Abstract
The multidrug ATP-binding cassette, subfamily G, 2 (ABCG2) transporter was recently identified as an important human urate transporter, and a common mutation, a Gln to Lys substitution at position 141 (Q141K), was shown to cause hyperuricemia and gout. The nature of the Q141K defect, however, remains undefined. Here we explore the Q141K ABCG2 mutation using a comparative approach, contrasting it with another disease-causing mutation in an ABC transporter, the deletion of Phe-508 (ΔF508) in the cystic fibrosis transmembrane conductance regulator (CFTR). We found, much like in ΔF508 CFTR, that the Q141K mutation leads to instability in the nucleotide-binding domain (NBD), a defect that translates to significantly decreased protein expression. However, unlike the CFTR mutant, the Q141K mutation does not interfere with the nucleotide-binding domain/intracellular loop interactions. This investigation has also led to the identification of critical residues involved in the protein–protein interactions necessary for the dimerization of ABCG2: Lys-473 (K473) and Phe-142 (F142). Finally, we have demonstrated the utility of using small molecules to correct the Q141K defect in expression and function as a possible therapeutic approach for hyperuricemia and gout.
Keywords: uric acid, BCRP, VRT-325
Uric acid in human blood is three to four times higher than in other mammals because of a loss of function in the uricase enzyme, the enzyme necessary to metabolize urate into allantoin (1, 2). The unusually high levels of uric acid in humans are close to the solubility threshold. Thus, when uric acid levels are further elevated (hyperuricemia), from a purine-rich diet or from genetic dysfunction, uric acid may precipitate as monosodium urate crystals accumulating in the joints and causing gout (2). Hyperuricemia also leads to kidney stones, chronic kidney disease (3), and hypertension (4) and may contribute to metabolic disorders (1, 5) and cardiovascular disease (6–8). The multifactorial nature of hyperuricemia and gout made discovering the underlying genes critical for urate handling difficult, and it was not until the use of genome-wide association studies that important urate transport molecules were characterized. Genome-Wide Association Studies (GWAS) suggested two previously unknown urate transporter genes, solute carrier family 2, member 9 (SLC2A9) and ATP-binding cassette, subfamily G, 2 (ABCG2), as having an outsized genetic contribution to regulating blood urate levels: SLC2A9 for reabsorption of urate and ABCG2 for urate excretion (9). In our previous work, we identified ABCG2 as a human urate efflux transporter physiologically important for hyperuricemia and gout. Additionally, we characterized the single nucleotide polymorphism resulting in a Gln to Lys substitution at position 141 (Q141K), as causal for gout resulting from a loss of urate transport function in ABCG2 (10).
The majority of work on ABCG2 [also know as the breast cancer resistance protein (BCRP)] has focused on its role as a multidrug transporter and specifically on its role as a xenotoxin and chemotherapeutic transport molecule, work that has included a description of the Q141K mutation’s effect on ABCG2 trafficking and function. Multiple groups reported that the Q141K mutation decreases protein expression and, coincidentally, xenotoxin transport function (11–16). This decrease of expression and function appears to be partially caused by the enhancement of the Q141K mutant’s susceptibility to proteasomal degradation (11–13). There is also recent evidence suggesting that a significant portion of the mutant Q141K protein accumulates in perinuclear aggresomes before being degraded (17). Conversely, the Q141K mutation has also been reported to reduce ABCG2 ATPase activity, suggesting that the mutation disrupts xenotoxin transport independently of expression or trafficking abnormalities (14, 16). However, how does the Q141K mutation affect urate transport? In our work, we found urate transport was significantly reduced by the Q141K mutation in Xenopus oocytes when transport was normalized to expression levels (10). In contrast, subsequent work by Matsuo et al. (18) confirmed ABCG2 as a urate transporter and that Q141K results in a loss of function. However, they postulated that the loss of urate transport function for the Q141K was specifically due to the reduction in protein expression (18) . Interestingly, the possibility that the Q141K mutation disrupts both function and trafficking/expression suggests intriguing parallels to other human disease causing ABC transporter mutations, namely the deletion of Phe-508 (ΔF508) in the cystic fibrosis transmembrane conductance regulator (CFTR). The position of the Phe-508 in CFTR is in a crucial structural area for the interactions between the soluble portion of the molecule and the membrane portion, an area known to be a mutational hotspot in ABC genes that cause human disease (19–21). However, it remains unclear why the Q141K mutation causes increased ubiquitination and proteasomal degradation or accumulation in aggresomes.
In this paper, we explore the nature of the Q141K defect and, specifically, its effect on expression in mammalian cells. By using a comparative approach to other ABC transporters, we identify the importance of the Q141 residue in nucleotide-binding domain (NBD) stability. Furthermore, we demonstrate that ABCG2 dimerization and expression is heavily dependent on interdomain interactions of separate ABCG2 molecules centered on the Phe-142 (F142) and Lys-473 (K473) residues, akin to the F508/Arg-1070 (R1070) interactions of CFTR. Finally, we demonstrate that correcting the gout causing Q141K ABCG2 defect can be accomplished with small molecules in a manner similar to the successful correction of the CFTR molecule as a treatment for cystic fibrosis.
Results
Temperature Correction of Q141K.
