Abstract
Hyperventilation-induced hypocapnia is common among asthma patients. This case study illustrates both methodology and results from a patient undergoing training in capnometry-assisted respiratory training (CART). CART is a 4-week training aimed at normalizing basal and acute levels of end-tidal carbon dioxide (PCO2) using a portable capnometer. In the presented case, basal levels of PCO2 increased from hypocapnic to normocapnic range over the course of treatment. Improvements were accompanied by improvements in lung function and reductions in diurnal lung function variability. Improvements remained stable throughout follow-up.
Keywords: Asthma, Hyperventilation, Breathing training, PCO2, Behavioral biofeedback
With an estimated 300 million sufferers worldwide, and 30 million in the US alone, asthma is among the four most common chronic diseases in adult populations and the most common among youth (Braman 2006). The economic cost of asthma in the US in 2010 was projected to be $20.7 billion, with $15.6 billion in direct healthcare costs accounting for the most sizable portion (National Heart, Lung and Blood Institute 2009). While the first-line treatment for asthma is medication, behavioral techniques have gained increasing attention as adjunctive treatments to improve asthma symptoms and lung function. Yet research on the additive gain of biofeedback techniques in asthma control or lung function (e.g., respiratory resistance, spirometry, trachea noise, frontalis electromyogram, heart rate, or inspiratory volume and accessory muscle electromyograms) is inconclusive at present partly due to methodological shortcomings (see Ritz et al. 2004, for review). However, two more recent trials have shown efficacy in improving asthma outcomes with biofeedback training, with one study involving heart rate variability biofeedback training demonstrating reductions in medication usage and respiratory resistance (Lehrer et al. 2004). The second trial, a recent pilot study of a capnometry-assisted respiratory training (CART) targeting hyperventilation in patients with asthma, also has shown promising results in symptom reduction and peak flow variability (Meuret et al. 2007).
Hyperventilation is breathing in excess of metabolic demands and is generally accompanied by hypocapnia, a reduction in partial pressure carbon dioxide (PCO2; Gardner 1996). Hypocapnic hyperventilation is indicated by levels of end-tidal PCO2 falling below 35 mmHg (Oakes 1996); severe hypocapnia is indicated when PCO2 levels are around or below 30 mmHg (Bass and Gardner 1985). Hypocapnia is related to symptoms of tingling, pins and needle feelings, dizziness, light-headedness and paresthesias even in healthy individuals (Kinsman et al. 1973; Rafferty et al. 1992). PCO2 is also an important regulator of cerebral blood flow, with hypocapnia leading to significant reductions in blood flow to the brain through vasoconstriction (Fried 1993), which can produce symptoms such as lightheadedness, dizziness, and feelings of faintness. Furthermore, acute hypocapnia leads to respiratory alkalosis (Gardner 1996), which causes the hemoglobin in the blood to bind more tightly to the oxygen. In other words, during acute hypocapnic states, less oxygen is released to the brain and tissues, which can also cause symptoms such as dizziness and tingling in the extremities (Gardner 1996). In chronic hyperventilation, respiratory alkalosis is often compensated, but severe symptoms persist and comprise activities and quality of life (Jack et al. 2004). It has been speculated that a hypersensitive suffocation alarm system may contribute to hyperventilation in panic disorder patients, although evidence on abnormal chemoreceptor sensitivity in chronic hyperventilation and panic remains inconclusive (Jack et al. 2004; Katzman et al. 2002).
Research has shown that asthma patients tend to have lower levels of PCO2 compared to healthy populations, with a significant number of patients experiencing symptoms of hypocapnic hyperventilation (Thomas et al. 2003; for reviews see Bruton and Holgate 2005; Meuret and Ritz 2010). Hyperventilation symptoms have been found in 29% of primary care asthma patients compared to 8% in other primary care patients (e.g., Thomas et al. 2001, 2005). Lower levels of PCO2 appear to be pervasive throughout daily life rather than only isolated to laboratory tasks. For example, in a 24-h ambulatory monitoring study, participants with asthma had systematically lower levels of PCO2 compared to healthy controls (Ritz et al. 2003). Basal and acute hypocapnia in asthma patients is associated with a decline in lung function (Butler et al. 1960; Newhouse et al. 1964; O’Cain et al. 1979; van den Elshout et al. 1991) and higher nonspecific airway hyperreactivity (Osborne et al. 2000). Also, hyperventilation symptoms in asthma exacerbations have been linked to a lower quality of life (Ritz et al. 2008).
Traditional breathing training for asthma focuses on slow, abdominal, and/or ‘‘deep’’ breathing (Girodo et al. 1992; Thomas et al. 2003, 2009). However, reductions in respiration rate may lead to compensatory deeper breathing; if this compensation is exaggerated, hyperventilation and respiratory symptoms intensify (Meuret et al. 2003). In consequence, traditional breathing retraining approaches could lead to hypocapnia and trigger asthma exacerbations. The Buteyko breathing method, a complementary medicine technique, attempts to raise PCO2 levels by reducing ventilation (Stalmatski 1997), which is expected to reduce the risk of bronchoconstriction, airway hyperreactivity, and inflammation. Patients are instructed in exercises to reduce ventilation by nasal, slow, shallow, regular breathing. However, some aspects of the technique are cumbersome and potentially aversive for patients, such as breath holding or mouth taping, and may lead to overcompensation by breathing deeper than necessary. To date, no study has targeted PCO2 levels directly, precluding a test of the basic rationale of this method. Published trials of the technique or variants of it have shown little change in physiological outcomes (such as mechanical lung function and airway resistance), although some have shown effects on symptoms, quality of life, and/or rescue inhaler use (Bowler et al. 1998; Cooper et al. 2003; Slader et al. 2006).
