Abstract
The chemokine CXC receptor 4 (CXCR4) is activated by stromal cell-derived factor (SDF-1α). CXCR4 may be part of a lipopolysaccharide (LPS) sensing co-clustering complex that modulates TLR4 activation and evidence suggest that SDF-1α can activate anti-inflammatory signaling pathways and suppress inflammation. In the present study we examined the hypothesis that the SDF-1α peptide analog and CXCR4 agonist CTCE-0214 is anti-inflammatory in three distinct models of murine systemic inflammation. Our findings demonstrate that CTCE-0214 in vivo significantly suppressed plasma tumor necrosis factor alpha (TNF-α) increases in acute endotoxemia and following zymosan-induced multiple organ dysfunction syndrome (MODS). In both models, CTCE-0214 did not suppress plasma increases in the anti-inflammatory cytokine interleukin (IL)-10. CTCE-0214 improved survival without antibiotics in a model of severe sepsis induced by cecal ligation and puncture (CLP). CTCE-0214 also decreased plasma increases in IL-6 but not TNF-α and IL-10 in response to CLP-induced inflammation. We demonstrated in a moderately severe model of CLP (one puncture) that IL-6 levels at 24 h were similar to sham controls. However in severe CLP (two punctures) plasma IL-6 levels were markedly elevated. Plasma SDF-1α levels varied inversely with the plasma IL-6. In addition to the beneficial effect of CTCE-0214 in these models of systemic inflammation in vivo, we also demonstrated that the analog dose dependently suppressed LPS-induced IL-6 production in bone marrow-derived macrophages. CTCE-0214 therefore may be beneficial in controlling inflammation sepsis and systemic inflammatory syndromes.
Keywords: CXCR4, SDF-1α, CTCE-0214, sepsis
INTRODUCTION
Pattern recognition receptors are fundamental to the innate immune response and in the pathophysiology of sepsis. It is well established that CD14 and MD-2 are cell surface receptor components necessary for TLR4 activation [1, 2]. Although the importance of the TLR4 sensing complex has been established, LPS sensing by other receptors is suggested by receptor clustering with TLR4 upon LPS stimulation [3, 4]. It has been demonstrated that CXCR4 is part of the LPS sensing co-clustering complex and that LPS can bind to CXCR4 resulting in the activation of signaling pathways [5]. Since CXCR4 activates heterotrimeric Gαi proteins, CXCR4 may be a TLR4 co-clustering receptor that modulates Gαi activation in endotoxemia and sepsis. The natural ligand for CXCR4 is stromal cell-derived factor (SDF-1α) also referred to as CXCL12. SDF-1α is a multifunctional cytokine that is expressed and secreted by several tissues, including endothelium and stromal cells [6]. Recent studies suggest that SDF-1α activates both CXCR4 and CXCR7 [7]. During the inflammation process, SDF-1α is released at the site of injury. It has been demonstrated that SDF-1α reverses antigen-induced T cells recruitment into inflammatory sites [8]. Evidence suggests that SDF-1α can activate anti-inflammatory signaling pathways and suppress inflammation [8–10]. SDF-1α therefore may function as an autocrine or paracrine ligand that suppresses inflammation.
Our previous studies and others demonstrated that TLR4 signaling is, in part, Gαi protein-coupled [11]. Gαi2(−/−) mice exhibit an enhanced pro-inflammatory phenotype to endotoxemia and CLP-induced sepsis compared with Gαi2(+/+) mice [12–14]. Thus, the predominant function of Gαi signaling pathways in sepsis appears to be anti-inflammatory.
The function of SDF-1α can be mimicked by small peptide agonists [15]. CTCE-0214 is a peptide analog of SDF-1α, which has been modified to improve plasma stability [16]. In the present study we examined the efficacy of CTCE-0214 as a CXCR4 agonist in vivo on the inflammatory response to acute endotoxemia, in a MODS model induced by peritoneal zymosan, and on mortality induced by CLP in mice. Since SDF-1α is the endogenous ligand for CXCR4, we also measured plasma SDF-1α in CLP models with differing sepsis severity. Additionally in vitro effects of CTCE-0214 were examined in LPS-stimulated BMDM.
MATERIALS AND METHODS
CTCE-0214 Peptide
CTCE-0214 is a peptide analog of SDF-1α, which links the N-terminal region (residue, 1–14), and the Cterminal region (residue, 55–67) of SDF-1α by a fourglycine linker. This analog is cyclized between amino acid residues at positions 20 and 24. Two Cys were replaced with Ala and Phe to improve plasma stability (Fig. 1) (Chemokine Therapeutics Corporation).