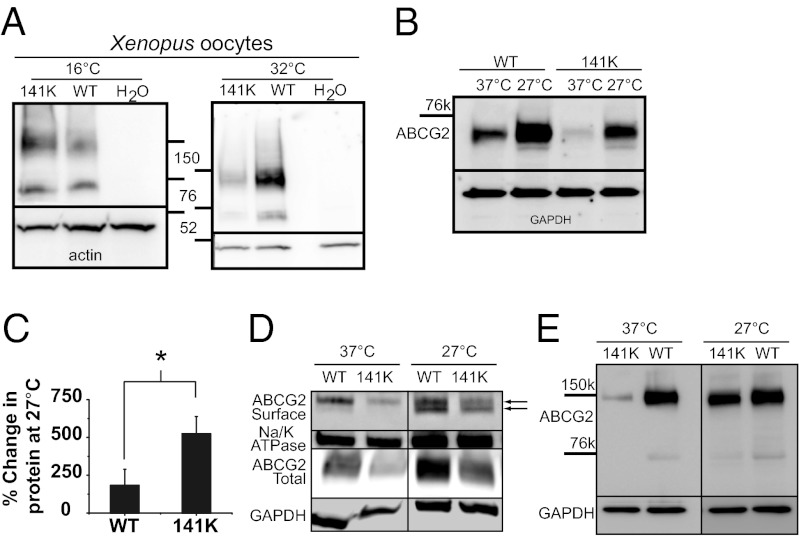
Previously, we showed that the Q141K mutation in ABCG2 was causal for gout and resulted in a 53% loss of urate transport function (10, 22). However, in our heterologous Xenopus expression system we did not observe a reduction in protein expression levels in the mutant protein (Fig. 1A), unlike what had been reported previously (12, 13, 15, 18). To investigate the opposing observations and to explore the possibility that both a loss of function and a expression defect could be caused by the Q141K mutation, we expressed our ABCG2 constructs in mammalian cell expression systems. We found, much like previous reports (12, 13, 15, 18), that, in multiple mammalian cell lines, transient expression of the Q141K mutation resulted in significantly less total protein (Fig. S1 A and B and Fig. 2D: 63.4 ± 0.1% decrease), surface protein (Fig. 1D and Fig. S1 C and D), and function (Fig. S1E: 63.08 ± 3.0% decrease) than wild-type ABCG2 [although with only slightly lower levels of unglycosylated protein (Fig. S2A): 27.3 ± 0.1% reduction; see Fig. S2B for ABCG2 glycosylation states]. In both the oocytes and mammalian cell lines, we used the same human ABCG2 constructs; thus the differences in relative expression may give a clue as to the nature of the Q141K defect. The oocyte incubation temperature is significantly lower than for mammalian cells, a property that may help fold and stabilize protein and protect it from endoplasmic reticulum-associated degradation (ERAD). To test the possibility that the Q141K mutant is being stabilized by the low incubation temperatures of the oocytes, we injected oocytes with mutant and wild-type ABCG2 mRNA and incubated the cells at either 16 °C or 32 °C (Fig. 1A). At 32 °C, there was an observable difference between the protein expression levels of the mutant and wild-type protein.
Fig. 1.
Temperature correction of Q141K ABCG2 expression defect. (A) ABCG2 expression in X. laevis oocytes, 3 d after injection, and incubated at 16 °C or 32 °C (representative of three experiments). (B) Western blot of transient expression of wild-type (WT) and Q141K ABCG2 in CHO cells incubated for 48 h at 27 °C or 37 °C. (C) Summary data of temperature rescue of protein expression (WT: n = 6; Q141K: n = 8). (D) Surface expression of biotinylated ABCG2 protein compared with Na+/K+ ATPase expression and total ABCG2 expression; black arrows identify the mature (Top) and immature unglycosylated (Bottom) form of ABCG2 protein (n = 4). (E) Dimer expression of WT and Q141K ABCG2 after 48 h of incubation at 27 °C or 37 °C (n = 4). Actin expression was used as a loading control for Xenopus oocyte Western blots; GAPDH expression was used as a loading control on all other Western blots. All means are ± SEM; *P < 0.05.
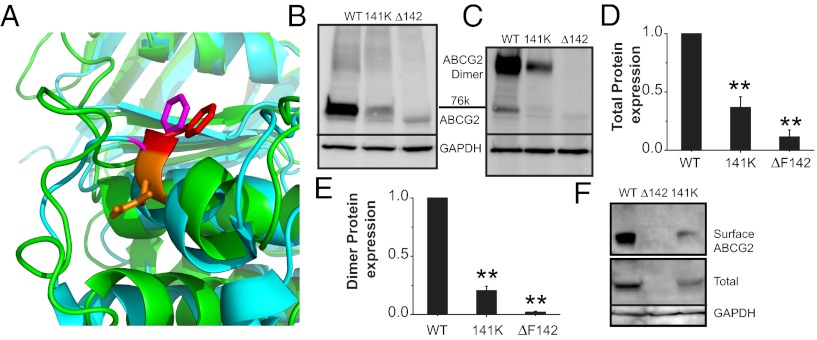
Fig. 2.
Q141K mutation occurs in mutational hotspot of ABC protein NBD. (A) Zoom-in view of superimposition of ABCG2 model (green) and human CFTR NBD1 crystal [cyan, Protein Data Bank (PDB) ID: 2PZE]. Orange, ABCG2 Q141; red, ABCG2 F142; magenta, CFTR F508. Western blots of transient total (B) and dimer expression (C) of WT, Q141K, and ΔF142 ABCG2 in HEK293 cells. Summary data of total expression of ABCG2 variants (D; n = 7 for each) and dimer expression (E; n = 6 for each). (F) Western blot of biotinylated surface and total ABCG2 expression. All means are ± SEM; **P < 0.01.
We next tested the reverse hypothesis: lowering the incubation temperature of the mammalian cells may rescue mutant protein expression. Fig. 1 B and C shows that the lowering of the incubation temperature of mammalian cells expressing wild-type ABCG2 and the Q141K mutant increases the expression of both; however, the percentage change in expression level is significantly greater in the mutant protein (Fig. 1C), a result consistent with low-temperature enhancement of folding and stabilizing mutant protein. Interestingly, the low temperature increases the lower unglycosylated wild-type and mutant ABCG2 bands (22–24) as well as the mature protein (Fig. S2C), suggesting that, although low temperature can rescue total mutant protein, it does not recover normal processing. Low temperature was also able to rescue the surface expression of the Q141K mutant (Fig. 1D), but again the majority of the rescued surface protein is the unglycosylated version of the protein (Fig. 1D, black arrows). Finally, we looked at the effect of low temperature on the dimerization of ABCG2. Litman et al. (24) demonstrated that removing the reducing agent from the running buffer preserves ABCG2 dimerization and allows it to be easily visualized on a Western blot. We found that the low-temperature incubation again preferentially increased the dimer mutant Q141K protein expression compared with wild type (Fig. 1E: dimer Q141K expression increased 242.98 ± 89.8% relative to wild type; n = 3). These results support the hypothesis that the Q141K mutant is unstable and that expression can be rescued with low-temperature incubation.
