Abstract
Epoxyeicosatrienoic acids (EETs) are arachidonic acid metabolites that importantly contribute to vascular and cardiac physiology. The contribution of EETs to vascular and cardiac function is further influenced by soluble epoxide hydrolase (sEH) that degrades EETs to diols. Vascular actions of EETs include dilation and angiogenesis. EETs also decrease inflammation and platelet aggregation and in general act to maintain vascular homeostasis. Myocyte contraction and increased coronary blood flow are the two primary EET actions in the heart. EET cell signaling mechanisms are tissue and organ specific and provide significant evidence for the existence of EET receptors. Additionally, pharmacological and genetic manipulations of EETs and sEH have demonstrated a contribution for this metabolic pathway to cardiovascular diseases. Given the impact of EETs to cardiovascular physiology, there is emerging evidence that development of EET-based therapeutics will be beneficial for cardiovascular diseases.
I. Overview of Eicosanoid Metabolic Pathways
Fatty acids are an essential dietary component, and it has long been recognized that arachidonic acid is an essential fatty acid. Arachidonic acid is a major component of cell membranes that resides in the sn-2 position of phospholipids. Once liberated from cell membrane phospholipids, this 20-carbon fatty acid is converted by a series of enzymes to numerous biological active metabolites termed “eicosanoids.” Cyclooxygenase (COX) metabolites that include the prostaglandins (PGs) were the first arachidonic acid metabolites to be extensively studied. A major breakthrough that highlighted the potential cardiovascular importance for eicosanoids was the discovery that aspirin inhibited COX enzymes and the formation of PGs (86). Since that time, drug therapies for cardiovascular diseases, pain, inflammation, and cancer have been developed that manipulate enzymes of the COX metabolic pathway, mimic or antagonize COX metabolites, and inhibit or activate COX metabolite receptors (15, 43, 113). Another arachidonic enzymatic pathway is the lipoxygenase (LOX) pathway that is responsible for the generation of hydroxyeicosatetraenoic acids (HETEs), lipoxins (LXs), and leukotrienes (LTs). These metabolites have been implicated in pulmonary responses to asthma, inflammation, and atherosclerosis (27, 240, 253). LT antagonists are currently being utilized for the treatment of asthma (240). The third eicosanoid enzymatic pathway is the cytochrome P-450 (CYP) pathway that contains two distinct enzymatic activities. CYP hydroxylase enzymes generate HETEs, such as 20-HETE, that have cardiovascular and proinflammatory activities (242, 295). Epoxyeicosatrienoic acids (EETs) derived from CYP epoxygenase enzymes also have cardiovascular actions and are anti-inflammatory (25, 89, 130, 264). This review focuses on the generation and regulation of the EETs and how these epoxides can impact cardiovascular physiology.
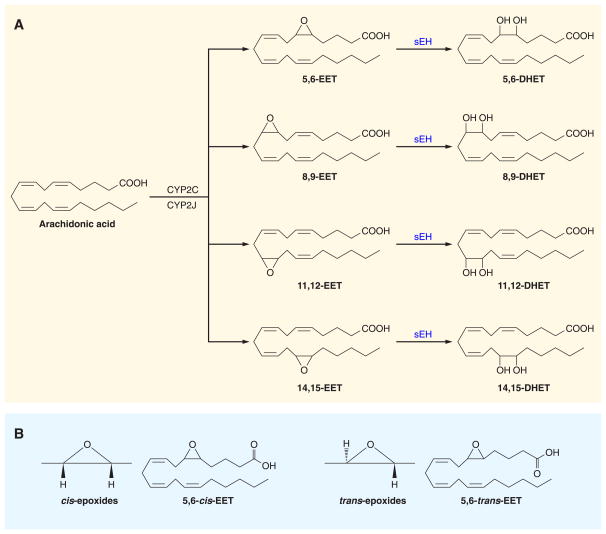
CYP epoxygenase enzymes belong to a complex superfamily of genes with a common evolutionary origin, a conserved peptide that provides them with a cysteine heme ligand, and the capacity to take an active form of atomic oxygen to ground state carbons (22–24). Epoxygenase enzymes generate cis-epoxides with a high degree of enantiofacial selectivity consisting of a mixture of (R,S) and (S,R) enantiomers (22, 263). Eventhough epoxygenase enzymes generate cis-EETs, both cis-EETs and trans-EETs have been found in plasma (148, 151). The biochemical pathway responsible for trans-EETs remains to be determined. As with other CYP enzymes, CYP epoxygenase enzymes are expressed in a gender-, age-, and organ-specific manner (22, 263). Hormonal and paracrine factors as well as environmental factors and diseases can alter CYP expression and activity (22, 25, 128, 263). The CYP epoxygenases that convert arachdionic acid to EETs are primarily members of the CYP2C and CYP2J classes (22–24, 316). These CYP epoxygenase enzymes are located in the endoplasmic reticulum and add an epoxide across one of the four double bonds in arachidonic acid to produce four EET regioisomers: 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET (22, 23) (FIG. 1).
Figure 1.
Cytochrome P-450 epoxygenase metabolic pathway. A: epoxides are generated from arachidonic acid. Arachidonic acid is converted to epoxyeicosatrienoic acid (EET) by cytochrome P-450 (CYP) epoxygenase. EETs primary metabolic fate is conversion to dihydroxyeicosatrienoic acids (DHETs) by the soluble epoxide hydrolase (sEH) enzyme. B: CYP epoxygenases generate cis-epoxides, whereas cis- and trans-epoxides exist in plasma (22, 151, 263). The enzymes that form trans-EETs remain unknown but could occur through radical-driven reactions (148, 151).
The CYP2C family of enzymatic proteins is responsible for the majority of the epoxide metabolite generation in mammals. Even though there is species variation of the CYP2C isoforms, there is a high degree of homology and catalytic activity between the human, rat, and mouse epoxygenase enzymes (23). Although homology between species is known to a certain degree, homologous human isoforms have not been identified for many of the rodent CYP enzymes (23). The main CYP epoxygenase in the humans, rats, and mice are as follows: humans, CYP2C8; rats, CYP2C11 and CYP2C23; mice, CYP2c40 and CYP2c44 (22, 25, 51, 64, 65, 277, 283, 318). This is not an exclusive list because a number of other CYP enzymes capable of epoxide activity have been identified in these species, but these CYP2Cs are the major contributors to EET generation based on sequence homology and regioisomeric epoxygenase activity. Human recombinant CYP2C8 enzyme generates 11,12-EET and 14,15-EET in equal amounts, whereas 5,6-EET and 8,9-EET were nondetectable (317). The regioisomeric EET profile for the rat CYP2C23 is 24% 8,9-EET, 60% 11,12-EET, and 11% 14,15-EET. The rat CYP2C11 generates a relatively equal percentage of 14,15-EET (39%) and 11,12-EET (41%) (21, 23, 156). Murine Cyp2c44 has a regioisomeric EET profile (20% 8,9-EET, 65% 11,12-EET, and 15% 14,15-EET) that is consistent with it being the major mouse kidney epoxygenase isoform (25, 283). Most importantly, the rat CYP2C23 and mouse Cyp2c44 have a very high degree of sequence homology with the human CYP2C8 epoxygenase (25, 156, 283).
The other CYP epoxygenase to consider is the CYP2J family because it has been identified as an enzyme that is capable of epoxide activity in rodents (316). Even though human CYP2J2, rat CYP2J3, and mouse Cyp2j5 isoforms all preferentially catalyze epoxidation of arachidonic acid at the 14,15-position, it is important to point out that the catalytic activity of the CYP2J to form epoxides of arachidonic acid is manyfold less than the CYP2C enzymes (21, 264). CYP2J2 generates 11,12-EET as a racemic mixture, whereas 14,15-EET is synthesized as 76% as the 14,15(R,S)-EET enantiomer (297). Human polymorphism studies indicate that CYP2J enzymes are associated with cardiovascular diseases and suggest that these epoxygenases contribute to cardiovascular function (56, 322).