Fig. 1.

Structures of SDF-1α and CTCE-0214.
Mice
Male CD-1 mice (7–8 weeks old) were purchased from Harlan laboratories. Investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and commenced with the approval of the Institutional Animal Care and Use Committee.
Cell Culture and Stimulation
Bone marrow-derived macrophages (BMDM) were isolated and cultured from CD-1 mice as previously described [17]. Briefly, mice were killed under anesthesia with ether, and both femurs were dissected free of adherent tissue. The proximal and distal femurs were removed and the marrow tissue was flushed by irrigation with culture medium. The marrow plugs were dispersed by passing through a 25-gauge needle, and the cells were suspended by vigorous pipetting and washed. Cells were cultured in DMEM containing 10% FBS, 50µg/ml penicillin/streptomycin and 25 ng/ml macrophage colony-stimulating factor (M-CSF; R&D system). Cells were incubated at 37°C in a humidified 5% CO2 atm and fresh medium with M-CSF was added every 3 days. After 9 days of culture a homogeneous population of adherent macrophages was obtained (>90% F4/80+ cells as determined by flow cytometry, data not shown). BMDMs were incubated with CTCE-0214 (28–280 nM) for 1 h followed by stimulation with LPS (100 ng/ml, Sigma) for 18 h. The supernatants were collected for assay of interleukin (IL)-6 production.
LPS Shock
LPS shock was induced by intraperitoneal injection of LPS (25 mg/kg). CTCE-0214 (1 to 25 mg/kg) was administrated by i.v. injection at the same time as LPS administration. Dosing of CTCE-0214 was chosen based on previous publication [18]. PBS was used as vehicle control. One hour after LPS injection the mice were killed. Control mice received saline injections. Plasma tumor necrosis factor alpha (TNF-α), IL-6 and IL-10 levels were determined by enzyme-linked immunosorbant assay (ELISA).
Zymosan-Induced Peritonitis Model
We employed a zymosan model, a clinically relevant model of sterile peritonitis that induces multiple organ dysfunction [19]. Zymosan peritonitis was induced by injection (i.p.) of zymosan (500 mg/kg). CTCE-0214 (25 mg/kg) was administered (i.v. at 1 and 3 h and i.p. at 6 h) post zymosan injection. Control mice received saline injections. Twenty-four hours after zymosan challenge, mice were killed and plasma TNF-α, IL-6, and IL-10 were measured by ELISA (eBioscience, San Diego, CA).
Cecal Ligation and Puncture
Cecal ligation and puncture (CLP) was performed in CD-1 mice as previously described [17]. Specifically, mice were anesthetized with ether and a midline incision was made below the diaphragm to expose the cecum. The cecum was ligated at the colon juncture with a 5–0 silk ligature suture without interrupting intestinal continuity and punctured once or twice with a 22-gauge needle. The cecum was returned to the abdomen, and the incision was closed in layers with a 5–0 silk ligature suture and wound clips. After the procedure, the animals were fluid resuscitated with a 1.0-ml sterile saline injected subcutaneously. Sham operation was performed the same as CLP except for the ligation and puncture of the cecum.
For the survival study, CD-1 mice (n=15/group) were subjected to severe CLP (two-puncture model) or sham operation. CTCE-0214 (25 mg/kg) was administrated by injection subcutaneously at 2, 18, 26, 42, and 50 h after CLP. Survival wasmonitored every 12 h for a total of 120 h.
To determine plasma TNF-α, IL-6, and IL-10 production, CD-1 mice (three per group) were subjected to sham and another two groups were subjected to CLP (four to five per group). CTCE-0214 (10 mg/kg) was administrated by injection subcutaneously at 2 and 6 h after CLP. Mice were killed at 24 h after CLP. Plasma TNF-α, IL-6, and IL-10 production were determined by ELISA.
For measurement of plasma SDF-1α and IL-6 levels, CD-1 mice were subjected to the moderate sepsis model (CLP induced by one puncture with 22-guage needle, which results in more than 50% survival at 120 h (data not shown)) and severe sepsis model (CLP induced by two punctures with 22-guage needle, which results in less than 10% survival at 120 h (data not shown)). Twenty-four hours after CLP, mice were killed.
Assay for TNF-α, IL-6, IL-10, and SDF-1α Production
TNF-α, IL-6 and IL-10 levels were measured using mouse TNF-α, IL-6 or IL-10 ELISA (eBioscience, San Diego, CA). Plasma SDF-1α levels also were determined by ELISA (R&D System).