Capnometry-assisted respiratory training, which was originally developed for patients with panic disorder (Meuret et al. 2001, 2008, 2010), teaches patients to normalize dysfunctional breathing patterns, specifically lower than normal levels of PCO2. In a first CART pilot study for patients with asthma: stable normalization in PCO2 and reductions in respiration rate, as well as reduced symptom frequency and distress, increased asthma control, and a reduction in mean diurnal peak expiratory flow variability was observed across the 4-week treatment and 1-month follow-up period (Meuret et al. 2007; Ritz et al. 2009). The CART technique aims to raise PCO2 directly by using feedback from a capnometer during training sessions and homework exercises to ensure that PCO2, a critical hyperventilation biomarker, is normalized (Meuret et al. 2007). The electronically monitored home-exercises help clients to transfer the skills they learn in the session to their daily lives and provide information about the patients’ compliance with the homework prescriptions. The merits of CART for asthma are being further investigated in an ongoing randomized-controlled intervention trial with a larger patient sample. Here we describe the treatment protocol and potential outcome using the example of one patient undergoing CART for asthma.
Treatment Description
Capnometry-assisted respiratory training focuses primarily on reducing the depth of breathing (i.e., tidal volume) by teaching patients to breathe more slowly, evenly, but most importantly, more shallowly. Nasal breathing (with the purpose to reduce the amount of air inspired) as well as abdominal breathing (as opposed to chest breathing, which can also aggravate sensations of chest tightness or pain) are also encouraged as means to further reduce breathing volume. In combination, these techniques comprise a powerful tool for raising end-tidal PCO2 levels with the goal of reaching the normocapnic range. The primary focus on depth is based on mediational studies showing that respiration rate does not drive PCO2 changes (Meuret et al. 2009, 2010) and that tidal volume increases alone can lead to hyperventilation (Ayala et al. 2010).
Treatment consists of 5 weekly 60–90 min sessions, conducted by trained personnel under supervision of a clinical psychologist. The first treatment session is dedicated to the presentation of the treatment rationale, familiarization with the portable capnometry device,1 and explanation of homework assignments. Patients are asked to practice their breathing techniques with the biofeedback device twice daily between sessions. These exercises are guided by standardized verbal prompts via an MP3 player. First, patients are asked to breathe without actively trying to control respiration for 2 min with their eyes closed in order to establish a baseline for the exercise. Patients are then instructed to match their breathing with pacing tones for 10 min. The pacing tones help patients focus on breathing regularly by providing a sound that rises in pitch to indicate inhalation and falls in pitch to indicate exhalation. Each week the tones are set to a progressively slower target rate (13, 11, 9, and 6 breaths per minute); the inhalation time is maintained at 40% of the total time per breath for each tone speed. During the pacing tones, patients are reminded to breathe through their nose with their mouths closed and to focus on breathing shallowly and abdominally. After 10 min of breathing with the tones, patients are asked to continue breathing for 5 min without auditory guidance. This ‘‘transfer period’’ is aimed at developing a sense of speed and regularity of breathing in absence of the pacing tones, to better facilitate the transfer of skills to daily life. Throughout paced and unpaced breathing, patients are instructed to try raising their PCO2 levels into a normocapnic range by using the PCO2 values from the capnometer display as feedback to guide necessary changes in respiration.
At the beginning of each weekly session, all exercises are downloaded from the electronic memory of the capnometer and then printed to provide feedback about the daily exercises in conjunction with symptom ratings from the patients’ diary log. At the end of the session, the therapist presents instructions for the next week’s tone speed, which is followed by further in-session practice. The final treatment session includes both the discussion of the previous week’s exercises as well as a summation of improvements and advice for maintenance of treatment gains. Post-treatment measurements, which take place within 1 week following the end of treatment, follow the same structure as the pre-treatment measurements. Follow-up appointments are scheduled for 1- and 6-months post-treatment.
Outcome Assessments
Lung Function
As part of the initial assessments, patients monitor symptoms and medication use at home for 1 week and measure their lung function six times daily with an electronic pocket spirometer (AM2?, Jaeger, Germany) in order to determine pre-treatment diurnal peak flow (PEF) variability, a common index of asthma control. High PEF variability is an indicator of low asthma control and is sometimes interpreted as a distal measure of airway hyperreactivity (Boezen et al. 1994). In separate assessment sessions at pre- and post-treatment, before each of the 5 weekly treatment sessions, and at 1-month and 6-months follow-up, measurements of respiration rate, PCO2, and forced expiratory volume in the first second (FEV1, by spirometry) are obtained. Except for the treatment session assessments, patients are instructed to not use their short-acting bronchodilator for 8 h and their daily dose of any long-acting bronchodilator should be delayed until after the assessment session.
Asthma Control
Self-reported experience of asthma control was measured by the Asthma Control Test (ACT, Nathan et al. 2004). The ACT is a five-item self-report instrument developed to parallel NHLBI (2007) guidelines of asthma control. The items explore symptoms, functional limitations, and bronchodilator use in the past 4 weeks (past week for the treatment sessions). Each item is rated on a 5-point scale, with 1 indicating the least amount of control and 5 indicating the greatest amount of control. The total of all items can range from 5 to 25. Internal consistency for the 5 items is good (a = .84), and agreement between ACT score and specialist’s ratings range from 71 to 78% (Nathan et al. 2004).