EET metabolism and cellular localization are important considerations, since these can influence their biological actions. EETs generated by epoxygenase enzymes or hydrolyzed from cell membrane phospholipids by phospholipase A2 (PLA2) can then be further metabolized. The main EET catabolic pathway is conversion to their corresponding diols by the soluble epoxide hydrolase (sEH) enzyme. 14,15-EET is the preferred substrate for sEH with 11,12-EET and 8,9-EET also being converted to their corresponding dihydroxyeicosatrieonic acids (DHETs) (32, 171, 208, 214, 221, 222) (FIG. 1). However, 5,6-EET is a poor substrate for sEH (208, 214). Other metabolic pathways include β-oxidation, ω-oxidation, and chain elongation (263). Chain elongation and β-oxidation are EET metabolic pathways that become prominent when tissue sEH activity is low or inhibited (78, 263). Chain elongation results in the formation of 22-carbon products from 11,12-EET and 14,15-EET (74, 263). 11,12-EET and 14,15-EET can also undergo β-oxidation resulting in 16-carbon epoxy fatty acids (73, 79, 263). CYP ω-oxidases have the capacity to add a methyl-terminal hydroxyl group to 8,9-EET, 11,12-EET, and 14,15-EET (263). Although glutathione-S-transferase can form EET glutathione conjugates, the biological significance for this EET metabolic pathway is questionable since the Km for EETs is in the high micromolar range (208, 214). On the other hand, EET metabolism by COX enzymes may have physiological relevance. Only 5,6-EET and 8,9-EET are substrates for COX, and 5,6-EET is converted to the PG analog 5,6-epoxy-PGE1 while 8,9-EET is converted to 11,12-hydroxy-8,9-EET (127, 263). These EET metabolic pathways can regulate EETs and their activity in organs.
Epoxy-derivatives can also be formed from unstable hydroxyperoxy intermediates of the LOX pathway. Arachidonic acid is converted to 15-hydroperoxyeicosatetraenoic acid (15-HPETE) by 15-LOX or 12-HPETE by 12-LOX (35). These HPETE intermediates can be metabolized to hydroxyepoxyeicosatrienoic acids (HEETAs), also referred to as hepoxylins, by hydroperoxide isomerases (36). Interestingly, CYP2J2 demonstrates hydroperoxidase isomerase activity and can generate 15-HEETA (36). HEETAs can be further metabolized by sEH to trihydroxyeicosatrienoic acids (THETAs) (35). Both HEETAs and THETAs appear to have biological activities including vascular actions that are distinct from EETs (35, 36, 103).
Localization of EETs to cell membranes and binding to proteins can also regulate EET actions (21, 263). EETs can be incorporated into membrane phospholipids, and this can account for plasma and tissue levels (291). Most of the phospholipid that contains EETs in the plasma is present in low-density lipoproteins (263). EETs found in human or rat tissues are almost exclusively located in the sn-2 position of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol (263, 291). Incorporation of EETs into phospholipids occurs through a coenzyme A-dependent process with the largest amount of EETs incorporated into phosphatidylcholine (263, 291). Even though EETs represent 0.01% of the total fatty acyl chains in phospholipids, changes in the amount of EETs could influence the lipid microenvironment in localized domains to have functional consequences. EETs incorporated in cell membrane phospholipids have the potential to be released as a diacyglycerol by phospholipase C (PLC) and acted upon by diacylglycerol lipase (DAGL) to form 2-epoxysatrienoylglycerols (2-EGs) (41). EETs also have the ability to bind to cytosolic fatty acid binding protein (FABP) (263, 294). FABP could then act as a transport protein for EETs and deliver them to specific intracellular enzymes or organelles. Interestingly, the primary metabolites of EETs, DHETs, incorporate into cell membrane phospholipids and bind to FABP in small amounts (263). This weak incorporation and binding of DHETs may explain why the majority of the DHETs formed in cells are released into the extracellular fluid.
II. Cardiac and Vascular Localization: CYP Epoxygenases
The localization and expression of CYP epoxygenases can determine their impact on function and regulation in response to paracrine and hormonal factors. Thus it is not surprising that specific CYP epoxygenases are localized to the heart and blood vessels and that the blood vessels within each organ can express different epoxygenases.
The CYP2J family appears to be the primary CYP epoxygenase isoform responsible for EET synthesis in the heart (316, 325). Human heart microsomes generate 8,9-EET and 14,15-EET with high enantioselectivitity for 14,15(R,S)-EET (217, 263, 326). Ventricular myocytes contain the CYP2J3 isoform, and 14,15-EET is the major EET regioisomer generated by recombinant CYP2J3 (263, 316). The CYP2J2 isoform is also expressed in cardiomyocytes producing 14,15-EET, 11,12-EET, and 8,9-EET (263, 316). Other isoforms are also known to be present in human heart. The human myocardium was determined to express CYP2C9 but not CYP2C8 by semiquantitative immunohistochemical analysis (64, 65). Mouse hearts express the novel Cyp2c50 epoxygenase enzyme but at much lower levels than the abundant liver expression (275, 277). The presence of the sEH enzyme in cardiac myocytes regulates the conversion of EETs to DHETs, and this regulation impacts on heart functions (65, 210, 250, 300).
Vascular localization has been extensively studied, and the epoxygenase enzymes and their regulation in physiological and pathophysiological states have been determined. Epoxygenase enzymes are present in endothelial and vascular smooth muscle cells, and in general, smaller resistance-sized arteries and arterioles have a greater capacity to generate EETs. Human arteries and arterioles express CYP2C8, CYP2C9, CYP2J2, and sEH enzymes (65, 313). Human coronary endothelial cells highly express sEH, and the presence of this enzyme is considerably lower in the vascular smooth muscle cell layers (63–65). Murine and bovine vascular expression has determined that the Cyp2C isoforms are primarily responsible for EET generation (84, 142, 208). Rat endothelial cells express CYP2C11 and CYP2C23 epoxygenase enzymes (142, 326). Renal microvessels have a higher CYP2C23 expression, whereas CYP2C11 predominates in others such as the mesenteric and coronary resistance arteries (142, 326). In mice, the predominant vascular epoxygenase enzyme is the Cyp2c44 (25, 247); however, vascular expression of the less active Cyp2c29 and Cyp2c38 enzymes have not been fully characterized (278, 317). To date, there is limited information on differences between blood vessel expression in the various organs and how epoxygenase activity is regulated in pathophysiological states.
Circulating blood cells could be a significant source for and have the potential to regulate EET levels. EETs are esterified in phospholipids in erythrocytes isolated from a number of species including humans (148–150). Epoxidation of arachidonic acid is catalyzed by hemoglobin in red blood cells (147, 151). Erythrocyte EETs can serve as a reservoir for release to vasodilate, inhibit platelet aggregation, and decrease inflammation (147). Likewise, red blood cells have sEH activity that hydrolyzes cis-EETs and trans-EETs (151, 255). Human peritoneal macrophages are another circulating cell that has the capacity to generate EETs and their corresponding DHETs (147, 235). CYP2J2 expression has been demonstrated in human alveolar macrophages and monocytic cells but not in neutrophils (212, 316). Stimulation of human monocytes with macrophage-colony stimulating factor upregulates CYP2J2 expression (212). These findings suggest that circulating cells have the capacity to generate EETs that influence cardiovascular function.
III. Genetic and Pharmacological Means to Manipulate EETs and sEH
A major roadblock for investigating EET cardiovascular and cell signaling actions is loss of the epoxygenase pathway in cell culture systems (87, 90,128). Another complexity that has hampered progress in the field has been that EETs are produced by multiple epoxygenase enzymes localized to different organs and cell types. Genetic manipulation has been conducted in cell cultures systems and mice over the last decade. This has resulted in a greater understanding for the contribution of EETs to cardiovascular function as well as cell signaling mechanisms responsible. Pharmacological manipulation of the epoxygenase pathway has also made great advances in the last 10–15 years. There are agonists, antagonists, and enzymatic inhibitors that can be used in cell culture and administered to animals chronically. Many of these pharmacological tools are commercially available and have been used by investigators worldwide to examine the contribution of EETs to cardiac and vascular function.
Many of the epoxygenase pathway enzymes have been knocked out using conventional gene deletion strategies in mice. One of the first deleted was the Cyp2j5 gene, and this resulted in a number of unexpected findings (8). First, deletion of the Cyp2j5 gene did not alter EET generation or epoxygenase activity in the animal or specific tissues (8). There was a gender-specific increase in blood pressure and enhanced renal vasoconstrictor responses to angiotensin and endothelin-1 in the female Cyp2j5 −/− mice (8). These changes in female Cyp2j5 −/− mice were associated with low plasma 17β-estradiol levels (8). Another interesting finding has been that deletion of the Cyp4a10 gene resulted in decreased Cyp2c44 enzyme expression and decreased EET generation (25). These mice had salt-sensitive hypertension as a consequence of the decreased EET production (25). Experimental studies describing the cardiovascular phenotype for mice with deletion of the Cyp2c44 gene, the major murine epoxygenase enzyme, have yet to be published. Deletion of the Ephx2 the gene responsible for production of the sEH enzyme has provided fewer surprises but somewhat controversial findings. The initial phenotype for the Ephx2 −/− mice was a gender-specific decrease in systolic blood pressure measured by tail cuff plethysmography in the male mice (259). There was also the expected increase in EET levels and the epoxide-to-diol ratio (259). Independent generation of Ephx2 −/− by other groups has not demonstrated this blood pressure phenotype when blood pressure was measured by indwelling catheters and radio-telemetry (190, 194). There has been a lack of development in regards to site-specific targeted or Tet-on gene deletion for the epoxygenase pathway genes. On the other hand, the genetic gene-deleted mice that have been generated have provided considerable information on the importance of the epoxygenase pathway to cardiovascular function and the contribution of EETs to disease states.