Statistical Analysis
Data are expressed as the mean±standard error of the mean. Statistical significance was determined by analysis of variance with Fisher’s probable least squares difference test or student t test using GraphPad Prism software. Log-rank (Mantel–Cox) Test was used for survival studies. p<0.05 was considered statistically significant.
RESULTS
CTCE-0214 Suppresses LPS-Induced Plasma TNF-α Production
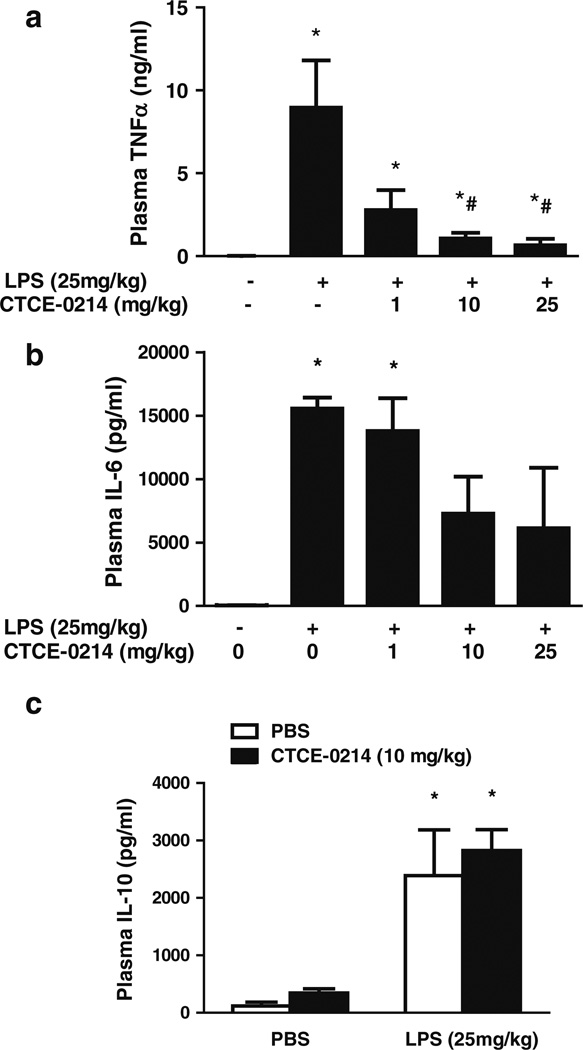
To test the potential beneficial effects of CTCE-0214, we determined its effect on LPS-induced shock. CTCE-0214 (1, 10, or 25 mg/kg) was administrated by i.v. injection at the same time as LPS administration. CTCE-0214 significantly decreased LPS-induced plasma TNF-α production in a dose dependent manor with a 93 ±4% reduction, p<0.05 (Fig. 2a). CTCE-0214 has no significant effect on LPS-induced plasma IL-6 and IL-10 production (Fig. 2b, c)
Fig. 2.
Effect of CTCE-0214 on LPS-induced plasma TNF-α, IL-6, and IL-10 production. CD-1 mice were injected with LPS (25 mg/kg, i.p.) with/without CTCE-0214 (1, 10, or 25 mg/kg, i.v.). One hour after the injections, the mice were killed and plasma TNF-α (a), IL-6 (b), and IL-10 (c) were determined by ELISA. *p<0.05 compared with control; #p<0.05 compared with LPS stimulation. N=3–5.
