pregnancy-specific stress measure has previously been shown to be a better predictor of adverse birth and child developmental outcomes than other nonpregnancy-specific (or general) stress measures.37
Obstetric risk, length of gestation, birthweight, and infant sex
Obstetric risk was defined as the presence of major medical complications in the index pregnancy, ie, gestational diabetes, vaginal bleeding, placenta abruptio, pregnancy-induced hypertension, preeclampsia, or infection. Risk conditions were ascertained by medical chart review and coded as a binary variable (presence or absence of obstetric risk), as previously described.38 Gestational age was determined by best obstetric estimate with a combination of last menstrual period and early uterine size, and was confirmed by obstetric ultrasonographic biometry <20 weeks using standard clinical criteria.39 Birthweight and infant sex were abstracted from the newborn medical record. Birthweight was adjusted for gestational age at birth before entering into the regression model.
Cord blood TL
Peripheral blood mononuclear cells (PBMCs) were isolated from cord blood using Ficoll (Sigma-Aldrich, St. Louis, MO) gradient centrifugation following established protocols.40 Whole DNA was isolated from cord blood PBMC samples using ethanol precipitation. Time from collection to processing the blood was <30 minutes. Aliquots of isolated DNA were shipped to the Blackburn Laboratory at the University of California, San Francisco, where TL assays were performed. Measurement of relative TL (telomere repeat copy number to single gene copy number [T/S] ratio) by quantitative polymerase chain reaction were adapted from a validated published method41 and performed as described previously.42 The conversion from T/S ratio to base pairs (bp) was calculated based on the mean telomeric restriction fragment length from Southern blot analysis and the slope of the plot of mean telomeric restriction fragment length vs T/S for these samples. This was expressed as the following formula: bp = 3274 + 2413 ⋆ (T/S).
Statistical analyses
Based on the Shapiro-Wilk test, TL data (T/S ratio) were normally distributed (W [Shapiro-Wilk test statistic] = 0.9676, P = .5400 for null hypothesis).
To examine the association between maternal psychosocial stress during pregnancy and the variance in newborn LTL adjusted for the effects of other potential determinants, a regression model was employed that simultaneously included the following predictors: maternal psychological stress, newborn gestational age at birth, birthweight (adjusted for gestational age), sex, and exposure to antepartum obstetric complications. Except for sex, these variables were selected a priori based on a review of the published literature on the determinants of newborn telomere biology across different tissues.29-34 Sex was also included in the model because it has been shown to be associated with adult LTL.35 Other sociodemographic (eg, race/ethnicity) and obstetric/medical (eg, preterm birth, mode of delivery) variables were not included because there was either no a priori hypotheses based on the existing literature regarding how they would influence TL, or we did not find an association with TL in our study sample. The coefficients for each predictor indicate the estimated independent effects of that predictor (ie, adjusting or controlling for the effects of the other predictors). Hence, the unstandardized regression (β) coefficient for maternal stress from this model indicates the change in the adjusted T/S ratio associated with a 1-U change in the pregnancy-specific stress score. Or, in other words, it estimates the magnitude of the effect of maternal stress on newborn TL in newborns of the same gestational age, weight, sex, and exposure to antepartum obstetric complications. Next, to determine the proportion of explained variance a Pearson correlation coefficient was calculated between maternal pregnancy-specific stress and the T/S ratio residualized for newborn gestational age at birth, birthweight (adjusted for gestational age), sex, and exposure to antepartum obstetric complications. Finally, to express the effect size in units of SD change we divided the study sample in 2 groups based on a median split on the pregnancy-specific stress score and then calculated Cohen's d statistic43 based on the difference between the 2 groups.
All statistical analyses were run using SPSS 18 (SPSS, Inc, Cary, NC), and the statistical significance level was set at α = 0.05.
Results
The mean cord blood TL was 2.50 ± 0.37 (mean ± SD) T/S ratio (equivalent to 9314 ± 887 bp), which is comparable to previous reports.44
The mean pregnancy-specific stress score was 2.40 ± 1.90 (SD) and ranged between 0–7.