Manipulating expression of epoxygenase pathway genes has been one approach to circumvent the loss of expression of this pathway in cell culture systems. This approach has been used in endothelial cells, vascular smooth muscle cells, and cardiac myocytes to generate EETs in these cell cultures. CYP2C and CYP2J epoxygenase enzymes have been successfully overexpressed in these cardiovascular as well as other cell culture systems (232, 249, 302). There have also been genetic manipulated enzymes that specifically generate a single regio- and stereo-selective epoxygenase metabolite. The F87V BM-3 enzyme, a mutant fatty acid hydroxylase isolated from Bacillus megaterium, produces 14,15(S,R)-EET (40). Kidney epithelial cells transfected with F87V BM-3 allowed for the determining the contribution of 14,15(S,R)-EET to mitogenic signaling responses (40). Likewise, expression of epoxygenase genes in vascular and heart cells has generated important information on cell signaling mechanisms involved in migration, proliferation, and responses to hormonal and paracrine factors (232, 249, 302). Genetic mice that express human epoxygenase CYP2C8 and CYP2J2 enzymes in endothelial cells have been generated, and the impact on blood pressure regulation and vascular inflammation has been examined (52, 172). These findings in cell culture and genetic mice will be detailed in later sections of this review.
The development of pharmacological tools to determine the contributions for epoxygenase metabolites to vascular and cardiac function has progressed considerably over the last two decades. Initially, chemical compounds that were general CYP inhibitors were used in the experimental setting. CYP inhibitors such as 17-ODYA were utilized, and their effects were compared with PLA2, COX, and LOX inhibitors (127, 242). A limitation for the use of general CYP inhibitors like 17-ODYA was that these compounds would not only inhibit CYP epoxygenase enzymes but would inhibit CYP hydroxylase enzymes (127, 242). Miconazole, ketaconazole, and econazole were used as more selective CYP epoxygenase inhibitors; however, these are antifungal agents and interpretation of results with these agents had to been done with great caution. In the mid 1990s, Dr. John R. Falck and co-workers (139) developed a series of compounds that were selective CYP epoxygenase or CYP hydroxylase inhibitors. CYP hydroxylase inhibitors included DDMS, and CYP epoxygenase inhibitors included PPOH and MS-PPOH. PPOH and MS-PPOH effectively inhibited renal epoxygenase activity with very little hydroxylase inhibitory activity at high micromolar concentrations (134, 139). This breakthrough allowed investigators to more accurately determine the contributions of 20-HETE and EETs to cardiovascular function.
The next big step in the development of pharmacological tools to evaluate the epoxygenase pathway was the design and synthesis of EET antagonists. EET antagonists are based on the general EET structure. The EET antagonist that is most widely used is 14,15-EE-5-ZE (aka. 14,15-EEZE) that is very similar to 14,15-EET but lacks an 8,9-and 11,12-olefin bond. 14,15-EEZE inhibits the vasodilation of all EET regioisomers, whereas 14,15-EEZE methyl-sulfonamide (14,15-EEZE-mSI) inhibits 14,15-EET and 5,6-EET but not 11,12-EET-mediated vasodilation (69, 100, 104). These findings provide evidence for unique structure-activity relationships for the various EET regioisomers and the possibility of multiple EET binding sites or receptors. Newer chemical compounds are being developed that can selectively antagonize the vascular actions of 11,12-EET or 14,15-EET (16). These newer pharmacological tools will allow investigators to distinguish the cardiovascular actions for specific EET regioisomers.
EET regioisomers have variable activities and potencies and utilize different cell signaling mechanisms. The development of EET regioisomeric specific agonists has been a major step towards investigating these differences and defining their structure-activity relationships. Initial modification to replace the carboxylic acid with a sulfonamide on various regioisomeric EETs was done to resist β-oxidation (69, 102). Several generations of EET analogs/agonists were synthesized that improved solubility and resisted auto-oxidation and metabolism by sEH (54, 70, 102, 132). Likewise, epoxide derivatives of docosahexaenoic acid have been synthesized and act as omega-3 EET analogs (310). Even though there has been great progress with the development of EET agonists that has enhanced our understanding of EET actions, the development of EET agonists that can be administered in vivo either acutely or chronically has been lacking until very recently. This has hampered progress toward evaluating the therapeutic potential for EET agonists.
Unlike EET agonists, sEH inhibitors have been extensively tested in vivo for cardiovascular therapeutic potential. The rapid development and testing of sEH inhibitors began with a breakthrough study published in 2000 that demonstrated that acute injection of an sEH inhibitor lowered blood pressure in spontaneously hypertensive rats (SHR) (314). Transition-state sEH inhibitors that were found to be potent and stable were amides, ureas, and carbamates (129, 162, 207). These sEH inhibitors inhibit the epoxide hydrolase activity of the COOH-terminal domain of the enzyme without affecting the NH2-terminal phosphatase domain (162, 207, 208). The functional importance of the NH2-terminal domain is unclear, but one potential function is the stabilization of epoxide hydrolase activity (276). Chronic sEH inhibitor administration was first achieved by intraperitoneal 1-cyclohexyl-3-dodecyl urea (CDU) injections into angiotensin hypertension rats (141). A second major milestone occurred when functional polar groups were incorporated into one of the alkyl chains of 1,3-disubstituted urea sEH inhibitors to improve their physical properties (161, 196, 209). This resulted in the design and synthesis of 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), which was the first sEH inhibitor to demonstrate oral bioavailability and blood pressure lowering effects when administered via this route (143). Significant testing of sEH inhibitors over the past 10 years demonstrates that they appear to be highly selective for sEH, lack significant toxicity, and have potential for use in humans (136, 196). Thus sEH inhibitors have been extensively studied for their ability to effect vascular and cardiac function and their potential therapeutic for cardiovascular diseases.
Genetic and pharmacological manipulation of EETs and sEH has contributed significantly to our understanding of their contribution to cardiovascular function. There is still significant progress to be made to have a better understanding. First, there needs to be additional progress with genetic rodent models including additional site-specific genetic manipulation. Another area is development of better CYP epoxygenase enzymatic inhibitors, EET antagonists, and EET agonists that can be administered in vivo acutely or chronically. These would allow for experiments to better define cell-signaling mechanisms by which EETs exert cardiovascular actions and have the potential to identify EET binding sites or receptors.
IV. EETs and sEH Regulation of Vascular Tone
The initial investigations into EET vascular actions involved determining diameter or organ blood flow responses when various regioisomeric EETs were added to the perfusate or bathing solution. In the first description of EET vascular actions, Proctor et al. (236) determined that bolus dose administration of 14,15-EET, 11,12-EET, or 8,9-EET increased intestinal arteriolar blood flow that was similar to that elicited by adenosine. Subsequent studies in coronary, renal, and cerebral vasculatures demonstrated that regioisomeric EETs resulted in increases in organ blood flow or vascular diameters (19, 54, 84, 142, 171). EET vasodilation has also been observed in blood vessels from bovine, canine, rodent, and rabbit species (87, 130, 242). More importantly, human blood vessels dilate in response to EETs (7, 172). There were also a few studies that found that certain EETs, usually 5,6-EET and 8,9-EET, resulted in vasoconstriction that was associated with COX metabolism (98, 138, 274). This is in agreement with the findings that 5,6-EET and 8,9-EET but not 11,12-EET and 14,15-EET are substrates for COX enzymes (127, 274). These COX-dependent EET metabolites resulted in vasoconstriction through activation of thromboxane (TP) receptors (98, 138). Another consistent finding with EETs was that EETs were more active than their corresponding diols, DHETs (270, 316). Coronary arteries are where DHETs have consistently had vascular activity (17, 69, 171). Early investigations demonstrated that EETs could have actions on both endothelial and vascular smooth muscle cells (87, 89, 117, 270). Overall, these initial studies demonstrated EETs were vasodilators and led to speculation that they could possibly serve as an endothelium-derived hyperpolarizing factor (EDHF).