Nature of the Q141K Defect.
Human disease, loss of function, reduced expression of mature protein, and temperature correction are characteristics that the Q141K mutation shares with exactly one other mutant ABC protein: ΔF508 CFTR, the most common cystic fibrosis-causing mutation (25). An alignment of the ABCG2 and CFTR proteins shows that both disease-causing mutations are in a homologous region of the NBD domain (Fig. S3A). However, the deletion of F508 in CFTR produces no mature glycosylated protein (25), whereas the Q141K defect in ABCG2 only reduces the amount of mature protein; thus the defects may be similar but not precisely homologous. To probe the homologous structural relationship of the Q141 of ABCG2 and the F508 of CFTR, we constructed a model of the ABCG2 NBD domain structure using various template crystal structures (see Materials and Methods for details). Our ABCG2 homology model (Fig. S3 A–C) demonstrates that the phenylalanine (F142) neighboring the Q141 residue is homologous to F508 CFTR (Fig. 2A). To test our model, we deleted F142 (Fig. S3 B and C, red side chain) and found that the deletion of F142 resulted in the expression of no mature protein and no surface protein, but only immature unglycosylated protein (Fig. 2 B–F and Fig. S4 A and C). These results support our model and the similar natures of the defects between Q141K ABCG2 and ΔF508 CFTR. Interestingly, the F142 deletion appears to disrupt the dimerization of the ABCG2 molecules (Fig. 2 C and E), which may explain why none of the protein progresses to the Golgi for glycosylation. ABCG2, as a half-molecule, must dimerize to traffic normally (26); CFTR does not require this, which may explain why low temperature does not rescue ΔF142 expression (Fig. S2D) whereas low temperature does rescue ΔF508 CFTR expression (27, 28).
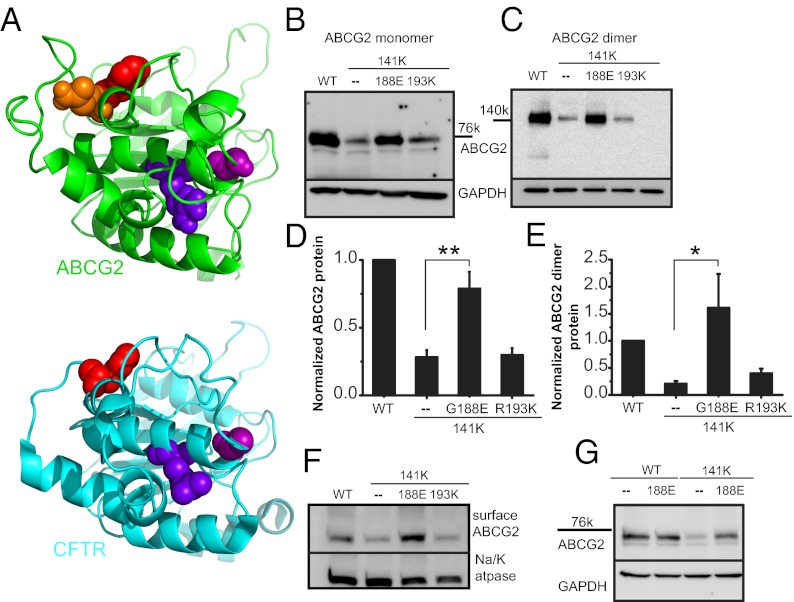
Extensive studies with other ABC molecules suggest that the region of the NBD containing the Q141 and F142 residues is important both for stabilizing the NBD domain and for the interdomain interactions between the transmembrane domains (TMDs) and the NBD domains (19–21). In CFTR, specific residues in the ABC signature sequence were found to be critical for NBD stability, and introduction of secondary suppressor mutations into the signature sequence could rescue the ΔF508 protein expression and specifically the stability of the isolated NBD1 domain (20, 21, 29–31). To test whether or not Q141K decreases stability of the ABCG2 NBD, we introduced stabilizing mutations into the signature sequence to try to rescue protein expression. We separately mutated two residues in the Q141K background—a Gly to Glu substitution at position 188 (G188E) and an Arg to Lys substitution at 193 (R193K) (homologous to the Gly-550 and Arg-555 residues of CFTR, shown in Fig. 3A)—and found that the G188E mutation acted as a suppressor mutation, significantly increased the amount of the Q141K total protein (Fig. 3 B–E and Fig. S4 E and G), and increased the dimer protein (Fig. 3 C and E and Fig. S4 F and H) and the surface protein (Fig. 3F); the R193K mutation did not act as a suppressor (P < 0.41; n = 10). The same G188E mutation introduced into the wild-type protein produced no change in expression (Fig. 3G) and did not recue the ∆F142 mutation (Fig. S2 E and F), supporting the hypothesis that the F142 deletion disrupts both stability and interdomain interactions. In contrast, stabilizing the NBD domain of Q141K ABCG2 rescues protein expression and suggests that primarily NBD stability may underlie the Q141K defect.
Fig. 3.
Suppressor mutation correction of Q141K expression defect. (A) Structural model of human ABCG2 NBD (green structure) and human CFTR NBD1 (blue structure, PDB ID: 2PZE). The NBD dimer interface is approximately on the right, and the NBD–TMD interface is approximately on the top. The following residues are shown as spheres (ABCG2/CFTR): orange, Q141; red, F142/F508; blue, R193/R555; purple, G188/G550. Western blot of transient total (B) and dimer (C) expression in CHO cells of suppressor mutations in the Q141K background. Summary data of suppressor rescue of Q141K ABCG2 total (D; n = 10) and dimer protein expression (E; n = 4). (F) Surface expression of biotinylated ABCG2 and Q141K variants and Na+/K+ ATPase, demonstrating strong rescue with the Q141K G188E mutation (n = 6). (G) The G188E mutation does not affect WT ABCG2 protein expression (n = 3). All means are ± SEM; *P < 0.05; **P < 0.01.