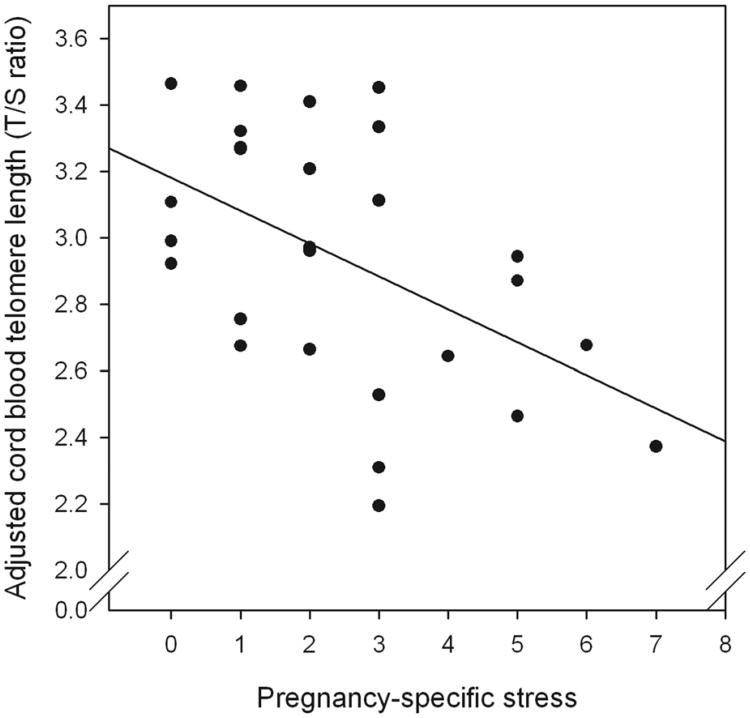
As hypothesized, after accounting for the effects of other potential determinants of newborn LTL (gestational age at birth, weight, sex, and exposure to antepartum obstetric complications), there was a significant, independent, linear effect of pregnancy-specific stress on new born LTL (β = −0.099; P = .04) (Table 2). This effect accounted for 25% (R2 = 0.25) of the variance in adjusted LTL (Figure). Based on a median split by pregnancy-specific stress, there was a 540-bp difference in newborn TL between mothers in the high- vs low-stress groups, which translates to a difference of 0.62 SD units (Cohen's d). We note that the unadjusted relationship between pregnancy-specific stress and newborn TL was not statistically significant (β = −0.062, P = .10).
Table 2. Fully adjusted regression model predicting newborn (cord blood) leukocyte telomere length (T/S ratio).
| Variable | Unstandardized β (95% CI) | SE of β | Significance (P value) | Standardized β | VIF |
|---|---|---|---|---|---|
| Pregnancy-specific stress | −l0.099 (−0.197 to −0.002) | 0.047 | .04 | −0.516 | 1.571 |
| Gestational age at birth | −0.008 (−0.124 to 0.107) | 0.056 | .88 | −0.033 | 1.268 |
| Birthweight (adjusted for gestational age) | −0.007 (−0.026 to 0.011) | 0.009 | .42 | −0.208 | 1.722 |
| Infant sex (0 = male, 1 = female) | 0.046 (−0.286 to 0.379) | 0.160 | .77 | 0.064 | 1.312 |
| Antepartum obstetric complications (0 = absent; 1 = present) | −0.218 (−0.554 to 0.118) | 0.162 | .19 | −0.285 | 1.190 |
CI, confidence interval; T/S, telomere repeat copy number to single gene copy number; VIF, variance inflation factor.
Figure. Pregnancy-specific stress and newborn cord blood telemore length.
Scatterplot of association between maternal pregnancy-specific stress and newborn (cord blood) telomere length (R2 = 0.25). T/S ratio is adjusted for covariates (newborn gestational age at birth, weight, sex, and exposure to antepartum obstetric complications).
T/S, telomere repeat copy number to single gene copy number.