Endothelial factors were first demonstrated to influence vascular tone and resistance in the 1980s. The discovery that nitric oxide was an endothelium-derived relaxing factor (EDRF) sparked interest in identifying other endothelium-derived factors. Although endothelium-dependent vasodilation to bradykinin and acetylcholine in large blood vessels such as the aorta were largely nitric oxide mediated, resistance-sized blood vessels demonstrated a nitric oxide-and COX-independent vasodilation (19, 47, 99, 135). This unknown nitric oxide- and COX-independent endothelial-derived factor was found to hyperpolarize vascular smooth muscle cells and coined EDHF (47). Additional experimental evidence suggested that EDHF was an arachidonic acid metabolite that activated vascular smooth muscle cell large-conductance calcium-activated K+ (KCa) channels (47, 81, 99). Campbell et al. (19) provided key experimental evidence that identified EETs as EDHFs. Bovine coronary artery relaxation and vascular smooth muscle cell hyperpolarization in response to methacholine was eliminated by the presence of epoxygenase enzyme inhibitors (19). Methacholine increased the metabolism of EETs by bovine coronary arteries (19). EETs bovine coronary artery relaxations were attenuated by inhibition of KCa but not ATP-sensitive potassium (KATP) channels, and 14,15-EET increased coronary arterial smooth muscle K+ channel activity (19). Further evidence for EETs as an EDHF was provided in a bioassay method with a donor and recipient coronary artery. These studies demonstrated that bradykinin releases a transferable CYP endothelial factor that activates KCa channels and hyperpolarizes coronary arterial smooth muscle cells (19). Another key publication identified CYP2C epoxygenase enzymes as an EDHF synthase in porcine coronary arteries (84). In these studies β-naphthol-flavone increased CYP2C expression as well as 11,12-EET generation and enhanced bradykinin-mediated vasorelaxation and membrane hyperpolarization (84). A CYP2C8/34 antisense oligonucleotide decreased CYP2C expression and abolished bradykinin-induced, EDHF-mediated hyperpolarization of coronary arterial smooth muscle cells (84). These studies provided significant evidence to support the notion that EETs were important endothelial factors and led to additional studies to further define EET vascular actions and cell-signaling mechanisms responsible for their vascular actions.
Numerous experimental studies determined the vascular actions of EETs in various organ beds. Although the occasional experimental study has failed to find vasodilation or vasoconstriction in response to EETs, the overwhelming majority of studies have found EETs to vasodilate. These investigations have also provided a better overall picture of how different regioisomeric cis-EETs contribute to vascular resistance and provided additional support that EETs act as an EDHF.
EET actions on the renal microvasculature have been extensively studied. Comparison of regioisomeric and stereoselective EETs were evaluated for their ability to vasodilate and activate afferent arterioles (138). 11,12-EET and 14,15-EET increased afferent arteriolar diameter to a greater extent than 8,9-EET and 5,6-EET resulted in vaso-constriction (138). In regards to steroselectivity, 11,12(R,S)-EET dilated rat interlobular and afferent arterioles and increased KCa activity in freshly isolated renal microvascular smooth muscle cells (138). This finding is consistent with CYP2C23 being the major epoxygenase enzyme in the kidney since 11,12(R,S)-EET is a major product. Additional experimental evidence determined that 11,12-EET acted directly on arteriolar smooth muscle cells and that the degradation product 11,12-DHET was devoid of dilator activity (138). The lack of DHET activity in renal arterioles suggests that sEH, which is highly expressed in the rodent and human renal vasculature, can importantly contribute to the regulation of EET actions. 5,6-EET serves as a poor substrate for sEH and appears to have unique renal vascular actions. Afferent arteriolar vasoconstriction to 5,6-EET was found to be dependent on COX metabolism and TP receptor activation (138). These findings were in agreement with an earlier study where the 5,6-EET-mediated increase renal vascular resistance and decrease in glomerular filtration rate was reversed to a renal vasodilation and increase in glomerular filtration rate in the presence of a COX inhibitor (274). On the other hand, 5,6-EET has been demonstrated to cause renal vasodilation that is either COX dependent or independent (28, 229, 230). EETs also contribute to acetycholine- and bradykinin-mediated afferent arteriolar dilation (99, 238). Various studies utilizing epoxygenase enzyme inhibitors, PPOH or miconazole, and the EET antagonist 14,15-EEZE have determined that EETs contribute to the nitric oxide- and COX-independent afferent arteriolar dilation (135, 238, 270, 282). Furthermore, bradykinin was also determined to increase renal microvascular EET production in a time-dependent manner between 5 and 30 min (135). These studies provide support to EETs being an EDHF; however, the contribution of specific EET regioisomers to the renal microvascular EDHF response remains unknown.
The cerebral vascular EET actions are unique in that EETs appear to be an important contributor to coupling blood flow in localized brain regions to the neuronal activity in that particular region (83, 114, 115, 164). This was based on the interesting observation that rat astrocytes express CYP2C11 and can synthesize EETs (4, 183, 211). Other experimental studies have demonstrated EET generation by rodent, rabbit, cat, and pig brains (83, 115, 164). Cat brains produce 5,6-EET, 8,9-EET, and 11,12-EET and cerebral arteries preconstricted with serotonin dilated to 8,9-EET and 11,12-EET to a greater extent than 5,6-EET (106). 11,12-EET-mediated vasodilation was blocked by K+ channel inhibition, and 11,12-EET increased cerebral arterial smooth muscle KCa channel activity (106). EET regioisomers increased diameters of piglet cerebral arteries with 5,6-EET being more potent regioisomer (177). 5,6-EET but not other EETs were found to vasodilate rabbit and cat pial arterioles. As observed with other vascular beds, cerebral arteriolar dilation in response to 5,6-EET was dependent on COX metabolism (60, 177). As for contributions to controlling regional cerebral blood flow, experimental studies have demonstrated that EETs generated by astrocytes in response to glutamate can act on astrocytes and cerebral vascular smooth muscle cells activating KCa channels resulting in hyperpolarization and vasodilation (4, 256). Likewise, epoxygenase inhibitors or EET antagonist can attenuate increases in cerebral blood flow upon neuronal stimulation of the brain cortex (184, 225, 226). Thus EETs generated by nonvascular cells appear to provide unique regulation of cerebral blood flow.
EET vascular actions on the coronary arteries have by far been the most extensively studied. Additionally, EETs have direct actions on cardiac myocytes and heart function, and this topic will be discussed in a separate section. Bovine and porcine coronary arteries were the blood vessels where CYP2C and EETs were implicated as major contributors to EDHF responses (19, 142). Coronary arteries dilate in response to all four EET regioisomers in the vast majority of the published studies (19, 169, 171, 243). There is also considerable evidence that DHETs possess vasodilatory activity in the coronary circulation (75, 76, 171, 223). Although in some cases DHETs were determined to be as potent as EETs, in most experimental settings the DHETs were less active compared with the EETs (242, 280). Experimental studies have demonstrated that DHET relaxes coronary arteries by activating smooth muscle cell KCa channels (171). Regulation of EET conversion to DHETs by sEH appears to take place in endothelial cells (171). Human coronary arteriolar responses to specific regioisomeic EETs are coordinated sEH present in endothelial cells. EETs and DHETs dilate human coronary arterioles and the dilatory response to 14,15-EET but not 11,12-EET is enhanced in the presence of sEH inhibition (171). This finding is consistent with 14,15-EET being the preferred substrate for the sEH enzyme. It also suggests that sEH can regulate the vascular actions of specific regioisomeric EETs and DHETs as well as the prevailing EET levels.
Even though the intestinal circulation was where EETs were first determined to be vasodilators, this vascular bed had some initial controversial findings in relation to EETs as an EDHF. An early study demonstrated that rat mesenteric artery acetylcholine nitric oxide-independent dilation and hyperpolarization was enhanced by CYP induction with β-naphthoflavone and significantly inhibited by the epoxygenase inhibitor clotrimazole (37). This finding supported the concept that EETs could serve as an EDHF in mesenteric resistance arteries. On the other hand, two other groups failed to demonstrate a contribution of the CYP epoxygenase pathway to the acetylcholine dilation and hyperpolarization in isolated rat mesenteric arteries (96, 279). In one study mesenteric artery dilation in response to 11,12-EET was observed and was inhibited by the KATP channel inhibitor glibenclamide (96). On the other hand, infusion of acetylcholine into mesenteric arteries resulted in the release of EETs, and 5,6-EET was demonstrated to elicit vasodilation that was inhibited by KCa channel blockade (1). More recent studies have consistently demonstrated that 11,12-EET and 14,15-EET dilate mesenteric resistance arteries (54, 59, 244, 311, 312). These studies also provide evidence that mesenteric arterial smooth muscle KCa and/or KATP channel is responsible for hyperpolarization (54, 59, 311, 312).