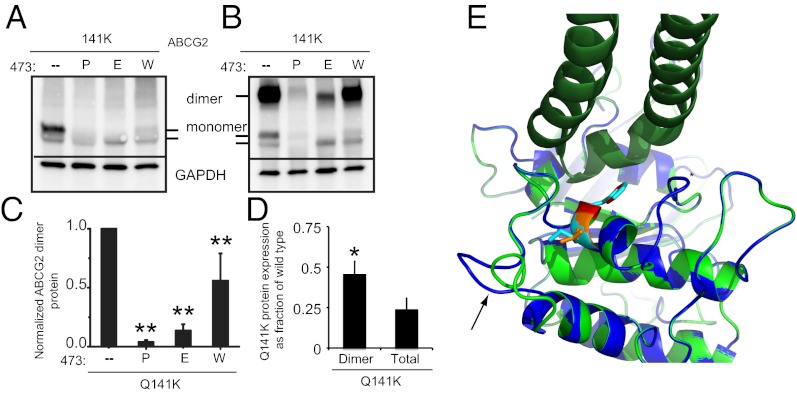
Even with considerable evidence suggesting that Q141K causes NBD instability, there remains the possibility that interdomain interactions may also be disrupted, as in the ΔF142 mutant and in the CFTR F508 deletion. The positively charged residues in intracellular loop 4 (ICL4) of CFTR are critical for these interdomain interactions, specifically for the R1070 residue that, when mutated to 1070W, can cause disease in the wild-type background but acts as a suppressor mutation for the F508 deletion (20, 30). Without the homology required for an alignment, our search for ABCG2 residues homologous to CFTR R1070 relied on finding ABCG2 residues with comparable characteristics. In the loops of ABCG2, there is only one positively charged residue, K473 (22), which is surrounded by hydrophobic residues, making it a prime target. Substitution of K473 in the wild-type ABCG2 with either proline or tryptophan—substitutions that at R1070 CFTR cause human disease (32)—disrupted the amount of total and dimer protein levels (Fig. S5 A–D). The reversal of charge with a K473E substitution also resulted in reduced expression and dimerization (Fig. S5 A–D). Both findings are consistent with the K473 residue being functionally homologous to the R1070 of CFTR. Significantly, introduction of the K473W substitution on the Q141K background did not rescue total, mature, or dimer levels (all were actually significantly less) (Fig. 4 A–C and Fig. S5E). The failure of the K473W mutation to rescue Q141K expression supports the hypothesis that the Q141K mutation does not interfere with interdomain interactions or dimerization of the ABCG2 protein. Furthermore, we found that the Q141K dimer protein expression, as a fraction of wild-type expression, is greater than the total expression (Fig. 4D), not less, suggesting that the mutant dimerizes normally (or marginally more efficiently than wild type; P ≤ 0.03; n = 7). Finally, modeling of ABCG2 with the Q141K substitution did not alter the F142 orientation or position in respect to the ICL residues, and K141 adopted the same orientation as Q141 (Fig. 4E). Interestingly, the presence of lysine is associated with a shifting outward of an adjacent loop (Fig. 4E, black arrow), which may disrupt hydrophobic packing and stability. However, it should be noted that side-chain orientation was limited by the template and derived from energy minimization and needs to be interpreted with caution. Taken together, our findings support the hypothesis that the Q141K mutation does not interfere with interdomain interactions or with dimerization of the ABCG2 protein.
Fig. 4.
Q141K does not disrupt interdomain interactions or dimerization. Western blots of total (A) and dimer (B) expression of Q141K ABCG2 with secondary mutations at K473 demonstrating that K473W cannot rescue Q141K expression. Summary data of K473 mutation on total (C; n = 4–6) Q141K expression. (D) Expression of total or dimer Q141K as a fraction of wild type (total or dimer); a greater proportion of total Q141K exists as a dimer than does wild type (n = 7). (E) Superimposition of ABCG2 NBD model (green, with Q141 as orange sticks and F142 as red sticks) and the model with Q141K substitution (blue, with K141 and F142 as cyan sticks). The Sav1866 crystal (PDB ID: 2HYD) was also superimposed, and one of its intracellular loops (dark green) was shown to indicate possible NBD–TMD interface mediated by F142. All means are ± SEM; *P < 0.05; **P < 0.01.
Small-Molecule Correction of Q141K.