CTCE-0214 Decreased Zymosan-Induced Plasma TNF-α but Not IL-10 Production
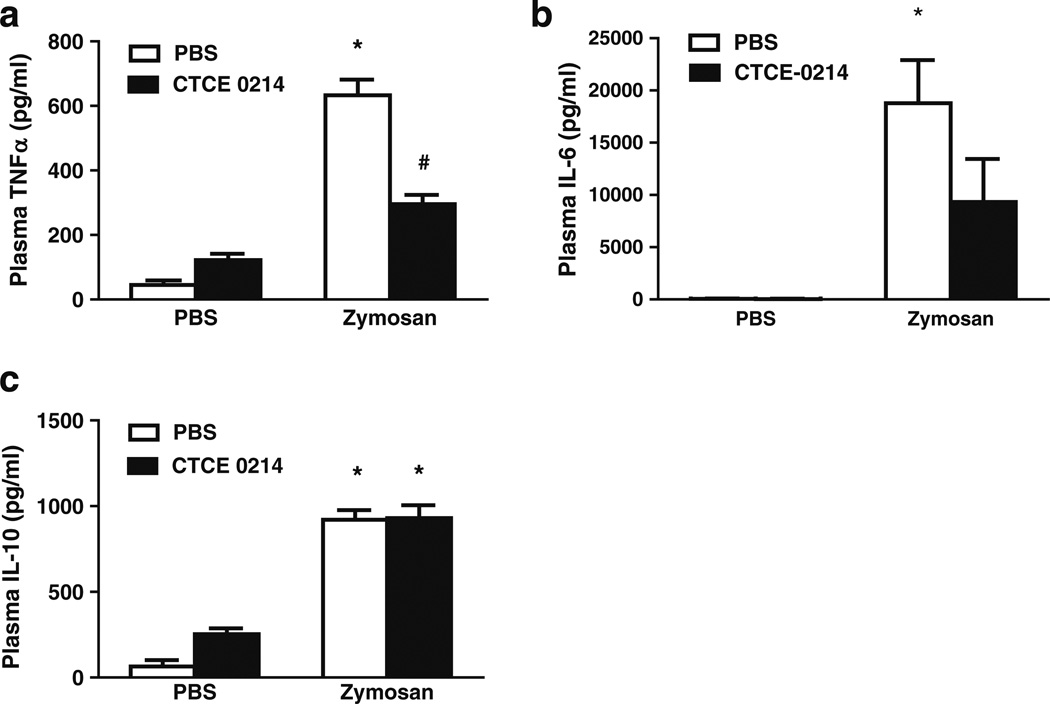
To further test the efficacy of CTCE-0214, peritonitis was induced using zymosan, a clinically relevant model of sterile peritonitis that induces multiple organ dysfunction [19]. Zymosan peritonitis was induced by injection (i.p.) of zymosan (500 mg/kg). CTCE-0214 (25 mg/kg) was administered (i.v. at 1 and 3 h and i.p. at 6 h) post zymosan injection.We found a significant reduction in plasma TNF-α (53±10% reduction, p<0.05) but not in IL-6 and IL-10 production in CTCE-0214-treated group compared to vehicle (PBS) group (Figs. 3a–c).
Fig. 3.
Effect of CTCE-0214 on zymosan-induced plasma TNF-α, IL-6, and IL-10 production. CD-1 mice were injected with zymosan (500 mg/kg, i.p.). One and three hours after zymosan injection, mice were injected with CTCE-0214 (25 mg/kg, i.v.). Six hours after zymosan injection, mice were injected with CTCE-0214 (25 mg/kg, i.p.). Twenty-four hours after zymosan challenge, mice were killed and plasma TNF-α (a), IL-6 (b), and IL-10 (c) were measured by ELISA. *p<0.05 compared with control; #p<0.05 compared with zymosan stimulation. N=3–6.
CTCE-0214 Suppresses Severe CLP-Induced Mortality
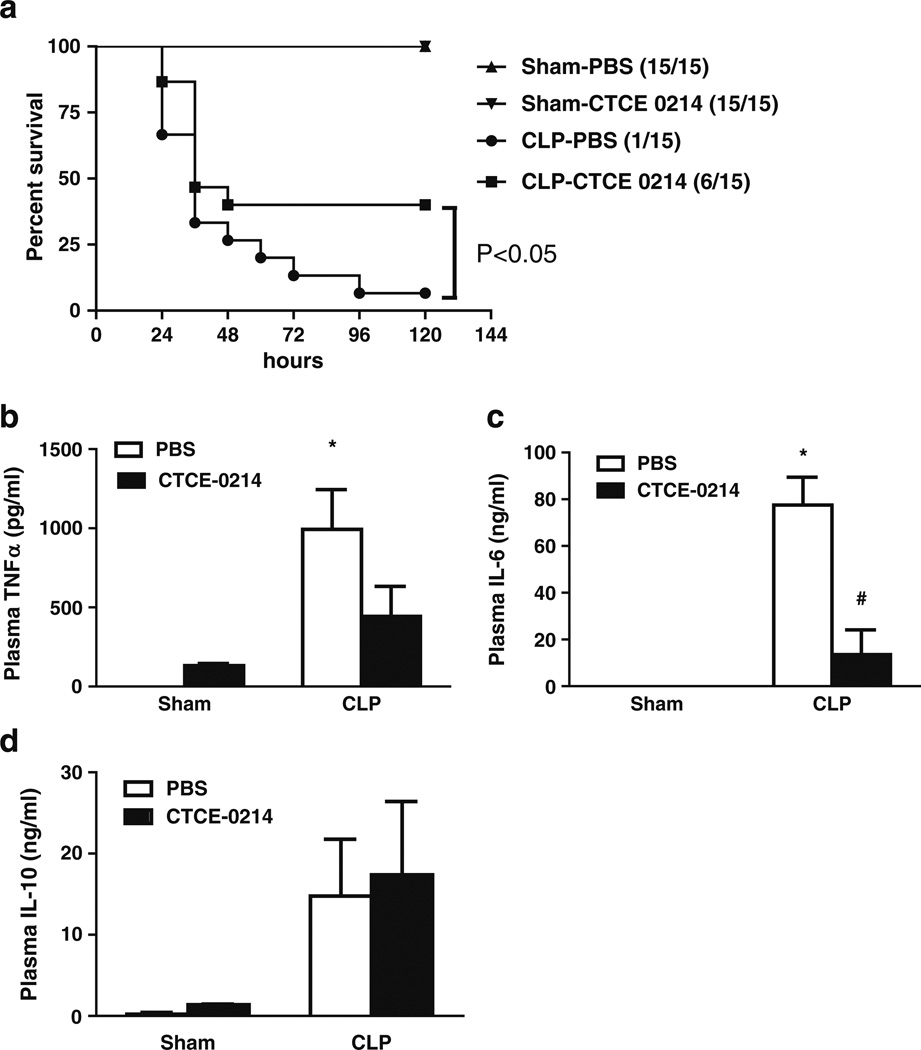
To determine the effect of CTCE-0214 in severe septic shock, severe sepsis was induced by CLP with two punctures with 22- gauge needle. This results in less than 10% survival at 120 h (Fig. 4a). CTCE-0214 (25 mg/kg) was administrated by injection subcutaneously at 2, 18, 26, 42, and 50 h after CLP. CTCE-0214 significantly reduced mortality induced by CLP (p<0.05, Fig. 4a). CTCE-0214 also significantly suppresses CLP-induced plasma IL-6 production but not TNF-α and IL-10 production compare to PBS group (Fig. 4b–d).