Client Description
In the following we will describe the case of John2 to illustrate the therapeutic process of CART. As a participant in an institutional review board-approved clinical trial testing the efficacy of CART, he signed the consent form after an initial explanation of the study and treatment process.
John is a 25-year-old male who was officially diagnosed with asthma in childhood. At treatment outset, he presented with symptoms of shortness of breath, wheezing, and chest tightness. John experienced these symptoms multiple times a week. Pollen, cigarette smoke, animal dander, and mold were described as his main asthma triggers. At the time treatment started, John was taking an inhaled corticosteroid for maintenance medication; he occasionally used his rescue inhaler (2 or 3 times per week, per patient report).
John’s ACT score was 22 at baseline, placing him in the well-controlled range. However, his baseline lung function FEV1 measurement was 3.40, which was only 76.9% of the expected lung function for a healthy male of his age and height. In terms of typical breathing patterns, he reported that he mostly breathed with his chest as well as yawned or sighed for no reason. Prior to treatment, his average baseline levels of PCO2 were in a hypocapnic range at 32.9 mmHg and his respiratory rate was at 14 breaths per minute.
Course of the Client’s Treatment
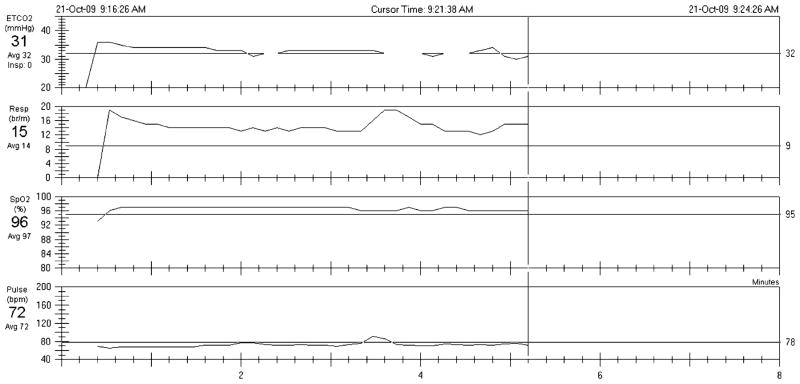
The therapist started the first session by explaining the treatment rationale, during which John was introduced to information about asthma and the role of breathing. Physiological and psychophysiological explanations of the production and maintenance of asthma symptoms were presented. As mentioned above, the phenomenon of hyperventilation and its possible impact on physical and psychological symptoms were discussed in detail. This included the consequences of fast, deep, and unstable breathing patterns, the benefits of symptom control through alternative breathing methods, and the goals of breathing training. Following the treatment rationale, the therapist showed John example levels of his PCO2, respiration rate, oxygen saturation, and pulse rate based on the results of his pretreatment recordings (see Fig. 1 for an example print out). His pretreatment recordings showed that though John’s respiration rate was within the normal range, his basal PCO2 level was in a hypocapnic range (32 mmHg). This highlights that a patient can be breathing in excess of their metabolic demands even if their respiration rate is normal. Breathing maneuvers to help John understand the relationship between his breathing and his symptoms were demonstrated and then emulated by the patient. The first breathing techniques taught were nasal and abdominal breathing. He learned abdominal breathing by placing one hand on his abdomen and the other hand on his chest in order to develop a sense of where his breathing primarily took place. While doing so he noted that his breath was predominantly in his chest and he was not accustomed to breathing abdominally. Then, pacing tones were added and John was instructed to breathe evenly and at 13 breaths per minute (br/min) with the aid of pacing tones from an MP3 player and the numeric feedback of the capnometer. Once achieved, he was asked to focus on his PCO2 levels by monitoring PCO2 levels on the capnometer. As he tried to breathe more shallowly, he noticed more sensations of shortness of breath, which tempted him to take a deep breath. However, according to the capnometer his PCO2 was below normal levels and his O2 was normal, indicating that John was taking in too much air. The following dialog illustrates a typical situation in which the patient has questions about how to correctly perform the breathing techniques.
Therapist: Now, try to breathe in as little air as possible
John: I’m trying, but I’m not sure what you mean
Therapist: Imagine a full glass of water. Instead of drinking the entire glass in one huge gulp, you could take little sips by reducing the amount of water you were taking in with each drink. Now apply the same principle to air, so instead of taking a huge gulp of air, you want to take little sips
John: It is really hard to take in less air because I feel like I need more air right after I breathe out
Therapist: This feeling of shortness of breath is normal because your body is not used to this breathing pattern. You can compare it to drinking too much coffee. If you start drinking less, your body will crave for more even though you’re doing something that is better for the body. After a while the craving will subside because your body gets used to it. Similarly, if your body is used to taking in large amounts of air, it will feel unnatural or even wrong to breathe less. You will feel short of breath, which is kind of like craving for more air. However, that sensation is not caused by a lack of oxygen but rather too low levels of PCO2. In fact, as you can see when looking at the values on your device, your levels of oxygen are in a normal range. If you take deep breaths, you may continue feeling the same sensation in spite of taking in more air because your gas levels are out of balance
John: So what you’re saying is, even though I feel like I’m not getting enough air during the shallow breathing, I need to continue breathing shallowly as long as my oxygen stays above 90%? Maybe I am so used to deep breathing that it is hard for me to change it
Therapist: Yes, this first week in particular will be challenging for you because you are not used to breathing this way, but your body will get accustomed to it, just like when you first start to exercise, it takes a while until it feels less strenuous. If you feel the sensation of shortness of breath and are worried you are not getting enough air, just check your oxygen levels on the capnometer. Most likely they will be just fine, and you are feeling short of breath due to your low PCO2
Fig 1.