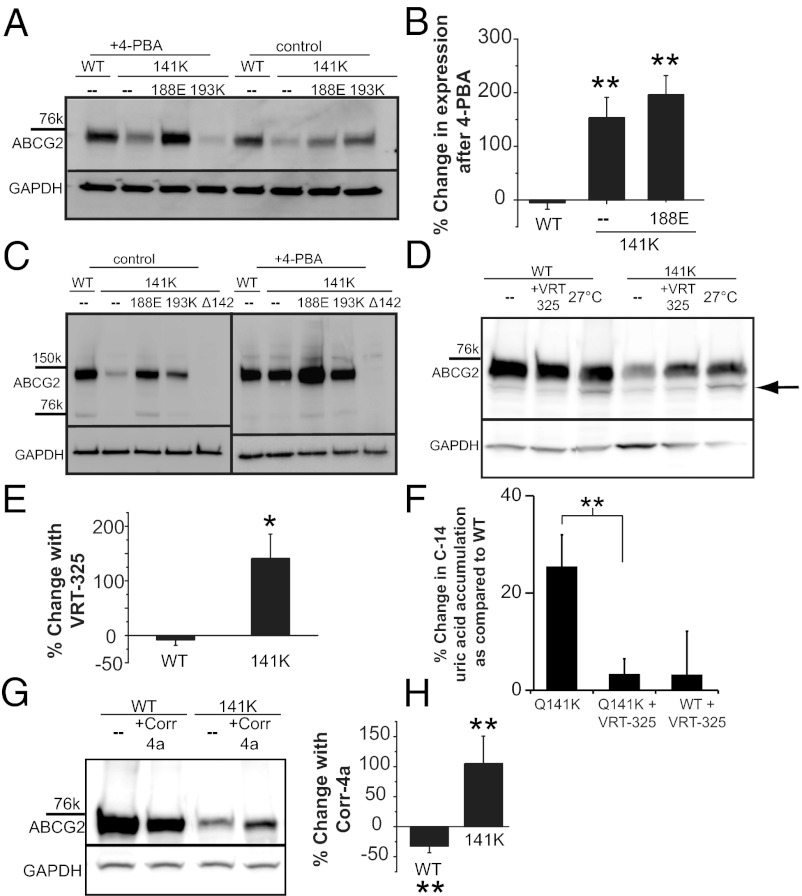
Recent advances in the understanding of how proteins are trafficked or what happens when misfolding occurs have led to the discovery of a large number of small molecules that can promote proper folding and chaperone a misfolded protein past the ERAD detection system, allowing the mutant protein to reach its intended destination. These molecules, when used to correct disease-causing trafficking mutations, represent a possible therapeutic strategy. As a demonstration of this principle and to learn more about the mechanism of dysfunction in the Q141K ABCG2 mutation, we attempted to correct the Q141K defect with small molecules. The histone deacetylase (HDAC) inhibitor 4-phenylbutyrate (4-PBA) rescues trafficking defects in other ABC proteins (33, 34) by increasing the HSP70 chaperone protein, thereby protecting misfolded proteins from being targeted to the ERAD pathway. We found that application of 4-PBA increased significantly the expression of the Q141K protein (Fig. 5 A and B) but had no effect on the wild type, consistent with the hypothesis that the ERAD pathway targets the Q141K protein. The Q141K monomer expression levels, even after correction, were not at levels comparable to the wild type (Fig. 5A). Conversely, the dimer form of the Q141K protein when treated with 4-PBA was corrected to expression levels not different from wild type (P < 0.147, n = 4), suggesting that the effect of 4-PBA is critical for the creation of the dimer form of the protein (Fig. 5C). Interestingly, we found that 4-PBA was also able to increase the amount of Q141K/G188E protein (Fig. 5 A–C), demonstrating that the G188E suppressor mutation is not a full rescue and that some protein is still targeted for degradation.
Fig. 5.
Small-molecule correction of gout-causing Q141K ABCG2 expression defect. (A) Western blot of ABCG2 variant expression in HEK293 cells demonstrating the efficacy of a 48-h treatment with 6 mM 4-PBA to correct mutant expression. (B) Summary data of 4-PBA rescue of Q141K expression (n = 3–6); 4-PBA has no effect on WT ABCG2 expression (P < 0.28; n = 4) compared with zero. (C) Western blots of ABCG2 variant dimer expression demonstrating efficacy of 48-h treatment with 6 mM 4-PBA to correct mutant dimer expression (n = 4). (D) Expression of WT and mutant ABCG2 in CHO cells and rescue with 24-h treatment with 10 µM VRT-325 or 27 °C incubation. Black arrow highlights the increase of unglycosylated protein with 27 °C incubation. (E) Summary data demonstrating efficacy of 24-h, 10-µM VRT-325 treatment for the rescue of mutant ABCG2 expression (n = 4). (F) Functional assay of ABCG2 function: percentage increase in accumulation of C-14 labeled uric acid in 2 h compared with WT accumulation (n = 5–11); higher accumulation represents lower efflux rates. (G) Western blot and summary data (H) of total Q141K ABCG2 expression rescue by 24-h treatment with 20 µM corr-4a (n = 4). GAPDH or actin expression used as a loading control on Western blots. All means are ± SEM; *P < 0.05; **P < 0.01.
The 4-PBA molecule is unlikely to interact directly with the ABCG2 protein, but instead alters the ERAD machinery, which, although instructive, is not an ideal therapeutic target. We next used the corrector molecule VRT-325, originally discovered in a high-throughput screen to identify molecules that would correct mutant CFTR (35, 36) and has subsequently been shown to bind directly to CFTR (37). The mutations that VRT-325 corrected in ABCB1 (P-glycoprotein) and CFTR were specific homologous mutations in the NBD; thus, we hypothesized that VRT-325 may stabilize the highly conserved NBDs of multiple ABC proteins including ABCG2. We found that incubation with the VRT-325 molecule significantly increased the amount of total (Fig. 5 D and E) and surface Q141K protein expression (Fig. S6A); VRT-325 had no effect on wild-type ABCG2 expression (P < 0.193, n = 3). Both low temperature and VRT-325 correction of Q141K produced increased levels of mature glycosylated monomer protein (Fig. 5D); however, unlike the low-temperature correction, VRT-325 did not increase the amount of immature unglycosylated protein (Fig. 5D and Fig. S6B). This suggests that VRT-325 may allow more of the Q141K protein to traffic normally, become glycosylated, and reach the cell membrane. In HEK293 cells expressing the Q141K mutant and treated with VRT-325, we also found that the accumulation of C-14–labeled uric acid was significantly less than in the untreated Q141K-expressing cells (Fig. 5F; P ≤ 0.004; n = 11, 10), demonstrating partial rescue of function along with expression of the Q141K mutant. Another small-molecule corrector found in CFTR screens was corr-4a (38), which, unlike VRT-325, does not interact directly with or correct P-glycoprotein mutations (37), making it possibly more specific to CFTR. Surprisingly, corr-4a (Fig. 5 G and H) increased the total protein levels of the Q141K ABCG2 mutant, but not wild-type protein, although the corr-4a (20 µM) correction appears to be less than the VRT-325 (10 µM) correction (Fig. S6C). Taken together, our results show that the Q141K protein level can be rescued using small corrector molecules, which is consistent with the hypothesis that the Q141K mutation is a misfolding mutation that increases degradation and consistent with the principle that the gout-causing Q141K mutation can be corrected with small molecules.