Fig. 4.
Effect of CTCE-0214 on severe CLP-induced sequelae. CD-1 mice were subjected to CLP in the absence of antibiotics. CTCE-0214 (25 mg/kg) or vehicle PBS was administrated by injection subcutaneously at 2, 18, 26, 42 and 50 h after CLP. Mortality was monitored every 12 h until 120 h (a). N=15/group. Statistics were determined with the log-rank (Mantel–Cox) test using GraphPad Prism software. *p<0.05 compared with the PBS group. CTCE-0214 (10 mg/kg) or vehicle PBS was also administrated by injection subcutaneously at 2 and 6 h after CLP. Plasma TNF-α (b), IL-6 (c), and IL-10 (d) were measured 24-h post-CLP. *p<0.05 compared with control, #p<0.05 compared with the PBS group. N=3–5.
SDF-1α Expression is Increased in Less Severe CLP but Inhibited in Severe CLP
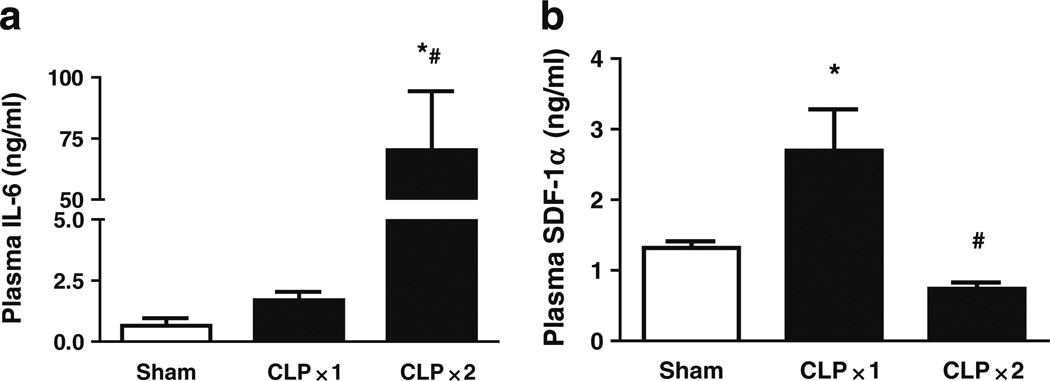
Mice were subjected to moderate sepsis, using one puncture with a 22-gauge needle, which results in more than 50% survival at 120 h (data not shown). Twenty-four hours after CLP, mice were killed and plasma was collected. Since plasma IL-6 levels have been shown to be an indicator of sepsis severity and correlate with sepsis outcome [20] plasma IL-6 was measured in relationship to plasma SDF-1α. Plasma IL-6 levels were the same as sham in the moderate CLP model but markedly increased in the severe CLP (Fig. 5a). SDF-1α levels were increased in the moderate CLP, but decreased back to sham levels in the severe CLP (Fig. 5b).