Illustration of the actual capnometer print-out from the tidal wave. The print-out shows the values for the 5-min pretreatment baseline recording. The top channel shows end-tidal PCO2, the 2nd respiration rate, the 3rd O2 saturation, and the bottom channel shows heart rate
This conversation is typical for asthma patients who are accustomed to breathing in excess of their metabolic demands (i.e., hyperventilating) even when resting. John acknowledged that the rationale made sense, but had great difficulty in controlling the depth of his breathing. The numeric feedback eased his worries about shortness of breath and he was reassured that with practice, sensations related to overbreathing should subside. Initial in-session practice was followed by detailed instructions on how to complete the between-session exercises (described previously) at home or elsewhere. He was instructed that the exercises were to be performed twice a day, preferably at different times. A detailed handout containing the therapy rationale was given to him for review.
In weeks 2–4, John was instructed to match his breathing movements with tones with a progressively slower respiration rate of 11, 9, and 6 breaths per minute, respectively. Self-report data for each exercise such as bronchodilator use, experience of asthma symptoms or attacks, levels of PCO2 and respiration rate observed during the exercise, as well as any outside influences affecting the exercise were recorded in provided diaries.
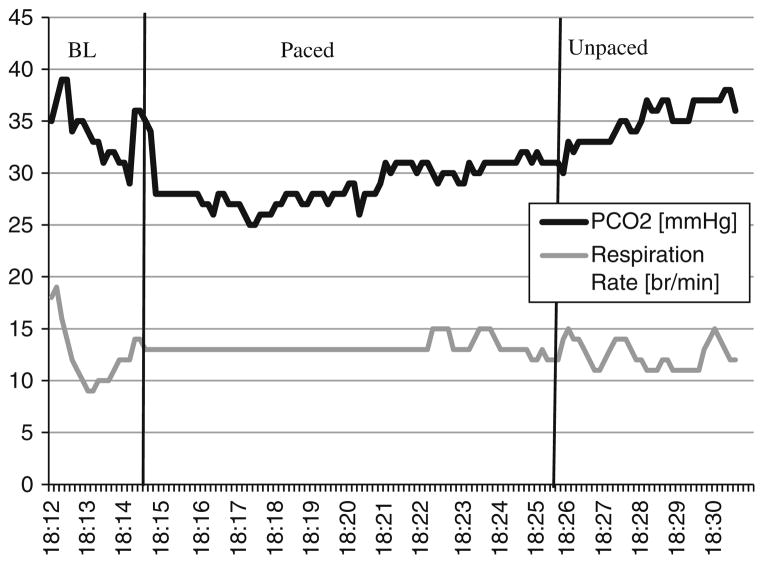
Treatment sessions 2–5, consisted of reviewing John’s physiological data from his homework breathing exercises and practicing with the next week’s tone speed. The sessions also included providing feedback on his maintenance of PCO2 and respiration rate levels, reassessing his implementation of the new breathing techniques (e.g., shallow and abdominal breathing), and discussing the application of new breathing skills to his daily life. The following conversation is an example from his second treatment session using printouts of recordings from John’s first week of breathing exercises (see Fig. 2 for a production of the data from John’s first exercise).
Therapist: Let’s look at your exercises together. This is your first exercise (Fig. 2). This is your PCO2 and respiration rate during the exercise. As you can see your exercise is divided into three phases: baseline, 10 min of paced breathing with the tones, and 5 min of breathing without tones. Your baseline recordings of this exercise, when you were not trying to control your breathing, show your average PCO2 level as 33 mmHg and your average rate of breathing was about 13 breaths per minute. Looking at your PCO2 levels for the exercise, your average PCO2 level is 29 during the 10 min with the tones, and your breathing rate is at 13 breaths per minute for most of time. During the 5-min transfer period, your average PCO2 level is 34 and the average respiration rate is 13. What was your experience during the exercises?
John: It was pretty challenging to slow down my breathing. I got off track easily when I wasn’t paying attention to my respiratory rate. Increasing my PCO2 level was even harder. I actually felt short of breath when I was focusing on increasing my PCO2 level
Therapist: This happens to many people during the first week. It is normal for you to feel short of breath as we discussed in our first session. Since your body is more accustomed to taking deeper breaths, it will take some time to get used to adopting a new breathing pattern. Have you noticed anything about your breathing when you were not doing the exercises?
John: I noticed when I check in with my respiration during the day that I am taking deep breaths. Before I started this training, I wouldn’t have even noticed. So, I feel that I am becoming more aware of my breathing and voluntarily try to change it even when I am not doing the exercises
Therapist: That’s very good that you are becoming more aware of your breathing! And what you are describing is called sigh breaths. Sighing often for no apparent reason can be a sign that your breathing pattern is irregular. A good rule of thumb is that if you can hear your breathing, in other words, if you can hear the air entering your nose, this indicates a sharp inhalation and may be a sign that you are breathing too quickly or deeply
John: Oh, ok, I’ll remember that. What about these times when I’m breathing right with the tones? It looks like my PCO2 is still decreasing then
Therapist: Decreasing your respiration rate can impact your PCO2. Your body wants to take deeper breaths to compensate for breathing more slowly, in fact, if you do not influence your breathing it switches automatically to deeper breathing when you breathe slower. However, if you take control of breathing you can overcome the urge to take deep breaths. Only by controlling both depth and rate of breathing can you move towards normalizing your PCO2
John: I thought I did take shallow breaths, but it doesn’t look like I did. How should I increase my PCO2?