Other vascular beds have also provided evidence that EETs are an EDHF. 11,12-EET elicited dilation in isolated pressurized rat cremaster muscle arterioles that was inhibited by the KCa channel blocker iberiotoxin (199). This study also demonstrated that the epoxygenase enzyme inhibitor MS-PPOH inhibited nitric oxide-independent dilation in response to acetylcholine (199). Human internal mammary arteries were determined to generate predominantly 11,12-EET, and this EET regioisomer dilated these arteries via activating smooth muscle large-conductance KCa channels (7). Tail artery vascular resistance decreased in response to 5,6-EET but not to the other EET regioisomers (30). 5,6-EET constricted rat gracilis arterioles, and this was dependent on COX and an intact endothelium (30). Guinea pig carotid arteries failed to relax in response to EETs, and the nonselective CYP inhibitor 17-ODYA did not attenuate acetylcholine endothelium-dependent hyperpolarization in these arteries (33). On the other hand, in isolated resistance arteries from hamster gracilis muscle, CYP2C metabolites importantly contribute to the EDHF response (14). Antisense oligonucleotides against the coding region CYP2C8/9 attenuated EDHF responses by 70% without altering the responses to the nitric oxide donor sodium nitroprusside or the vasoconstrictor norepinephrine (14). These experimental studies in other circulations such as the skeletal muscle have provided initial information on EETs and their contribution to vascular tone, but much remains to be done.
The pulmonary circulation is unique in that blood flow needs to be directed to alveoli that have higher oxygen concentrations. Thus many hormonal and paracrine factors including PGs will have the opposite effect on pulmonary vascular tone compared with other organ circulations. Pulmonary blood vessels in rabbit vasoconstrict in response to 5,6-EET, and this response is dependent on an intact endothelium and COX (269). Likewise, 14,15-EET also constricts isolated perfused rabbit pulmonary arteries (269). Rabbit pulmonary arteries and lungs were demonstrated to express CYP2J and CYP2C proteins (316). On the other hand, there are reports that 5,6-EET dilates pulmonary arteries in piglets, dogs, and rabbits in a COX-dependent manner (97, 268, 269). These differences in pulmonary vascular responses may be related to the size of the blood vessels being investigated. Stephenson et al. (269) compared smaller pulmonary arteries and resistance vessels to larger extralobar pulmonary arteries in rabbits and found that 5,6-EET dilates larger arteries and constricts smaller resistance-sized arteries, and these vascular responses were found to be COX dependent. This finding is consistent with smaller sized vessels having the function to direct blood flow to alveoli that have a high oxygen concentration, whereas blood flow for other functions needs to be directed to areas that require oxygen for activity. Along these lines, the acute hypoxic pulmonary vasoconstrictor response in mice is abolished by the CYP epoxygenase inhibitor MS-PPOH or the EET antagonist 14,15-EEZE (228). 11,12-EET increased pulmonary artery pressure in a concentration-dependent manner via a transient receptor potential (TRP)-dependent signaling pathway (158). Lastly, sEH inhibition or Ephx2 −/− mice increased lung EET production and enhanced the pulmonary vasoconstrictor response to acute pulmonary hypoxia (158). EETs have also been implicated in pulmonary vascular remodeling associated with chronic hypoxia. 11,12-EET levels are elevated and CYP epoxygenase inhibitors decrease pulmonary vascular remodeling in chronic hypoxia (58, 228). Taken together, these experimental studies demonstrate the importance of the epoxygenase pathway for regulation of blood flow in the lungs.
V. EET Cell Signal Transduction Mechanisms and Vascular Tone
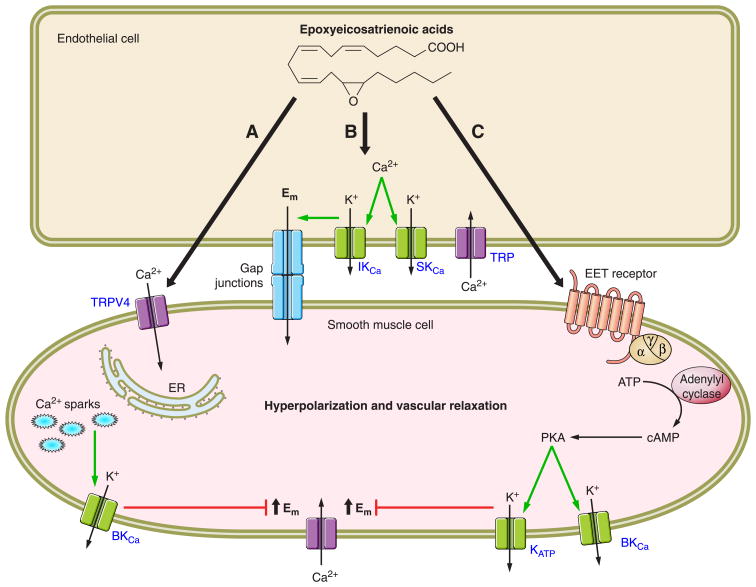
Vascular smooth muscle cell signaling mechanisms utilized by EETs have been extensively investigated using a wide array of techniques and blood vessels from many different organ circulations. An overriding conclusion from these studies is that EETs activate vascular smooth muscle cell K+ channels and in particular the large-conductance KCa channels resulting in cell membrane hyperpolarization (18, 90, 270). Another consistent finding has been increases in cAMP in response to EETs, and this signaling pathway has been associated with vasodilation (18, 90, 270). As with other paracrine factors, the EET/EDHF cell-signaling cascade has expanded and become more complex as investigators continue to explore the epoxygenase pathway (FIG. 2).
Figure 2.
EETs vasodilation is mediated by multiple mechanisms. A summary of vascular cell signaling mechanisms utilized by regioisomeric EETs in various organs is given. A: EETs generated by endothelial cells activate TRPV4 channels on vascular smooth muscle cells. Calcium influx through TRPV4 channels causes Ca2+ sparks from the endoplasmic reticulum. Ca2+ sparks activate large-conductance Ca2+-activated K+ channels (BKCa) resulting in K+ efflux from the smooth muscle cell and membrane hyperpolarization. B: EETs activate endothelial cell transient receptor potential (TRP) channels resulting in Ca2+ Influx. An increase in endothelial cell Ca2+ activates small-conductance (SKCa) and intermediate-conductance (IKCa) K+ channels to cause membrane hyperpolarization. Endothelial membrane hyperpolarization spreads to the vascular smooth muscle cell via gap junctions. C: EETs released by endothelial cells activate an unidentified receptor stimulating cAMP production via activation of adenylyl cyclase by the guanine nucleotide protein Gαs. Subsequent protein kinase A (PKA) activation by cAMP results in activation of BKCa and KATP, K+ efflux, and smooth muscle cell hyperpolarization.
EETs were demonstrated to be a transferable factor released by endothelial cells to act on vascular smooth muscle cells to elicit vasodilation (19, 84). Experimental studies have focused on cell signaling mechanisms responsible for EET activation of KCa channels. Electrophysiological studies using whole cell and cell-attached patch-clamp configurations in vascular smooth muscle cells demonstrated that 8,9-EET, 11,12-EET, and 14,15-EET activate large-conductance KCa channels (7, 19, 54, 84, 106). Bovine coronary arteries transfected with adenoviral F87V BM-3 to produce 14,15(S,R)-EET was demonstrated to be sufficient as an endogenous activator of large-conductance KCa channels (20). 5,6-EET analogs that resist metabolism by COX have also been demonstrated to relax bovine coronary arteries and activate vascular smooth muscle cell large-conductance KCa channels (307). Likewise, vasodilation of bovine coronary arteries and renal afferent arterioles in response to EETs is abolished by large-conductance but not small-conductance KCa inhibition (19, 132). The activation of large-conductance KCa channels by EETs is not due to direct binding, since 11,12-EET activates the KCa channel in cell-attached patches but is without effect in inside-out patches (334). Experimental evidence demonstrates that EETs and EDHF require a guanine nucleotide binding protein (G protein) to achieve vasorelaxation (179, 181).