Comment
To the best of our knowledge, the current finding represents the first report in human beings to suggest that the effects of prenatal stress exposure on cellular aging may begin during intrauterine life and may already be evident at the time of birth. After accounting for the potential effects of gestational age at birth, weight, sex, and exposure to antepartum obstetric complications, maternal pregnancy-specific psychosocial stress was significantly and independently associated with newborn LTL. There was a 540-bp difference in newborn TL between mothers in the high- vs low-stress groups (based on a median split), which translates to a difference of 0.62 SD units. Extrapolations of available data from studies in adults of clinical conditions and adult TL were performed by converting TL bp differences to SD units (Cohen's d statistic) by dividing the difference between the 2 groups by the pooled SD of the whole group. These extrapolations suggest the magnitude of the effect in the current report is equivalent to or greater than that of smoking, obesity, diabetes, or hypertension on adult TL.16,45-49 We note, however, that LTL is highly variable in our newborn cohort, which is consistent with previous reports.44,50 Thus, the clinical significance of this effect remains to be determined in future longitudinal studies. Because the initial (newborn) setting of TL is one of the key 2 determinants of subsequent TL17 (the other major determinant being rate of TL attrition over time), and because adult TL is, in turn, a determinant of aging, disease risk and mortality, it is therefore plausible that variations in newborn TL may confer clinically meaningful differential risks for subsequent TL-related health outcomes. Nevertheless, given the relatively small sample size, conclusions are preliminary and independent replication of these results is warranted.
The present finding extends our earlier work on prenatal psychosocial stress exposure and TL in young adults.25 Evidence from animal and human studies linking other adverse conditions during fetal development with subsequent TL provides further biological plausibility for this relationship.32,34,51-53 For example, a recent study in chickens found that exposure to high levels of yolk corticosterone, a potent stress hormone, is associated with short telomeres in the offspring.54 Yet, other studies in human beings have found associations between exposure to a variety of adverse conditions in infancy and childhood with subsequent TL.55-61
In terms of biological mechanisms, prenatal psychosocial stress exposure could affect fetal telomere biology through several interrelated biological pathways: maternal stress induces the release of maternal and placental hormones (eg, cortisol, placental corticotrophin-releasing hormone) and of inflammatory and oxidative stress mediators that can enter the fetal circulation or induce changes in placental physiology including alterations in blood flow and metabolism.27,62 These changes, in turn, may impact processes underlying the initial setting and regulation of telomerase activity and TL via many of the same epigenetic and other phenomena that regulate constitutive gene expression across various cell and tissue types.63
It is possible that an alternate explanation for the observed effect is that maternal stress may preferentially alter the distribution of cell populations among leukocytes such that cells with longer TL are reduced under conditions of stress. However, although the analysis of TL in the current study was restricted to average newborn cord white blood cells (PBMCs), the findings may be applicable to other cell types and tissues. Previous studies have reported that in newborns TL is highly concordant between cord white blood cells and other tissues,44 and correlations of TL are very high across different hematopoietic cells.64
Although we statistically controlled for other potential determinants of newborn TL, the possibility of residual confounding cannot be excluded. Furthermore, we suggest that it also is important in this context to address the effects of potential interactions because the effects of stress likely occur in the context of other conditions such as biophysical, medical, nutritional, and behavioral factors.37
Among the various components of psychosocial stress previous studies have established that pregnancy-specific stress (the measure selected by us in the present study) seems to best capture those aspects of stress most closely and consistently related to pregnancy and birth outcomes.37,65 The use of biological measures of stress in conjunction with the psychosocial measures would have further strengthened the study, however, biological stress measures were not available in the current cohort.
Thus, in addition to replicating this finding in an independent sample, we suggest that future studies should examine interactions between maternal stress and other factors such as obstetric risk conditions during pregnancy and adverse birth outcomes; putative biological mediators of the effect of maternal stress on newborn TL such as stress-related endocrine, immune, and oxidative state measures; the complementary role of variation in telomerase expression; and potential epigenetic and other mechanisms of transduction.
In summary, our finding provides the first preliminary evidence in human beings that maternal psychological stress may exert a “programming” effect on the newborn telomere biology system. Given the critical importance of the initial setting of TL for subsequent health, disease risk, aging, and longevity-related outcomes, it is plausible that in utero telomere biology represents a molecular mechanism whereby stress exposure in this critical period before birth can impact aging and subsequent health and disease susceptibility over the lifespan.