Therapist: Try to breathe through your nose as much as possible. To reduce the amount of air you inhale, imagine you are breathing through a coffee straw. You will take in less air that way
John: It feels like I’m barely taking in air
Therapist: You may feel like that now, but if you continue breathing shallowly through your nose, you will be more comfortable and can increase your PCO2
John: I have a question about abdominal breathing. When I tried to breathe with my abdomen, it was hard to increase my PCO2. Why is that happening?
Therapist: You might have taken deeper breaths as you were breathing abdominally. Abdominal breathing helps slow down your respiration rate, but you may need to be careful about deep breathing. So you don’t want your stomach to completely inflate with air for each breath, but rather just have a very slight movement. Always remember, you are targeting both the speed and the volume of your breathing
Fig 2.
Illustrated are the data of the first breathing exercise (week 1) demarcated by the 2-min baseline (BL), 10 min of pacing tones (paced), and 5 min of unpaced breathing (unpaced)
Over the course of treatment John progressively altered his breathing pattern and extended the new breathing techniques to situations where he felt symptomatic or was confronted with his asthma triggers. The following conversation is from the last treatment session.
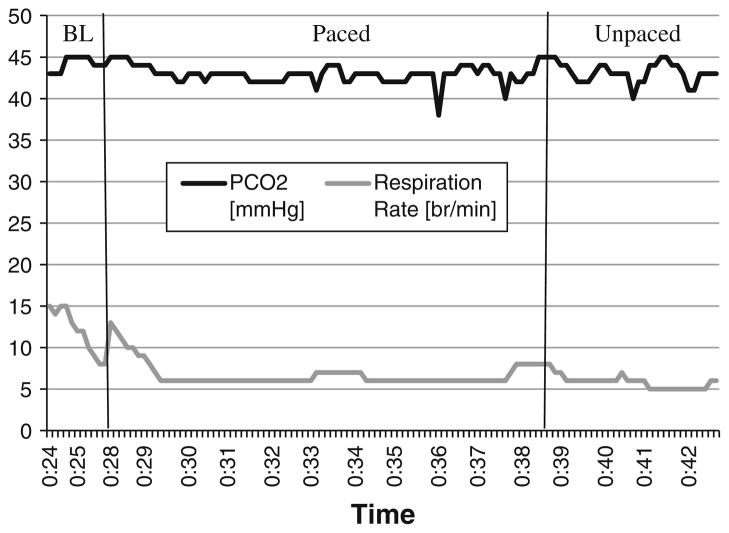
Therapist: Let’s take a look at your final exercise of your last week of training (see Fig. 3 for a production of the data from John’s last exercise). What do you think about your respiratory rate and PCO2?
John: I think they both look really good!
Therapist: They do! Let’s also take a look at your baseline for this exercise when you weren’t trying to control your breathing (Fig. 3). What do you notice from this printout?
John: Again, I think they both look really good! My PCO2 is in the normal range and my respiration rate is nice and slow
Therapist: Yes indeed! It looks like you were very successful at breathing both shallowly and slowly compared to the beginning of the treatment. How do you feel about your breathing?
John: It feels like I have a lot more control over my respiration now than before I started this treatment. At the beginning, it was very hard to be able to do the exercises and I thought it was impossible
Therapist: Yes, I remember when you said you were not sure whether it was working for you after the first week. What have you noticed about the frequency or severity of your asthma symptoms?
John: I really haven’t had any major symptoms and didn’t use my rescue inhaler much in the last week
Therapist: Yes, although our breathing training cannot cure your asthma because it is a chronic disease, it can help you to manage your asthma better. How do you control your asthma symptoms now as compared to before you started this treatment?
John: Now when I feel symptomatic, I first try to breathe slowly and shallowly instead of automatically grabbing my rescue inhaler. I usually feel quite sick around this time of year due to the pollen but things have been much better in the past weeks and I definitely feel less symptomatic and dependent on my inhaler
Fig 3.
Illustrated are the data of the final breathing exercise (week 4) demarcated by the 2-min baseline (BL), 10 min of pacing tones (paced), and 5 min of unpaced breathing (unpaced)
Outcome and Prognosis
John completed his treatment, which included pretreatment, five treatment sessions, post-treatment, and 1- and 6-months follow-up assessments.
Weekly Home Exercise Effects on PCO2 and Respiration Rate
John showed high treatment compliance in that he attended all treatment sessions and completed 10–12 (out of 13) exercises weekly. John improved his breathing pattern over the 4 weeks of treatment. His improvement was evidenced by increases in his PCO2 levels while keeping his respiration rate slow and regular. John’s average baseline PCO2 increased across the 4 weeks [mean (SD); 36.3 (2.9), 38.9 (1.6), 40.0 (2.5), and 42.4 (2.6) mmHg] and average baseline respiration rate remained slow as expected [13.1 (3.0), 11.5 (2.6), 13.1 (2.7), and 9.6 (2.4) br/min]. During the 10 min with the pacing tones, his PCO2 and respiration rate levels were also improved [36.2 (2.9), 37.5 (1.8), 38.6 (2.6), and 40.6 (2.5) mmHg for PCO2; 13.3 (1.0), 11.4 (1.0), 10.0 (1.6), and 6.5 (0.7) br/min for respiration rate]. During the 5 min without the pacing tones, his improvements on both PCO2 and respiration rate levels were improved [36.5 (2.6), 37.7 (2.2), 39.0 (2.5), and 41.2 (2.5) mmHg for PCO2; 12.4 (1.8), 10.8 (1.8), 9.8 (1.8), and 6.3 (1.5) br/min for respiration rate]. Increases in his PCO2 and reductions in his respiration rate were steady across treatment weeks.