Fig. 5.
CLP-induced plasma IL-6 and SDF-1α production. CD-1 mice were subjected to moderate sepsis model (CLP X1) or severe sepsis model (CLP X2). Twenty-four hours after CLP, mice were killed and whole blood was collected. Plasma levels of IL-6 (a) and SDF-1α (b) were measured by ELISA. *p<0.05 compared with sham control; #p<0.05 compared with moderate CLP. N=3.
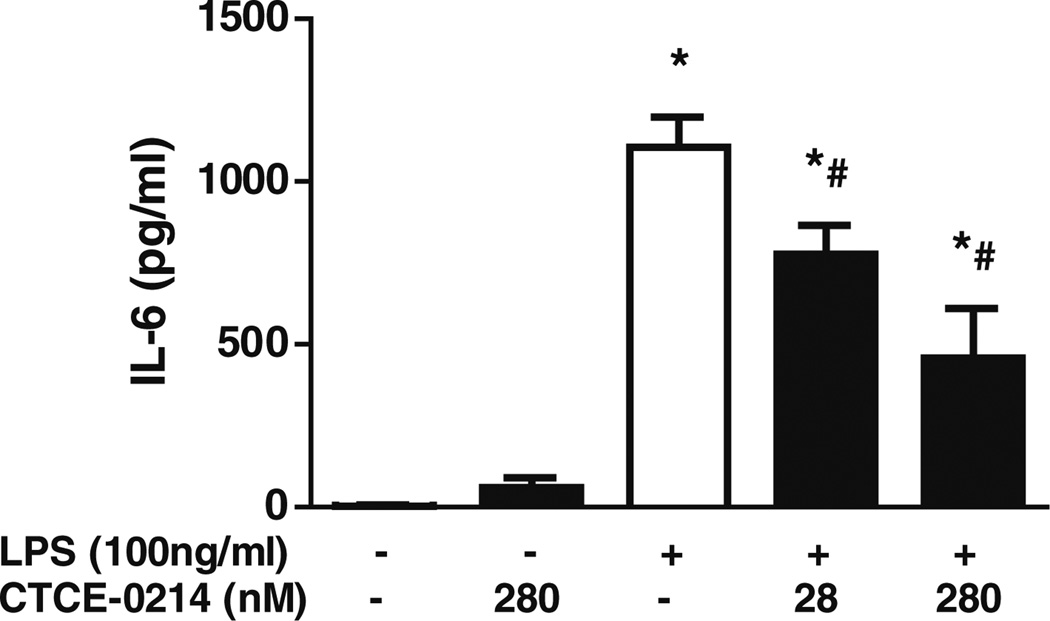
CTCE-0214 Inhibited LPS-Induced IL-6 Production in BMDM
BMDMs were incubated with CTCE-0214 (28–280nM) for 1 h followed by stimulation with LPS (100 ng/ml) for 18 h. CTCE-0214 significantly decreased LPS-induced IL-6 production (Fig. 6).
Fig. 6.
Effect of CTCE-0214 on LPS-induced IL-6 production in BMDM. BMDMs from CD-1 mice were incubated with CTCE-0214 (28–280nM) for 1 h followed by activation with LPS (100 ng/ml, Sigma) for 18 h. LPS-induced IL-6 production was examined by ELISA. *p<0.05 compared with basal control; #p<0.05 compared with LPS stimulation. N=3.
DISCUSSION
Our findings demonstrate that the SDF-1α analog and CXCR4 agonist CTCE-0214 is effective in vivo in suppressing plasma TNF-α in acute endotoxemia and zymosan-induced MODS. In both models CTCE-0214 did not suppress plasma increases in the anti-inflammatory cytokine IL-10. CTCE-0214 improved survival without antibiotics in a severe model of sepsis induced by CLP. CTCE-0214 also suppressed plasma increases in IL-6 but not TNF-α and IL-10 in response to CLP. Plasma IL-6 has been shown to be a predictor of sepsis severity [20]. We demonstrated in a moderately severe model of CLP (one puncture) that IL-6 levels at 24 h were similar to sham controls. However in severe CLP (two punctures) plasma IL-6 levels were markedly elevated. Interestingly plasma SDF-1α levels varied inversely with the plasma IL-6. Studies have demonstrated that inflammatory cytokines suppress SDF-1α production and CXCR4 expression [21]. In addition to the beneficial effect of CTCE-0214 in these models of systemic inflammation, we also demonstrated that the analog suppressed LPS-induced IL-6 production in BMDM.