Discussion
Identifying ways to increase ABCG2 expression or therapies to correct mutations in ABCG2 is a new prospect for researchers. For decades, ABCG2 has been targeted for inhibition to aid in the effectiveness of chemotherapeutics and loss-of-function mutations as something to exploit. However, the findings that ABCG2 plays a significant physiological role in urate excretion and blood type characterization (39, 40), and that loss of function causes human diseases warrants a greater focus on ABCG2's specific role in human disease. Seeing ABCG2 as an important urate transporter and the Q141K mutation as a loss-of-function mutation led naturally to a comparison with other disease-causing mutations in human ABC transporters, a comparison that lead inevitably to CFTR. The parallels between the Q141K mutation in ABCG2 and the deletion of the Phe-508 in CFTR, a mutation that occurs in ∼90% of all individuals with cystic fibrosis (25), are striking.
Recent work in CFTR has shown that the ΔF508 mutation causes two defects, instability of the NBD1 domain and disruption in the interactions between the ICL4 and NBD1 (19, 20, 29, 30). Critical for this discovery was the identification of secondary mutations that increased NBD1 stability (19, 20). Here we tested homologous NBD mutations in Q141K ABCG2 and found the G188E mutation rescued Q141K expression, suggesting the Q141K mutation results in increased ABCG2 NBD instability. Interestingly, the R193K mutation, homologous to the suppressor mutation R555K in CFTR, didn’t increase Q141K ABCG2 expression; but did appear to decrease the insoluble fraction of Q141K protein (Fig. S6D). This result is consistent with the conclusions of Roxo-Rosa et al. that G550E and the four R to K mutations, or 4RK, (including R555K) suppressors rescue mutant CFTR by different mechanisms (41). It may be that R193K could not rescue mature protein but actually enhanced the degradation of misfolded protein by preventing the formation of the large insoluble aggresomes as reportedly seen by other groups (17).
Our CFTR ΔF508 and Q141K ABCG2 comparison also revealed the importance of F142 in ABCG2. The F142 deletion causes a severe decrease in expression but, unlike the Q141K mutation, the ΔF142 cannot be rescued with temperature or with suppressor mutations. Interestingly, the homologous ΔF508 mutation in CFTR can be rescued by temperature (27, 28) and by suppressor mutations (20, 21, 29–31). This discrepancy may result from the fundamental differences in protein topography; namely, ABCG2 is a half-transporter and CFTR is not. This difference translates into the apparent failure of ΔF142 ABCG2 to dimerize and fold properly, a failure with important consequences for trafficking and expression (11, 26). We probed this idea by introducing mutations in the first ICL at residue K473, the residue that we believe to be functionally homologous to R1070 in CFTR ICL4, and found that mutations of K473 could disrupt ABCG2 dimerization much like ΔF142. We therefore propose the site of action between the F142 and K473 as a critical site of the protein–protein interaction necessary for dimerization, much like the F508 and R1070 are key residues in CFTR folding. This possibility that protein–protein interactions are key for ABCG2 dimerization is a relatively new idea. Early work (24, 42) showed that the simple withdrawal of reducing agent from the Laemmli buffer allowed the visualization of the ABCG2 dimer on a Western blot and proposed therefore that disulfide bonds between cysteines, specifically the C603 residue, was the critical residue in ABCG2 dimerization (43–45). However, more recent work has showed that the cysteines were not necessary for dimerization and that in vitro ABCG2 dimerized and trafficked without the need for disulfide bonds (43, 46–48), suggesting the importance of protein–protein interactions in ABCG2 dimerization.
Hyperuricemia and gout therapy continue to focus on blocking the production of uric acid even though the physiological defect is most often decreased excretion (2). A therapy focused directly on the primary uric acid secretion transport mechanism in the kidney and gut (49) may lead to better outcomes and fewer adverse effects. Early attempts to correct aberrant mutant CFTR focused on small-molecule drugs that disrupted the ERAD machinery, allowing misfolded CFTR to the surface, like the Food and Drug Administration-approved HDAC inhibitor 4-PBA (34), which we here demonstrate can rescue mutant ABCG2. Other HDAC inhibitors have also been shown to rescue mutant ABC proteins. Hutt et al. (50) used multiple HDAC inhibitors to rescue ∆F508 CFTR surface expression and function and proposed that inhibiting HDAC7 function may alter chaperone levels including Hsp 70, similar to the proposed action of 4-PBA (33, 34, 50). Interestingly, Basseville et al. (17) recently found that a different suite of HDAC inhibitors rescued Q141K ABCG2 function and expression levels but did not observe any changes in Hsp 70 or Hsp 90 levels. Taken together, these findings suggest that molecules that can alter the proteostasis of mutant protein (51) may represent a viable therapeutic approach for treating diseases caused by misfolded proteins, including hyperuricemia and gout. However, the observed discrepancy in the HDAC inhibitor mechanism indicates that caution is warranted in their therapeutic use.
Recent corrector screens have yielded more specific molecules capable of correcting ΔF508 CFTR. Both VRT-325 (35, 36) and corr-4a (38) have been shown to cause a significant, if small, rescue of the mature, complex glycosylated form of the ΔF508 CFTR molecule. The VRT-325 compound appears to work by directly binding to the ΔF508 molecule (37) and by promoting interactions between the two halves of the ΔF508 CFTR molecule (52). Specifically, Yu et al. (53) recently showed that VRT-325 stabilizes the ΔF508 CFTR NBD1 domain, protecting the isolated domain from limited proteolysis, but had no effect on the second half of the ΔF508 CFTR molecule. Thus, the VRT-325 molecule corrects in ΔF508 CFTR the exact defect that we describe here for Q141K ABCG2, and indeed, we found that VRT-325 significantly increased total, dimer, and surface expression of the Q141K protein. Importantly, we also found that the increase in protein expression corresponded to an increase in function as well. The success of the VRT-325 and corr-4a molecules in correcting mutant Q141K protein expression clearly demonstrates that a better understanding of the way in which ABCG2 folds and dimerizes can lead to drug discovery of new therapies for gout and hyperuricemia. Going forward, it will also be important to discern why the Q141K mutation leads to a functional defect, even after expression level is normalized (10, 16), and whether NBD instability reduces binding or hydrolysis of ATP.