Supplementary Material
Acknowledgments
Supported by US Public Health Service (National Institutes of Health) grants R01 HD-060628 and P01 HD-047609 (P.D.W.), HD-065825 (S.E.), and R01 HD-041663 and R01 HD-052732 (H.N.S.).
Footnotes
E.H.B., J.L., and E.S.E. are cofounders of Telomere Health Inc, a company focused on telomere measurement. The conduct of assays and all other research activities for the current report are, however, unrelated to this company. The other coauthors report no potential conflict of interest.
Contributor Information
Dr Sonja Entringer, Department of Pediatrics, University of California, Irvine, School of Medicine, Irvine, CA.
Dr Elissa S. Epel, Department of Psychiatry, University of California, San Francisco, School of Medicine, San Francisco, CA.
Dr Jue Lin, Department of Biochemistry and Biophysics, University of California, San Francisco, School of Medicine, San Francisco, CA.
Dr Claudia Buss, Department of Pediatrics, University of California, Irvine, School of Medicine, Irvine, CA.
Dr Babak Shahbaba, Department of Statistics, University of California, Irvine, School of Medicine, Irvine, CA.
Dr Elizabeth H. Blackburn, Department of Biochemistry and Biophysics, University of California, San Francisco, School of Medicine, San Francisco, CA.
Dr Hyagriv N. Simhan, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Dr Pathik D. Wadhwa, Departments of Pediatrics, Psychiatry and Human Behavior, Obstetrics and Gynecology, and Epidemiology, University of California, Irvine, School of Medicine, Irvine, CA.
References
- 1.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2010;17:507–16. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 4.Moyzis RK, Buckingham JM, Cram LS, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85:6622–6. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–10. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaskelioff M, Muller FL, Paik JH, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–6. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–65. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epel ES, Merkin SS, Cawthon R, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2009;1:81–8. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 11.Kimura M, Hjelmborg JV, Gardner JP, et al. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H, Belcher M, van der Harst P. Healthy aging and disease: role for telomere biology? Clin Sci (Lond) 2011;120:427–40. doi: 10.1042/CS20100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 14.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–3. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 15.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 16.Benetos A, Gardner JP, Zureik M, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–5. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 17.Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63:979–83. doi: 10.1093/gerona/63.9.979. [DOI] [PubMed] [Google Scholar]
- 18.Codd V, Mangino M, van der Harst P, et al. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42:197–9. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prescott J, Kraft P, Chasman DI, et al. Genome-wide association study of relative telomere length. PLoS One. 2011;6:e19635. doi: 10.1371/journal.pone.0019635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy D, Neuhausen SL, Hunt SC, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107:9293–8. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damjanovic AK, Yang Y, Glaser R, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179:4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphreys J, Epel ES, Cooper BA, Lin J, Blackburn EH, Lee KA. Telomere shortening in formerly abused and never abused women. Biol Res Nurs. 2012;14:115–23. doi: 10.1177/1099800411398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks CG, Miller DB, McCanlies EC, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–60. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Entringer S, Epel ES, Kumsta R, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108:E513–8. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Entringer S, Buss C, Swanson JM, et al. Fetal programming of body composition, obesity, and metabolic function: the role of intrauterine stress and stress biology. J Nutr Metab. 2012;2012:632548. doi: 10.1155/2012/632548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–43. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 29.Friedrich U, Schwab M, Griese EU, Fritz P, Klotz U. Telomeres in neonates: new insights in fetal hematopoiesis. Pediatr Res. 2001;49:252–6. doi: 10.1203/00006450-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Biron-Shental T, Sukenik Halevy R, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A. Telomeres are shorter in placental trophoblasts of pregnancies complicated with intrauterine growth restriction (IUGR) Early Hum Dev. 2010;86:451–6. doi: 10.1016/j.earlhumdev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Biron-Shental T, Kidron D, Sukenik-Halevy R, et al. TERC telomerase subunit gene copy number in placentas from pregnancies complicated with intrauterine growth restriction. Early Hum Dev. 2011;87:73–5. doi: 10.1016/j.earlhumdev.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Davy P, Nagata M, Bullard P, Fogelson NS, Allsopp R. Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta. 2009;30:539–42. doi: 10.1016/j.placenta.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izutsu T, Kudo T, Sato T, et al. Telomerase activity in human chorionic villi and placenta determined by TRAP and in situ TRAP assay. Placenta. 1998;19:613–8. doi: 10.1016/s0143-4004(98)90022-4. [DOI] [PubMed] [Google Scholar]