Evidence for EETs and G protein activation of large-conductance KCa channels has been established using 11,12-EET regioisomer on bovine coronary arteries. 11,12-EET activation of large-conductance KCa in bovine coronary artery vascular smooth muscle cells in the inside-out patch configuration required the addition of GTP to the cytoplasmic surface (179). Furthermore, the G protein inhibitor GDPβS and an anti-Gαs antibody inhibited 11,12-EET and GTP activation of the large-conductance KCa (179, 182). These studies suggest that 11,12-EET activates vascular smooth muscle cell large-conductance KCa by a Gαs-dependent mechanism. These findings were in agreement with those demonstrating that bradykinin-dependent EDHF responses and activation of pig coronary artery vascular smooth muscle cell large-conductance KCa were inhibited by GDPβS (179). Another study further implicated 11,12-EET and 14,15-EET in the EDHF responses. The next series of experiments by Li et al. (180) demonstrated that 11,12-EET could alter GTP binding to Gαs. 11,12-EET was demonstrated to stimulate ADP-ribosylation of the G protein and activates Gαs in vascular smooth muscle cells (180, 181). Another finding consistent with Gαs in EET-mediated vasodilation is that 11,12-EET analogs increase cAMP accumulation in renal microvascular and mesenteric resistance artery smooth muscle cells (54, 132). Likewise, afferent arteriolar dilation to 11,12-EET is significantly attenuated by protein kinase A (PKA) but not PKG or guanylyl cyclase inhibition (137). The findings that cAMP and PKA contribute to EET-mediated vasodilation are in harmony with findings in platelets that the anti-aggregatory actions mediated by 11,12-EET utilize this cell signaling mechanism (168). Although it is becoming clear that EET-mediated activation of vascular smooth muscle cell large-conductance KCa requires G protein Gαs and cAMP/PKA, there is evidence emerging for TRP channels and localized increases in intracellular calcium in the vasodilation elicited by EETs (18).
There has been significant evidence that EETs increase intracellular calcium levels in a number of cell types including vascular smooth muscle cells. Fang et al. (80) demonstrated in pig aortic smooth muscle cell cultures that the four regioisomeric EETs increased intracellular Ca2+. The increase in intracellular Ca2+ was due to influx, since removal of extracellular Ca2+ or verapamil abolished 14,15-EET-me-diated increases in Ca2+ (80). Further evidence was presented that this increase in Ca2+ may modulate EET-induced vasorelaxation (80). Additional evidence has suggested that EETs increase vascular smooth muscle cell Ca2+ levels through activation of vallinoid type 4 TRP (TRPV4) channels (287). Ca2+ influx through TRPV4 channels would activate ryanodine receptors on the sarcoplasmic reticulum resulting in a Ca2+ spark and activation of large-conductance KCa channels (287). This is supported by data in smooth muscle cells from rat cerebral arteries where 11,12-EET and the TRPV4 agonist 4α-phorbol-12,13-didecanoate increase TRPV4 currents (57). 11,12-EET increases in TRPV4 currents are suppressed by antisense oligonucleotides that decrease TRPV4 expression (59). 11,12-EET-mediated dilation of mesenteric resistance arteries is absent in TRV4 gene-deficient mice (59). TRPV4 gene-deficient mice also provide evidence that EETs are linked to TRPV4 and flow-induced vasodilation, since the CYP component is missing in these mice (59). Additional experimental evidence supported the notion that flow-induced dilations were mediated by 5,6-EET activation of vascular smooth muscle cell TRPV4 channels (287). Thus TRPV4 channels on vascular smooth muscle cells appear to contribute to EET-mediated vasodilation.
To date, there is no evidence that the EET activation of vascular smooth muscle cell TRPV4 channels and resulting activation of large-conductance KCa channels is linked to the G protein Gαs and cAMP/PKA signaling cascades. These mechanisms appear to be exclusive and separate at this point. Future experimental studies are necessary to further define vascular smooth muscle cell signaling mechanisms by which EETs act as EDHFs. Providing another layer of complexity are the experimental findings that EETs acting on endothelial cells alter vascular tone.
There is accumulating evidence that EETs can hyperpolarize endothelial cells and that this is a potential mechanism contributing to vascular smooth muscle cell relaxation (18,90). 14,15-EEZE and CYP inhibitors can inhibit bradykinin-mediated Ca2+ influx in human endothelial cells (91). Likewise, sEH inhibition to increase endothelial cell EETs can enhance endothelial cell Ca2+ influx (91, 281). In contrast to EET activation of large-conductance KCa channels in vascular smooth muscle cells, EETs appear to activate small- and intermediate-conductance KCa channels in endothelial cells (91). TRP channels, specifically the TPRC3 and TRPC6 channels, have also been implicated in EET-induced Ca2+ influx and KCa channel activation in endothelial cells (91, 187). Additionally, 11,12-EET TRP channel translocation to caveolae to modulate Ca2+ influx is dependent on PKA activation (91). Pulmonary vasoconstriction to acute hypoxia or 11,12-EET does not occur in TRPC6 gene-deficient mice (158). There is also evidence that the endothelium participates in 11,12-EET-mediated vasodilation and is dependent on activation of endothelial cell small-and intermediate-conductance KCa channels (293). These findings are consistent with the idea that EETs acting on endothelial cells can hyperpolarize vascular smooth muscle cells via gap junctions and the oubain-sensitive Na+-K+-ATPase.
There is a large volume of evidence that EETs can activate endothelial and vascular smooth muscle cell KCa channels; however, there is also evidence for a KATP channel contribution to EET-mediated vasorelaxation. 11,12-EET and 14,15-EET dilation of mesenteric resistance arteries are mediated by KATP channel activation (311, 312). EETs activate a glibenclamide-sensitive KATP channel current in freshly isolated rat mesenteric vascular smooth muscle cells (312). Likewise, 11,12-EET applied extracellulary activated KATP channels in mesenteric arterials (188). EET activation of KATP channels appears to be linked to the G protein Gαs and cAMP/PKA signaling cascades (188). Other studies have provided evidence for KCa channels or heme oxygenase release of carbon monoxide and guanylyl cyclase activation in mesenteric resistance arteries (244, 245). Further studies are needed to resolve the involvement of KATP channels and interactions with other EET vasodilatory signal transduction mechanisms.
VI. EET Interactions with Hormonal and Paracrine Factors
EETs also influence vascular responses to mechanical stimuli as well as hormonal and paracrine constrictor and dilator factors. Numerous studies have demonstrated a contribution for EETs to acetycholine- and bradykinin-elicited EDHF-mediated vasodilation (18, 84, 135). Likewise, induction of vascular CYP2C11 and CYP2C23 by fenofibrate or endothelial expression of human CYP2J2 or CYP2C8 enhances renal microvascular dilation to acetylcholine (172, 328). Interestingly, in eNOS gene-deficient mice,CYP epoxygenase metabolites account entirely for skeletal muscle arteriolar dilations to acetylcholine (123). EETs are also an important component to the conducted dilation in response to acetylcholine in arterioles (118). In this case, the conduction of the hyperpolarization along the arteriole is dependent on CYP-derived epoxygenase metabolites (118). There is also a contribution of EETs to shear stress-dependent hyperpolarization and dilation of skeletal muscle arterioles (121, 122, 271). Thimerosol is another endothelium-dependent vasodilator that has a nitric oxide- and PG-independent dilatory component (42). EETs appear to mediate the EDHF portion of thimerosol renal vasodilation (42). EETs have also been demonstrated to contribute to vasodilation in response to adenosine activation of the adenosine A2A receptor (29, 45, 213). Adenosine increases EET generation in blood vessels, and epoxygenase inhibition greatly attenuates the vasodilation to adenosine or A2A receptor agonists (29). Experimental studies in rat preglomerular microvessels further demonstrated that adenosine A2A receptor activation is coupled to EET release upstream of adenylyl cyclase activation (29). On the other hand, adenosine-mediated dilation of cerebral arterioles has been linked to adenosine A2B receptor activation and EET release (256). This finding in the cerebral circulation is consistent with EETs contribution to functional hyperemia in the whisker barrel cortex (256). Thus EETs appear to be very important for the regulation of vasodilatory responses to physiological stimuli.