To the best of our knowledge these results are the first to demonstrate in vivo beneficial effects of an SDF-1α analog in these murine models of severe inflammation. The mechanisms whereby CTCE-0214 mediates an anti-inflammatory effect remain unclear. SDF-1α has been shown to have multifunctional effects on varied cell types. In T cells SDF-1α has been shown to suppress antigen-induced recruitment to inflammatory sites [8]. SDF-1α suppresses LPS- stimulated tissue factor, and decreases phytohemagglutinin-induced inflammatory mediator MCP-1, IL-8, and matrix metalloproteinase-9 production in peripheral blood mononuclear cells from angina patients [9]. SDF-1α is protective in ischemia/reperfusion injury [10]. In that study infusion of SDF-1α prior to murine coronary artery occlusion followed by 4 h of reperfusion was accompanied by a reduction in infarct size. A CXCR4 antagonist abrogated this protection. In isolated myocytes, SDF-1α induced ERK1/2 and AKT activation but suppressed JNK and p38 phosphorylation. The authors concluded that SDF-1α and its receptor might afford protection from hypoxic stimuli by recruiting anti-apoptotic kinases ERK and AKT. In sepsis the PI3K-AKT pathway negatively regulates inflammatory processes and inhibition of PI3K-AKT signaling increases susceptibility to polymicrobial sepsis [22]. Thus, SDF-1α and its cognate chemokine receptor may play a key role in modulating the PI3K-AKT anti-apoptotic and anti-inflammatory pathways in sepsis. In addition to these signaling pathways hemoxygenase-1 (HO-1) appears to be important in hypoxic stress and mice deficient in HO-1 are more susceptible to polymicrobial sepsis [23]. Both vascular endothelial growth factor (VEGF) and SDF-1α may mediate transfected HO-1 protection in mice with ischemic infarction [24]. HO-1 transfection of cardiomyocytes induces VEGF and SDF-1α expression whereas neutralizing antibodies to VEGF and SDF-1α abrogated the protective effect of HO-1 in ischemic injury.
Pattern recognition receptors and proximal signaling events could also be altered by CTCE-0214. CXCR4 has been implicated as a sensing apparatus for LPS in addition to the TLR4, MD2, CD14 complex which forms a co-clustering complex of CXCR4 and TLR4 in lipid rafts upon LPS stimulation [3, 5]. Since LPS may directly activate CXCR4, CTCE-0214 could antagonize LPS binding. Activation of CXCR4 by SDF-1α in transfected HEK cells has been shown to suppress nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation in response to LPS stimulation whereas receptor antagonism of CXCR4 enhanced LPS-induced NF-κB activation. Thus the reduced TNF-α production in vivo observed with CTCE-0214 in acute endotoxemia may in part be a consequence of receptor antagonism or inhibition of NF-κB activation. However, zymosan-induced MODS and polymicrobial sepsis involve multiple TLRs and other protein recognition receptors in addition to TLR4. Also, SDF-1α binds to CXCR7. It is possible that CTCE-0214 binds to CXCR7 and regulates the LPS response. However, this possibility remains to be furhter investigated. Since CXCR4 is a Gαi protein coupled receptor, activation of Gαi signaling pathways may regulate TLR activation [12–14]. Of key relevance to the present findings is our previous observation that Gαi2(−/−) mice exhibit a profound pro-inflammatory phenotype to endotoxemia and CLP-induced sepsis [12, 25]. It is speculated that genetic deletion of Gαi removes the protective function of CXCR4 in MODS and sepsis. Thus activation of CXCR4 by autocrine or paracrine mechanisms may constitute a negative feedback pathway to control the systemic inflammatory response in critical illness. The present studies demonstrate that CTCE-0124 is beneficial in three distinctly different models of murine inflammation.
ACKNOWLEDGMENTS
This work was supported in part by NIH GM027673 (JAC), NIH A1079248 (HF), and Chemokine Therapeutics Corporation.