Data from his posttreatment, 1- and 6-months follow-up assessments indicated that John’s improvement on both PCO2 and respiration rate remained in healthy ranges (PCO2 = 38.0, 38.4, and 40.2 mmHg; respiration rate = 7.0, 9.3, and 6.4 br/min, for posttreatment, 1- and 6-months follow-up, respectively).
Treatment Effects on Self-Report and Physiological Measurement
John’s ACT scores remained relatively stable across time points. However, his lung function steadily improved [FEV1 (% predicted); 3.40 (76.9%), 3.29 (74.4%), 3.47 (78.5%), 3.62 (81.9%), 3.73 (84.4%), 3.90 (88.2%), 3.88 (87.8%), 3.91 (88.5%), and 3.64 (82.4%) across assessment time points of pre-, 5 weekly treatment sessions, posttreatment, 1- and 6-months follow-ups]. John’s measures of 3-day PEF variability showed decreases from 13.2% at pretreatment to 5.9% at post-treatment. These gains were not fully retained at 1 month follow up (11.2%) and 6-months follow up (10.9%).
Treatment Challenges
An integral part of CART is using the numerical biofeedback of the portable capnometer to correct low PCO2 levels. It is important that patients understand the relationship between their breathing maneuvers and the numeric feedback. In order to raise PCO2 from a hypocapnic range, a person should take in less air; the concept of taking in less air to raise a value can be confusing. Confusion can be lessened if the therapist asks questions to assure the patient understands what the goals of treatment are and how to attain those goals. Understanding of the goals can be also be consolidated by writing them out clearly in an easily accessible location for future reference. The therapist can also guide patients through hands-on demonstrations of each concept. For example, the therapist can instruct the patient to breathe normally while observing the numeric feedback and can then instruct the patient to breathe as deeply as possible for a few breaths while continuing to observe the capnometer display, which at this point will show a sharp drop in PCO2. This type of demonstration can help solidify the understanding of the association between deep breathing and decreases in PCO2.
For CART to be effective, patients must overcome the difficulties encountered from raising PCO2. At first, taking in less air can increase patients’ perception of shortness of breath or worries about not getting enough oxygen. Patients report feeling like they need to take a deep breath; however, doing so further lowers PCO2. It is important for therapists to warn patients about the initial difficulties of raising their PCO2 and encourage them to resist the urge to take a deep breath. The capnometery device also includes a transcutaneous pulse oximeter, which allows patients to reassure themselves that they are indeed getting enough oxygen and can continue shallow breathing. In order for the urge to take a deep breath to subside, patients must practice shallow breathing regularly.
Our initial findings indicate that it takes 4 weeks of systematic practice for patients to become accustomed to the new breathing pattern and effectively raise PCO2 (Ritz et al. 2009). Therefore, compliance with homework exercises is an integral part of treatment. If patients have trouble completing the exercises, using an analogy such as learning to drive can be a helpful illustration.
Therapist: This treatment is like learning to drive. Someone can talk to you for hours about what it feels like to drive and the correct way to do it, but it’s really impossible to learn without getting in the car and driving for yourself. It’s the same way with the exercises, to really get the maximum benefits out of this treatment you have to practice what we talk about in our sessions
Finally, the breathing exercises require patients to be able to breathe through their noses, since the respiratory biofeedback is measured through a nasal cannula. Suffering from allergies can cause patients to experience nasal congestion and post-nasal drip, which makes breathing through the nasal cannula and thereby completing exercises difficult. Patients should be encouraged to clear their nasal passages to the extent possible and complete the exercises to the best of their ability.
Summary
John’s initial asthma symptoms and respiratory patterns are typical of those seen in our ongoing and past pilot study (Meuret et al. 2007; Ritz et al. 2009). His experience and improvement with the presented breathing training exemplifies how CART can be beneficial to asthma patients, especially those who have abnormal respiratory patterns such as hyperventilation. John started out with hypocapnic and irregular breathing, but successfully modified his breathing pattern to bring his PCO2 to a normocapnic range through slow, shallow, abdominal breathing. In addition to correcting his respiratory pattern, he experienced increased lung function as well as an improvement in lung function variability.
Some caution should be used when interpreting the findings. John was encouraged to not alter his breathing pattern during the quiet sitting periods, but may have learned to breathe a certain way when attached to the capnometer. As we did with John, emphasis should be placed on trying to apply the exercises to daily life and when feeling symptomatic in order to generalize learning beyond the exercises.
The case described here took place in the context of a larger randomized controlled trial. The weekly hourly sessions were administered by a CART-trained therapist. To provide the necessary feedback of PCO2 and RR, a portable capnometry device is required. The model used for this study can be purchased for roughly $2,700, but cheaper models that provide PCO2 and RR can be purchased for as low as $1,200. Patients can also keep track of their lung function using inexpensive, pocket-sized PEF meters.
Capnometry-assisted respiratory training shows promise as a useful adjunctive treatment to medication management of asthma. Patients receive feedback from the capnometer during their home exercises, likely increasing their sense of control and accountability during treatment. Reviewing printouts of exercises also likely increases patient’s compliance to treatment. Furthermore, it allows therapists and patients to monitor and reduce patients’ hypocapnia systematically, distinguishing this technique from earlier breathing treatments. John’s treatment outcome is a result of correcting both hyperventilation and irregularity of breathing by adding a shallow breathing component with objective measurement of his PCO2 levels.