Finally, the similarities between ΔF508 CFTR and Q141K ABCG2 extend beyond the work presented here. They may provide a window into the evolutionary persistence of mutational hotspots in the NBD domains of human ABC transporters. In both cases, the disease-causing mutation appears to persist at elevated levels in specific human populations because it confers some heterozygotic advantage. For CFTR, the dysfunction of the ΔF508 mutation may provide some protection from cholera infection (54), whereas in ABCG2 the Q141K mutation may persist to increase blood urate levels of particular human populations beyond what even the loss of uricase function achieved. The persistence of the structural weakness in the NBD exposed in these two human diseases may speak to the larger question of why hotspots across protein families persist: they represent a mechanism for the protein to adapt to changing selective environments with single point mutations.
Materials and Methods
Detailed methods and method references can be found in SI Materials and Methods.
ABCG2 NBD Homology Model.
See SI Materials and Methods for details and references. The homology model of the ABCG2 NBD (residues 45–275) was built using the sequence of Sav1866 as a template. The refined model has a Z-score of −2.179, no residues with unfavorable φ- and ψ-angles, and an rmsd value of 0.6 Å.
C-14 Uric Acid Accumulation Studies.
Confluent transfected cells were treated with 10 µM VRT-325 or vehicle for 24 h. They were then incubated for 2 h in DMEM containing 500 μM cold urate and 75 μM C-14–labeled urate, washed, and lysed, and the lysate radioactivity was counted with a scintillation counter (LS600 LL; Beckman Coulter Inc.).
Other Reagents.
VRT-325 (corrector C3) and Corr-4a (corrector C4) were obtained from Cystic Fibrosis Foundation Therapeutics, and 4-PBA was a generous gift from by Neeraja Sharma (The Johns Hopkins University School Of Medicine, Baltimore, MD).
Supplementary Material
Acknowledgments
The authors thank Tatia C. Woodward, Anna Köttgen, Paul A. Welling, and Feng Qian for assistance with and discussions on the manuscript. These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK32753.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214530110/-/DCSupplemental.
References
- 1.Johnson RJ, et al. Lessons from comparative physiology: Could uric acid represent a physiologic alarm signal gone awry in Western society? J Comp Physiol B. 2009;179(1):67–76. doi: 10.1007/s00360-008-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillinger MH, Rosenthal P, Abeles AM. Hyperuricemia and gout: New insights into pathogenesis and treatment. Bull NYU Hosp Jt Dis. 2007;65(3):215–221. [PubMed] [Google Scholar]
- 3.Wright AF, Rudan I, Hastie ND, Campbell H. A ‘complexity’ of urate transporters. Kidney Int. 2010;78(5):446–452. doi: 10.1038/ki.2010.206. [DOI] [PubMed] [Google Scholar]
- 4.Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008;75(Suppl 5):S13–S16. doi: 10.3949/ccjm.75.suppl_5.s13. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RJ, et al. Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30(1):96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergamini C, Cicoira M, Rossi A, Vassanelli C. Oxidative stress and hyperuricaemia: Pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail. 2009;11(5):444–452. doi: 10.1093/eurjhf/hfp042. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, et al. Hyperuricemia and risk of stroke: A systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–892. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaffo AL, Edwards NL, Saag KG. Gout. Hyperuricemia and cardiovascular disease: How strong is the evidence for a causal link? Arthritis Res Ther. 2009;11(4):240. doi: 10.1186/ar2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köttgen A, et al. LifeLines Cohort Study CARDIoGRAM Consortium DIAGRAM Consortium ICBP Consortium MAGIC Consortium Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–154. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward OM, et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA. 2009;106(25):10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa H, et al. Ubiquitin-mediated proteasomal degradation of ABC transporters: A new aspect of genetic polymorphisms and clinical impacts. J Pharm Sci. 2011;100(9):3602–3619. doi: 10.1002/jps.22615. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa T, et al. Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations. Pharm Res. 2009;26(2):469–479. doi: 10.1007/s11095-008-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo C, et al. Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res. 2004;21(10):1895–1903. doi: 10.1023/b:pham.0000045245.21637.d4. [DOI] [PubMed] [Google Scholar]
- 14.Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int J Cancer. 2004;109(2):238–246. doi: 10.1002/ijc.11669. [DOI] [PubMed] [Google Scholar]
- 15.Imai Y, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1(8):611–616. [PubMed] [Google Scholar]
- 16.Morisaki K, et al. Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol. 2005;56(2):161–172. doi: 10.1007/s00280-004-0931-x. [DOI] [PubMed] [Google Scholar]
- 17.Basseville A, et al. Histone deacetylase inhibitors influence chemotherapy transport by modulating expression and trafficking of a common polymorphic variant of the ABCG2 efflux transporter. Cancer Res. 2012;72(14):3642–3651. doi: 10.1158/0008-5472.CAN-11-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuo H et al. (2009) Common defects of ABCG2, a high-capacity urate exporter, cause gout: A function-based genetic analysis in a Japanese population. Sci Transl Med 1(5):5ra11. [DOI] [PubMed]