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: Cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. Journal of Immunology. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 3.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nature Immunology. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews. Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 5.Triantafilou M, Lepper PM, Briault CD, Ahmed MA, Dmochowski JM, Schumann C, Triantafilou K. Chemokine receptor 4 (CXCR4) is part of the lipopolysaccharide "sensing apparatus". European Journal of Immunology. 2008;38:192–203. doi: 10.1002/eji.200636821. [DOI] [PubMed] [Google Scholar]
- 6.Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. American Journal of Physiology. Cell Physiology. 2007;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 7.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. The Journal of Experimental Medicine. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poznansky MC, Olszak IT, Foxall R, Evans RH, Luster AD, Scadden DT. Active movement of T cells away from a chemokine. Natural Medicines. 2000;6:543–548. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- 9.Damås JK, Waehre T, Yndestad A, Ueland T, Müller F, Eiken HG, Holm AM, Halvorsen B, Frøland SS, Gullestad L, Aukrust P. Stromal cell-derived factor-1alpha in unstable angina: Potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002;106:36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: Role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan H, Peck OM, Tempel GE, Halushka PV, Cook JA. Toll-like receptor 4 coupled GI protein signaling pathways regulate extracellular signal-regulated kinase phosphorylation and AP-1 activation independent of NFkappaB activation. Shock. 2004;22:57–62. doi: 10.1097/01.shk.0000129759.58490.d6. [DOI] [PubMed] [Google Scholar]
- 12.Fan H, Zingarelli B, Peck OM, Teti G, Tempel GE, Halushka PV, Cook JA. Lipopolysaccharide- and gram-positive bacteria-induced cellular inflammatory responses: Role of heterotrimeric G{alpha}i proteins. American Journal of Physiology. Cell Physiology. 2005;289:C293–C301. doi: 10.1152/ajpcell.00394.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lentschat A, Karahashi H, Michelsen KS, Thomas LS, Zhang W, Vogel SN, Arditi M. Mastoparan, a G protein agonist peptide, differentially modulates TLR4- and TLR2-mediated signaling in human endothelial cells and murine macrophages. Journal of Immunology. 2005;174:4252–4261. doi: 10.4049/jimmunol.174.7.4252. [DOI] [PubMed] [Google Scholar]
- 14.Solomon KR, Kurt-Jones EA, Saladino RA, Stack AM, Dunn IF, Ferretti M, Golenbock D, Fleisher GR, Finberg RW. Heterotrimeric G proteins physically associated with the lipopolysaccharide receptor CD14 modulate both in vivo and in vitro responses to lipopolysaccharide. The Journal of Clinical Investigation. 1998;102:2019–2027. doi: 10.1172/JCI4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tudan C, Willick GE, Chahal S, Arab L, Law P, Salari H, Merzouk A. C-terminal cyclization of an SDF-1 small peptide analogue dramatically increases receptor affinity and activation of the CXCR4 receptor. Journal of Medicinal Chemistry. 2002;45:2024–2031. doi: 10.1021/jm0104015. [DOI] [PubMed] [Google Scholar]
- 16.Li K, Chuen CK, Lee SM, Law P, Fok TF, Ng PC, Li CK, Wong D, Merzouk A, Salari H, Gu GJ, Yuen PM. Small peptide analogue of SDF-1alpha supports survival of cord blood CD34+ cells in synergy with other cytokines and enhances their ex vivo expansion and engraftment into nonobese diabetic/severe combined immunodeficient mice. Stem Cells. 2006;24:55–64. doi: 10.1634/stemcells.2005-0082. [DOI] [PubMed] [Google Scholar]
- 17.Fan H, Bitto A, Zingarelli B, Luttrell LM, Borg K, Halushka PV, Cook JA. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez LE, Alpdogan O, Shieh JH, Wong D, Merzouk A, Salari H, O'Reilly RJ, van den Brink MR, Moore MA. Increased plasma levels of stromal-derived factor-1 (SDF-1/CXCL12) enhance human thrombopoiesis and mobilize human colony-forming cells (CFC) in NOD/SCID mice. Experimental Hematology. 2004;32(3):300–307. doi: 10.1016/j.exphem.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Volman TJ, Hendriks T, Goris RJ. Zymosan-induced generalized inflammation: experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock. 2005;23:291–297. doi: 10.1097/01.shk.0000155350.95435.28. [DOI] [PubMed] [Google Scholar]
- 20.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: Interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. Journal of Immunology. 2001;166:5749–5754. doi: 10.4049/jimmunol.166.9.5749. [DOI] [PubMed] [Google Scholar]
- 22.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- 23.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. Journal of Clinical Investigation. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin HH, Chen YH, Chang PF, Lee YT, Yet SF, Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. Journal of Molecular and Cellular Cardiology. 2008;45:44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Fan H, Li P, Zingarelli B, Borg KT, Halushka PV, Birnbaumer L, Cook JA. Heterotrimeric Gαi proteins are regulated by lipopolysaccharide and are anti-inflammatory in endotoxemia and polymicrobial sepsis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011 doi: 10.1016/j.bbamcr.2011.01.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]