- 34.Menon R, Yu J, Basanta-Henry P, et al. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 2012;7:e31136. doi: 10.1371/journal.pone.0031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aviv A. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J Mol Med (Berl) 2002;80:689–95. doi: 10.1007/s00109-002-0377-8. [DOI] [PubMed] [Google Scholar]
- 36.Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999;18:333–45. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 37.Wadhwa PD, Entringer S, Buss C. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 2011;38:351–84. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wadhwa PD, Garite TJ, Porto M, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–9. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–5. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- 40.Ulmer AJ, Scholz W, Ernst M, Brandt E, Flad HD. Isolation and subfractionation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation on Percoll. Immunobiology. 1984;166:238–50. doi: 10.1016/S0171-2985(84)80042-X. [DOI] [PubMed] [Google Scholar]
- 41.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraemer HC, Morgan GA, Leech NL, Gliner JA, Vaske JJ, Harmon RJ. Measures of clinical significance. J Am Acad Child Adolesc Psychiatry. 2003;42:1524–9. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr Res. 2002;52:377–81. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Zee RY, Castonguay AJ, Barton NS, Germer S, Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl Res. 2010;155:166–9. doi: 10.1016/j.trsl.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29:283–9. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 47.Bhupatiraju C, Saini D, Patkar S, Deepak P, Das B, Padma T. Association of shorter telomere length with essential hypertension in Indian population. Am J Hum Biol. 2012;24:573–8. doi: 10.1002/ajhb.22264. [DOI] [PubMed] [Google Scholar]
- 48.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham heart study. Aging Cell. 2006;5:325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 49.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 50.Akkad A, Hastings R, Konje JC, Bell SC, Thurston H, Williams B. Telomere length in small-for-gestational-age babies. BJOG. 2006;113:318–23. doi: 10.1111/j.1471-0528.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 51.Tarry-Adkins JL, Chen JH, Smith NS, Jones RH, Cherif H, Ozanne SE. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 2009;23:1521–8. doi: 10.1096/fj.08-122796. [DOI] [PubMed] [Google Scholar]
- 52.Tarry-Adkins JL, Martin-Gronert MS, Chen JH, Cripps RL, Ozanne SE. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J. 2008;22:2037–44. doi: 10.1096/fj.07-099523. [DOI] [PubMed] [Google Scholar]
- 53.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett. 1999;448:4–8. doi: 10.1016/s0014-5793(99)00336-1. [DOI] [PubMed] [Google Scholar]
- 54.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc Biol Sci. 2012;279:1447–56. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67:531–4. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life care-giving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Donovan A, Epel E, Lin J, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–71. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drury SS, Theall K, Gleason MM, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry. 2012;17:719–27. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry. 2010;68:e21–4. doi: 10.1016/j.biopsych.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kananen L, Surakka I, Pirkola S, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Surtees PG, Wainwright NW, Pooley KA, et al. Life stress, emotional health, and mean telomere length in the European prospective investigation into cancer (EPIC)-Norfolk population study. J Gerontol A Biol Sci Med Sci. 2011;66:1152–62. doi: 10.1093/gerona/glr112. [DOI] [PubMed] [Google Scholar]
- 62.Huizink AC, Mulder EJ, Buitelaar JK. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull. 2004;130:115–42. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- 63.Blasco MA. The epigenetic regulation of mammalian telomeres. Nat Rev Genet. 2007;8:299–309. doi: 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- 64.Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Exp Hematol. 2010;38:854–9. doi: 10.1016/j.exphem.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–58. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.