- 19.Rabeh WM, et al. Correction of both NBD1 energetics and domain interface is required to restore ΔF508 CFTR folding and function. Cell. 2012;148(1–2):150–163. doi: 10.1016/j.cell.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendoza JL, et al. Requirements for efficient correction of ΔF508 CFTR revealed by analyses of evolved sequences. Cell. 2012;148(1–2):164–174. doi: 10.1016/j.cell.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serohijos AW, et al. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci USA. 2008;105(9):3256–3261. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodward OM, Köttgen A, Köttgen M. ABCG transporters and disease. FEBS J. 2011;278(18):3215–3225. doi: 10.1111/j.1742-4658.2011.08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozvegy C, et al. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun. 2001;285(1):111–117. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 24.Litman T, et al. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta. 2002;1565(1):6–16. doi: 10.1016/s0005-2736(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 25.Guggino WB, Stanton BA. New insights into cystic fibrosis: Molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7(6):426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi-Nakao K, Tamura A, Furukawa T, Nakagawa H, Ishikawa T. Quality control of human ABCG2 protein in the endoplasmic reticulum: Ubiquitination and proteasomal degradation. Adv Drug Deliv Rev. 2009;61(1):66–72. doi: 10.1016/j.addr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Kwon SH, Pollard H, Guggino WB. Knockdown of NHERF1 enhances degradation of temperature rescued DeltaF508 CFTR from the cell surface of human airway cells. Cell Physiol Biochem. 2007;20(6):763–772. doi: 10.1159/000110436. [DOI] [PubMed] [Google Scholar]
- 28.Denning GM, et al. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 29.Hoelen H, et al. The primary folding defect and rescue of ΔF508 CFTR emerge during translation of the mutant domain. PLoS ONE. 2010;5(11):e15458. doi: 10.1371/journal.pone.0015458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibodeau PH, et al. The cystic fibrosis-causing mutation deltaF508 affects multiple steps in cystic fibrosis transmembrane conductance regulator biogenesis. J Biol Chem. 2010;285(46):35825–35835. doi: 10.1074/jbc.M110.131623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teem JL, et al. Identification of revertants for the cystic fibrosis delta F508 mutation using STE6-CFTR chimeras in yeast. Cell. 1993;73(2):335–346. doi: 10.1016/0092-8674(93)90233-g. [DOI] [PubMed] [Google Scholar]
- 32.Krasnov KV, Tzetis M, Cheng J, Guggino WB, Cutting GR. Localization studies of rare missense mutations in cystic fibrosis transmembrane conductance regulator (CFTR) facilitate interpretation of genotype-phenotype relationships. Hum Mutat. 2008;29(11):1364–1372. doi: 10.1002/humu.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suaud L, et al. 4-Phenylbutyrate stimulates Hsp70 expression through the Elp2 component of elongator and STAT-3 in cystic fibrosis epithelial cells. J Biol Chem. 2011;286(52):45083–45092. doi: 10.1074/jbc.M111.293282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest. 1997;100(10):2457–2465. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loo TW, Bartlett MC, Clarke DM. Rescue of DeltaF508 and other misprocessed CFTR mutants by a novel quinazoline compound. Mol Pharm. 2005;2(5):407–413. doi: 10.1021/mp0500521. [DOI] [PubMed] [Google Scholar]
- 36.Van Goor F, et al. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Loo TW, Bartlett MC, Clarke DM. Correctors promote maturation of cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by binding to the protein. J Biol Chem. 2007;282(46):33247–33251. doi: 10.1074/jbc.C700175200. [DOI] [PubMed] [Google Scholar]
- 38.Pedemonte N, et al. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115(9):2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zelinski T, Coghlan G, Liu XQ, Reid ME. ABCG2 null alleles define the Jr(a-) blood group phenotype. Nat Genet. 2012;44(2):131–132. doi: 10.1038/ng.1075. [DOI] [PubMed] [Google Scholar]
- 40.Saison C, et al. Null alleles of ABCG2 encoding the breast cancer resistance protein define the new blood group system Junior. Nat Genet. 2012;44(2):174–177. doi: 10.1038/ng.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roxo-Rosa M, et al. Revertant mutants G550E and 4RK rescue cystic fibrosis mutants in the first nucleotide-binding domain of CFTR by different mechanisms. Proc Natl Acad Sci USA. 2006;103(47):17891–17896. doi: 10.1073/pnas.0608312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kage K, et al. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97(5):626–630. doi: 10.1002/ijc.10100. [DOI] [PubMed] [Google Scholar]
- 43.Ni Z, Bikadi Z, Rosenberg MF, Mao Q. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2) Curr Drug Metab. 2010;11(7):603–617. doi: 10.2174/138920010792927325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriksen U, Fog JU, Litman T, Gether U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem. 2005;280(44):36926–36934. doi: 10.1074/jbc.M502937200. [DOI] [PubMed] [Google Scholar]
- 45.Kage K, Fujita T, Sugimoto Y. Role of Cys-603 in dimer/oligomer formation of the breast cancer resistance protein BCRP/ABCG2. Cancer Sci. 2005;96(12):866–872. doi: 10.1111/j.1349-7006.2005.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDevitt CA, Collins R, Kerr ID, Callaghan R. Purification and structural analyses of ABCG2. Adv Drug Deliv Rev. 2009;61(1):57–65. doi: 10.1016/j.addr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Shigeta J, Katayama K, Mitsuhashi J, Noguchi K, Sugimoto Y. BCRP/ABCG2 confers anticancer drug resistance without covalent dimerization. Cancer Sci. 2010;101(8):1813–1821. doi: 10.1111/j.1349-7006.2010.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni Z, Mark ME, Cai X, Mao Q. Fluorescence resonance energy transfer (FRET) analysis demonstrates dimer/oligomer formation of the human breast cancer resistance protein (BCRP/ABCG2) in intact cells. Int J Biochem Mol Biol. 2010;1(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- 49.Ichida K, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. doi: 10.1038/ncomms1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutt DM, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol. 2010;6(1):25–33. doi: 10.1038/nchembio.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319(5865):916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 52.Loo TW, Bartlett MC, Clarke DM. Correctors enhance maturation of DeltaF508 CFTR by promoting interactions between the two halves of the molecule. Biochemistry. 2009;48(41):9882–9890. doi: 10.1021/bi9004842. [DOI] [PubMed] [Google Scholar]
- 53.Yu W, Kim Chiaw P, Bear CE. Probing conformational rescue induced by a chemical corrector of F508del-cystic fibrosis transmembrane conductance regulator (CFTR) mutant. J Biol Chem. 2011;286(28):24714–24725. doi: 10.1074/jbc.M111.239699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guggino SE. Gates of Janus: Cystic fibrosis and diarrhea. Trends Microbiol. 1994;2(3):91–94. doi: 10.1016/0966-842x(94)90541-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.