Acknowledgments
This research was supported by grant 5R01HL89761 from the National Heart, Lung, and Blood Institute.
Footnotes
The TIDAL WAVE® Sp uses the CAPNOSTAT® CO2 sensor which passes an infrared light beam through the gas sample. The CO2 concentration of the sample is directly related to the specific wavelength of infrared light energy absorbed by the CO2 molecules. A photodetector measures the remaining light energy not absorbed by the sample and compares that to the infrared source thus measuring the CO2 concentration of the sample. End tidal CO2 is calculated continuously on a breath by breath basis.
Name and identifying information were altered or removed for anonymity.
There are no conflicts of interest to report.
Contributor Information
Ashton M. Jeter, Department of Psychology, Southern Methodist University, P.O. Box 750442, Dallas, TX 75275, USA
Hwacha C. Kim, Department of Psychology, Southern Methodist University, P.O. Box 750442, Dallas, TX 75275, USA
Erica Simon, Department of Psychology, Southern Methodist University, P.O. Box 750442, Dallas, TX 75275, USA.
Thomas Ritz, Department of Psychology, Southern Methodist University, P.O. Box 750442, Dallas, TX 75275, USA.
Alicia E. Meuret, Email: ameuret@smu.edu, Department of Psychology, Southern Methodist University, P.O. Box 750442, Dallas, TX 75275, USA
References
- Ayala ES, Meuret AE, Ritz T. Confrontation with blood and disgust stimuli precipitates respiratory dysregulation in blood-injury-injection phobia. Biological Psychology. 2010;84:87–97. doi: 10.1016/j.biopsycho.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Bass C, Gardner WN. Respiratory and psychiatric abnormalities in chronic symptomatic hyperventilation. British Medical Journal. 1985;290:1387–1390. doi: 10.1136/bmj.290.6479.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boezen HM, Schouten JP, Postma DS, Rijcken B. Distribution of peak expiratory flow variability by age, gender and smoking habits in a random population sample aged 20–70 yrs. The European Respiratory Journal. 1994;7:1814–1820. doi: 10.1183/09031936.94.07101814. [DOI] [PubMed] [Google Scholar]
- Bowler SD, Green A, Mitchell CA. Buteyko breathing techniques in asthma: A blinded randomised controlled trial. The Medical Journal of Australia. 1998;169:575–578. doi: 10.5694/j.1326-5377.1998.tb123422.x. [DOI] [PubMed] [Google Scholar]
- Braman SS. The global burden of asthma. Chest. 2006;130:4S– 12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- Bruton A, Holgate ST. Hypocapnia and asthma: A mechanism for breathing retraining? Chest. 2005;127:1808–1811. doi: 10.1378/chest.127.5.1808. [DOI] [PubMed] [Google Scholar]
- Butler J, Caro CG, Alcala R, Dubois AB. Physiological factors affecting airway resistance in normal subjects and in patients with obstructive respiratory disease. The Journal of Clinical Investigation. 1960;39:584–591. doi: 10.1172/JCI104071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Oborne J, Newton S, Harrison V, Thompson Coon J, Lewis S, et al. Effects of two breathing exercises (buteyko and pranayama) in asthma: A randomized controlled trial. Thorax. 2003;58:674–679. doi: 10.1136/thorax.58.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried R. The psychology of physiology of breathing. New York: Plenum Press; 1993. [Google Scholar]
- Gardner WN. The pathophysiology of hyperventilation disorders. Chest. 1996;109:516–534. doi: 10.1378/chest.109.2.516. [DOI] [PubMed] [Google Scholar]
- Girodo M, Ekstrand KA, Metivier GJ. Deep diaphragmatic breathing: Rehabilitation exercises for the asthmatic patient. Archives of Physical Medicine and Rehabilitation. 1992;73:717–720. [PubMed] [Google Scholar]
- Jack S, Rossiter HB, Pearson MG, Ward SA, Warburton CJ, Whipp BJ. Ventilatory responses to inhaled carbon dioxide, hypoxia, and exercise in idiopathic hyperventilation. American Journal of Respiratory and Critical Care Medicine. 2004;170:118–125. doi: 10.1164/rccm.200207-720OC. [DOI] [PubMed] [Google Scholar]
- Katzman MA, Struzik L, Vijay N, Coonerty-Femiano A, Mahamed S, Duffin J. Central and peripheral chemoreflexes in panic disorder. Psychiatry Research. 2002;113:181–192. doi: 10.1016/s0165-1781(02)00238-x. [DOI] [PubMed] [Google Scholar]
- Kinsman RA, Luparello T, O’Banion K, Spector S. Multidimensional analysis of the subjective symptomatology of asthma. Psychosomatic Medicine. 1973;35:250–267. doi: 10.1097/00006842-197305000-00008. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Scardella A, Siddique M, et al. Biofeedback treatment for asthma. Chest. 2004;126:352–361. doi: 10.1378/chest.126.2.352. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Ritz T. Hypocapnia in anxiety and respiratory disease: Empirical evidence and clinical strategies. International Journal of Psychophysiology. 2010;78:68–79. doi: 10.1016/j.ijpsycho.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Ritz T, Wilhelm F, Roth WT. Targeting PCO2 in asthma: Pilot evaluation of a capnometry-assisted breathing training. Applied Psychophysiology and Biofeedback. 2007;32:99–109. doi: 10.1007/s10484-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Hofmann SG, Suvak MK, Roth WT. Changes in respiration mediate changes in fear of bodily sensations in panic disorder. Journal of Psychiatric Research. 