The interaction between EETs and the other major endothelial factor nitric oxide has been demonstrated but not extensively studied. It is clear that eNOS-deficient mice have intact vasodilation to shear stress and an attenuated vasodilation to acetylcholine (123). These findings suggest that EETs have the capacity to increase their contribution to decrease vascular resistance when endothelial nitric oxide levels are decreased (123). There is an inhibitory interaction between nitric oxide and EETs such that nitric oxide inhibits endothelial epoxygenase generation of EETs (123). Likewise, a product of the nitric oxide metabolic pathway, hydrogen peroxide (H2O2), has been demonstrated to inhibit EET production by human recombinant CYP2C9 and CYP2J2 epoxygenase enzymes (170). These studies also found that H2O2 was a major contributor to bradykinin EDHF dilation in human coronary arterioles and that in the presence of catalase to decrease H2O2 production an EET contribution to the dilation was unmasked (170). This suggested that redox control of CYP epoxygenases by H2O2 modulates vascular bioavailability of EETs. Interestingly, there is also evidence that CYP2C9 is a significant source of reactive oxygen species in coronary arteries (87). Antisense oligonucleotides against CYP2C abolished the EDHF-mediated responses but potentiated nitric oxide relaxation of coronary arteries in response to bradykinin (87). Furthermore, inhibition of CYP2C9 with sulfaphenazole improved forearm blood flow responses to acetylcholine in patients with coronary artery disease (87). This improved vasodilatory response in patients with coronary artery disease was related to an increase in nitric oxide bioavailability (87). Experimental findings in humans have also determined that nitric oxide and CYP epoxygenases regulate arterial stiffness in response to flow variations (12); however, how nitric oxide and EETs could interact to regulate arterial stiffness remains to be explored. Contrary to evidence that decreased EETs potentiate nitric oxide relaxation, CYP epoxygenase expression or EETs increased nitric oxide synthase activity in bovine endothelial cells (284, 305). CYP102 F87V mutant, CYP2C11-CYPOR, and CYP2J2 tranfected into endothelial cells increased nitric oxide synthase (NOS)-dependent conversion of L-arginine to L-citrulline (284). These studies implicated mitogen-activated protein kinase (MAPK) and protein kinase C pathways (284, 305). However, CYP epoxygenase transfection or EETs increased Thr495 phosphorylation of endothelial NOS that would decrease enzymatic activity (284). Thus the regulation of NOS activity by EETs and NO regulation of CYP epoxygenases remain controversial. On the whole, these studies suggest a complex relationship between endothelial cell CYP epoxygenase generation of EETs, nitric oxide generation, and regulation of reactive oxygen species that can significantly impact vasodilatory responses.
CYP epoxygenases can also have a profound influence on vasoconstrictor responses. Renal microvascular responses to angiotensin II are potentiated by CYP epoxygenase inhibition (131). There is also an upregulation in vascular sEH that decreases EET bioavailability in angiotensin II-infused hypertension that enhances vascular reactivity in this pathophysiological state (126, 141, 142). Consistent with this finding, increased afferent arteriolar reactivity to angiotensin II in hypertension is blunted by 11,12-EET or sEH inhibition (142, 329). Angiotensin II upregulation of vascular sEH appears to be at the transcriptional level (2). Angiotensin II activation of the transcriptional factor AP-1 in endothelial cells that subsequently activates the promoter region on Ephx2 resulted in an increased sEH protein expression (2). ACE inhibition in hypertension has also been demonstrated to improve EET-dependent EDHF mesenteric resistance artery responses (108). Likewise, rats treated with angiotensin converting enzyme (ACE) inhibitors have increased renal microvascular dilation to bradykinin that is mediated by CYP epoxygenases (197). This appears to be preglomerular, since efferent arteriolar dilation to bradykinin in ACE inhibited rats was nitric oxide dependent but not influenced by CYP eicosanoids (197). On the other hand, in rabbit there is evidence that EETs produced by the glomerulus or efferent arteriole contribute to bradykinin dilation of the efferent arteriole (238). Evidence also suggests that angiotensin type 2 receptors are coupled to EET generation and blunting of angiotensin II constriction of the afferent arteriole (167). Even though significant evidence for interactions in blood vessels between the CYP epoxygenase and renin-angiotensin system exist, the cellular pathways and mechanisms that determine the interactions remain to be defined.
An interaction with endothelin-1 and endothelial generation of EETs can influence vascular tone. The epoxygenase inhibitor MS-PPOH enhanced endothelin-1 arteriolar constrictor responses but did not alter endothelin-1 calcium responses in freshly isolated vascular smooth muscle cells (139). This finding is consistent with the concept that endothelin-1 results in endothelial generation of EETs that oppose vasoconstriction. In agreement with these findings, endothelin-1 vasoconstrictor responses are attenuated in mice that have transgenic CYP2C8 or CYP2J2 expression in endothelial cells (172). Lastly, the myogenic constriction in response to increases in perfusion pressure is enhanced in the presence of CYP epoxygenase inhibitors (134). This observation suggests that the release of endothelial-derived EETs in response to increases in transmural pressure attenuates the adjustments in arteriolar caliber. Taken together, these studies demonstrate that EETs can oppose vasoconstrictor stimuli to maintain proper vascular tone.
Experimental evidence has provided convincing evidence that endothelial-derived EETs can influence responses to mechanical, hormonal, and paracrine stimuli with regard to vascular tone. EETs in general contribute to endothelial-dependent dilator responses and oppose vasoconstrictor responses. The fact the EETs can modulate hormonal responses and that many hormones such as angiotensin II can influence vascular proliferation as well as vascular tone suggested that EETs could also modulate vascular growth processes.
VII. EETs and sEH Regulation of Vascular Homeostasis
As EETs were being investigated as a regulator of vascular tone, more and more evidence suggested that epoxygenase metabolites contribute importantly to vascular homeostasis. EETs were demonstrated to be anti-inflammatory, anti-aggregatory, and angiogenic (18, 89, 130, 263). In addition, EET generation and metabolism in circulating blood cells such as macrophages and erythrocytes have been determined (147, 151, 235). Thus EETs have multiple roles and are a key component for studies in vascular biology.
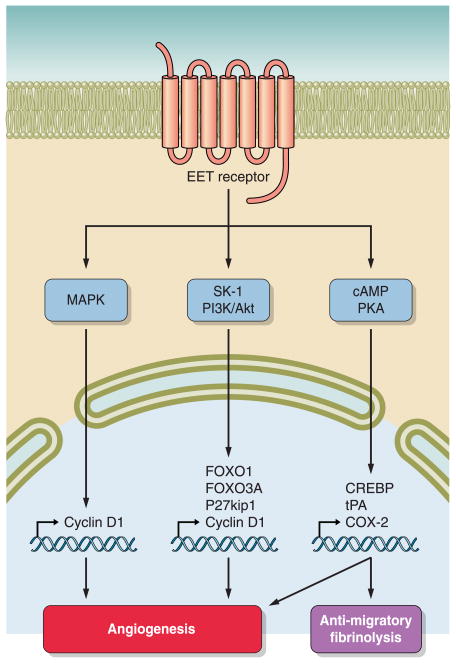
CYP epoxygenase metabolites have actions on endothelial and vascular smooth muscle cells to effect proliferation and migration (89, 130, 263). In regards to endothelial cells, CYP2C overexpression or EETs result in human or murine endothelial cell proliferation (231, 301). All four EET regioisomers have been reported to increase endothelial cell proliferation (89, 231). Cell signaling pathways that have been implicated in EET-mediated proliferation include the inositol-3-kinase (PI3K)/Akt pathway, the MAPK pathway, and the cAMP/PKA pathway (89, 231, 319). EET activation of the EGF receptor appears to be upstream of these cell-signaling pathways (203). 14,15-EET elicits the release of heparin-binding EGF-like growth factor (HB-EGF) in renal epithelial and cancer cells (39, 44). Although not yet identified, EET transactivation of the EGF receptors in endothelial cells appears to involve a similar mechanism (203). EGF receptor activation results in activation of Akt and subsequent increased expression of cyclin D1 (44, 203, 231). CYP2C9 overexpression in human umbilical vein endothelial cells (HUVECs) resulted in activation of p38MAPK and inactivation of c-Jun NH2-terminal kinase (JNK) resulting in increased cyclin D1 expression (203). Furthermore, 11,12-EET-induced proliferation in HUVECs involved a reduction in the cyclin D1 inhibitory protein, P27Kip1, and PI3K/Akt inactivation forkhead transcription factors, FOXO1 and FOXO3a (231). CYP2C9-induced endothelial cell proliferation can be inhibited by administration of a dominant negative Akt or a constitutively active FOXO3a (231). Another signaling pathway implicated in EET increases in cyclin D1 is MAPK phosphatase-1 (MKP-1) that decreases JNK activity (232). Therefore, EGF receptor transactivation and increased cyclin D1 appear to be important signaling mechanisms by which EETs induce endothelial cell proliferation (FIG. 3).
Figure 3.
EETs intracellular signaling pathways resulting in angiogenesis. An overview of intracellular pathways that have been demonstrated to be activated by regioisomeric EETs to promote angiogenesis is given. Mitogen-activated protein kinase (MAPK) can increase nuclear cyclin D1 generation. Sphingosine kinase-1 (SK-1) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways can activate transcription factors and the generation of cell cycle modulators. Protein kinase A (PKA) acting via the cAMP/PKA response element binding protein (CREBP) results in COX-2 production that influences angiogenesis, cell migration, and fibrinolysis.