2009;43:634–641. doi: 10.1016/j.jpsychires.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Seidel A, Bhaskara L, Hofmann SG. Respiratory and cognitive mediators of treatment change in panic disorder: Evidence for intervention specificity. Journal of Consulting and Clinical Psychology. 2010;78:691–704. doi: 10.1037/a0019552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training for treating panic disorder. Useful intervention or impediment? Behavior Modification. 2003;27:731–754. doi: 10.1177/0145445503256324. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of end-tidal PCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research. 2008;42:560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Respiratory biofeedback-assisted therapy in panic disorder. Behavior Modification. 2001;25:584–605. doi: 10.1177/0145445501254006. [DOI] [PubMed] [Google Scholar]
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: A survey for assessing asthma control. American Academy of Allergy, Asthma and Immunology. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (NHLBI) Expert panel report 3: Guidelines for the diagnosis and management of asthma (NIH Publication No. 07-4051) U.S. Department of Health and Human Services, National Institutes of Health (NIH); 2007. National Asthma Education and Prevention Program. Retrieved from http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. [Google Scholar]
- National Heart, Lung, and Blood Institute (NHLBI) Morbidity & mortality: 2009 chart book on cardiovascular, lung and blood diseases. U. S. Department of Health and Human Services, National Institute of Health; 2009. Available at: http://www.nhlbi.nih.gov/resources/docs/2009_ChartBook.pdf. [Google Scholar]
- Newhouse MT, Becklake MR, Macklem PT, McGregor M. Effect of alterations in end-tidal CO2 tension on flow resistance. Journal of Applied Physiology. 1964;19:745–749. doi: 10.1152/jappl.1964.19.4.745. [DOI] [PubMed] [Google Scholar]
- Oakes DF. Clinical practitioner’s pocket guide to respiratory care. 4. Old Town, MN: Health Educator Publications Inc; 1996. [Google Scholar]
- O’Cain CF, Hensley MJ, McFadden ER, Jr, Ingram RH., Jr Pattern and mechanism of airway response to hypocapnia in normal subjects. Journal of Applied Physiology. 1979;47:8–12. doi: 10.1152/jappl.1979.47.1.8. [DOI] [PubMed] [Google Scholar]
- Osborne CA, O’Connor BJ, Lewis A, Kanabar V, Gardner WN. Hyperventilation and asymptomatic chronic asthma. Thorax. 2000;55:1016–1022. doi: 10.1136/thorax.55.12.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty G, Saisch S, Gardner W. Relation of hypocapnic symptoms to rate of fall of end-tidal PCO2 in normal subjects. Respiratory Medicine. 1992;86:335–340. doi: 10.1016/s0954-6111(06)80033-8. [DOI] [PubMed] [Google Scholar]
- Ritz T, Dahme B, Roth WT. Behavioral interventions in asthma biofeedback techniques. Journal of Psychosomatic Research. 2004;56:711–720. doi: 10.1016/S0022-3999(03)00131-4. [DOI] [PubMed] [Google Scholar]
- Ritz T, Meuret AE, Wilhelm F, Roth WT. End-tidal PCO2 levels in asthma patients in the laboratory and at home. Biological Psychology. 2003;62:233–234. [Google Scholar]
- Ritz T, Meuret AE, Wilhelm F, Roth WT. Changes in PCO2, symptoms, and lung function of asthma patients during capnometry-assisted breathing training. Applied Psychophysiology and Biofeedback. 2009;34:1–6. doi: 10.1007/s10484-008-9070-1. [DOI] [PubMed] [Google Scholar]
- Ritz T, Rosenfield D, Meuret AE, Bobb C, Steptoe A. Hyperventilation symptoms are linked to a lower perceived health in asthma patients. Annals of Behavioral Medicine. 2008;35:97–104. doi: 10.1007/s12160-007-9014-7. [DOI] [PubMed] [Google Scholar]
- Slader C, Reddel H, Spencer L, Belousova E, Armour C, Bosnic-Anticevich S, et al. Double blind randomised controlled trial of two different breathing techniques in the management of asthma. Thorax. 2006;61:651–656. doi: 10.1136/thx.2005.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmatski A. Freedom from asthma: Buteyko’s revolutionary treatment. London: Kyle Cathie; 1997. [Google Scholar]
- Thomas M, McKinley RK, Freeman E, Foy C. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: Cross sectional survey. British Medical Journal. 2001;322:1098–1100. doi: 10.1136/bmj.322.7294.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, McKinley RK, Freeman E, Foy C, Price D. The prevalence of dysfunctional breathing in adults in the community with and without asthma. Primary Care Respiratory Journal. 2005;14:78–82. doi: 10.1016/j.pcrj.2004.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, McKinley RK, Freeman E, Foy C, Prodger P, Price D. Breathing retraining for dysfunctional breathing in asthma: A randomised controlled trial. Thorax. 2003;58:110–115. doi: 10.1136/thorax.58.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, McKinley RK, Mellor S, Watkin G, Holloway E, Scullion J, et al. Breathing exercises for asthma: A randomized controlled trial. Thorax. 2009;64:55–61. doi: 10.1136/thx.2008.100867. [DOI] [PubMed] [Google Scholar]
- van den Elshout FJ, van Herwaarden CL, Folgering HT. Effects of hypercapnia and hypocapnia on respiratory resistance in normal and asthmatic subjects. Thorax. 1991;46:28–32. doi: 10.1136/thx.46.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]