Other pathways have also been implicated in EET-induced proliferation and migration. 11,12-EET administered to HUVECs has been demonstrated to induce proliferation through activation of sphingosine kinase-1 (SK1), resulting in transactivation of the EGF receptor and activation of Akt kinase (301). Expression of a dominant negative SK1 or siRNA targeting SK1 substantially reduced 11,12-EET-induced endothelial cell proliferation, migration, and tube formation (301). EETs also contribute to vascular endothelial growth factor (VEGF)-stimulated proliferation and tube formation in lung endothelial cells (53, 200). VEGF increased Cyp2c44 expression and production of EETs (306). Additionally, a siRNA targeting Cyp2c44 significantly reduced VEGF-induced endothelial proliferation that was associated with decreases in phosphorylation of the ERK1/2 and Akt kinases (306). 14,15-EET has been also demonstrated to increase VEGF expression via Src-signal transducer and activator transcription-3 (STAT-3) signaling in human dermal microvascular endothelial cells (46). These findings suggest that a positive feedback mechanism between EETs and VEGF could exist to induce endothelial cell proliferation. There also appears to be an interaction between EETs and COX-2 expression in endothelial cells that could contribute to angiogenesis (90). CYP2C9 overexpression or stimulation with 11,12-EET increased cAMP levels and stimulated binding of the cAMP-response element-binding protein (90). PKA inhibition attenuated CYP2C9-induced increase in COX-2 promoter activity and protein expression (90). Endothelial tube formation in CYP2C9 overexpression was also attenuated by COX-2 inhibition (90). Although the exact coordination of these endothelial cell signaling mechanisms to processes of proliferation, migration, and tube formation remain to be determined, it is clear that EETs are angiogenic and have effects on vascular smooth muscle cells.
The actions of EETs on vascular smooth muscle cells are not defined to the same degree as those on endothelial cells. One of the early studies found that 14,15-EET potentiated platelet-derived growth factor (PDGF)-induced vascular smooth muscle cell proliferation (77). This experimental study also determined that 14,15-EET decreased PGE2 levels as a potential mechanism for potentiating proliferation (77). On the other hand, a majority of the studies have established that EETs have antimigratory effects on cultured vascular smooth muscle cells (50, 273). 5,6-EET, 11,12-EET, or 14,15-EET inhibits PDGF-induced rat aortic smooth muscle cell migration (273). Adenoviral overexpression of CYP2J2 in vascular smooth muscle cells inhibited serum-and PDGF-induced migration (273). Additional evidence demonstrated that 11,12-EET and CYP2J2 overexpression increased vascular smooth muscle cell cAMP levels, and 11,12-EET migration was blocked by cAMP or PKA inhibition (273). On the whole, there is emerging evidence that EETs can act on vascular smooth muscle cells and contribute to angiogenesis, but the detailed cell-signaling mechanisms await future experimental studies.
The majority of experimental studies in endothelial and vascular smooth muscle cells provide convincing evidence that EETs have actions on proliferation and migration that would promote angiogenesis. A clearer picture of EETs and angiogenesis has emerged over the last decade, and in vivo data provided definitive evidence (201, 203, 233, 306, 320). EETs induced angiogenesis in chick chorioallantoic membranes (203). Experimental studies have also demonstrated angiogenesis in EET-impregnated Matrigel plugs in rodents (233, 288, 306). Ischemia models have provided further support that EETs are angiogenic. Overexpression of CYP epoxygenases CYP2C11 or CYP2J2 increased skeletal muscle capillary density in the ischemic rat hindlimb model (286). Likewise, capillary density in stroke-prone SHR rats was increased by chronic sEH inhibition, and pancreatic vascular density increased in insulin resistance in Ephx2 −/− mice (189, 258). These studies provided initial evidence that EET-induced angiogensis can protect organs from hypoxic ischemic injury.
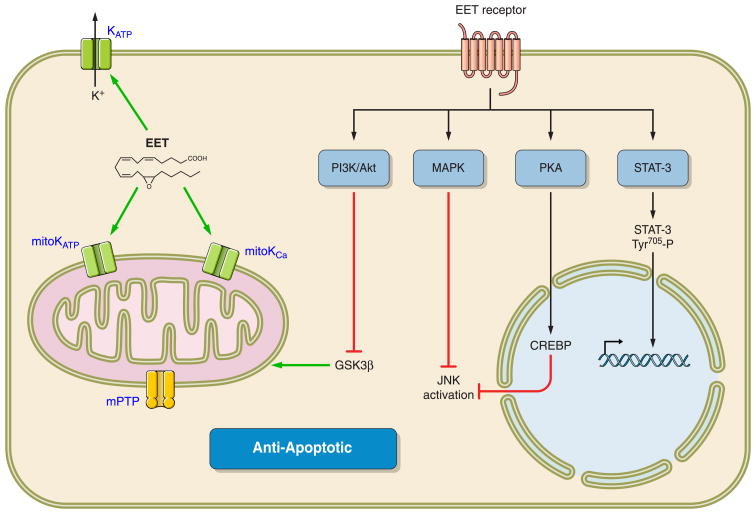
EETs and CYP epoxygenases also appear to protect cells from apoptosis. Endothelial cell apoptosis induced by TNF-α was attenuated by overexpresion of CYP epoxygenases (305). Decreased apoptosis was associated with EET inhibition of ERK dephosphorylation and activation of the PI3K/Akt signaling pathway (305). Inhibition of sEH has also been demonstrated to protect the brain from ischemic injury by protecting neurons from cell death (55, 140, 165, 258, 324). Gene expression of several antiapoptotic members of the Bcl-2 family, inhibitors of Fas-induced apoptosis (Cflar and Faim), and the antioxidant Prdx2 increased in response to sEH inhibition, whereas TNF receptors and Sphk2 were decreased (258). Endothelial cells from the coronary and pulmonary vasculatures also are protected from apoptosis by EETs (53, 191). These studies demonstrated a contribution or the MAPK and PI3/Akt signaling pathways to cell cytoprotection (53). CYP2J2 overexpression in bovine aortic endothelial cells protects against hypoxia-reoxygenation injury, and these actions are in part mediated by antioxidant actions (302). The combination of increased capillary density in response to ischemia and protections from apoptosis suggests that EETs protect organs from damage in cardiovascular disease states.
Findings that EETs have angiogenic properties also suggest that EETs could have the unwanted effect of promoting tumorigenesis. Indeed, mice lacking the Cyp2c44 epoxygenase gene had marked reductions in tumor volume, mass, and vascularization (234). PPARα activation downregulates endothelial cell CYP2C epoxygenases and blunts proliferation and tumor angiogensis and growth (315). CYP epoxygenase inhibitors also reduce capillary formation and tumor size in brain tumors. Thus epoxygenase inhibitors or EET antagonists have the potential to decrease angiogenesis to act as an anti-cancer therapy.
EETs also contribute to vascular homeostasis through their anti-inflammatory actions. The notion of EETs as having vascular anti-inflammatory properties was first described in a landmark publication by Node et al. (218). 11,12-EET decreased the number of adherent and rolling mononuclear cells in mice carotid arteries following TNF-α injection (218). This decrease in mononuclear adherent cells induced by 11,12-EET was similar to that observed after administration of a VCAM-1 blocking antibody (218). Additional data presented demonstrated CYP2J2 expression in endothelial cells of human coronary arteries as the potential epoxygenase-generating enzyme (218). Transfection with CYP2J2 or 11,12-EET administration to bovine endothelial cells decreased VCAM-1 expression and NFκB promoter activity (218). Likewise, CYP2J2 overexpression in mouse aortic endothelial cells attenuates the pro-atherosclerotic actions of hyperhomocysteinemia induction of matrix metalloproteinase-9 (MMP-9) via inhibition of NFκB (218). In agreement with these findings are experimental findings demonstrating proinflammatory mediators like cytokines and lipopolysaccharide (LPS) decrease endothelial epoxygenase expression and EET generation (125, 160). Conversely, TNF-α or chemokine receptor-2 inhibition results in an increase in CYP2C protein expression in rodents (61, 62). These findings demonstrate that EETs are important regulators of vascular inflammation.
There is also significant evidence that EETs and sEH inhibitors have anti-inflammatory effects against acute and chronic inflammation (130, 136). Mice that are deficient in sEH or sEH inhibition decreased inflammation and increased survival following LPS injection (248). This antiinflammatory effect was associated with an increase in the EET-to-DHET ratio (248). Interestingly, the plasma levels of the inflammatory COX metabolite PGE2 were reduced following sEH inhibition to mice treated with LPS (248). Inflammatory pain in response to LPS or carrageenan is also significantly reduced by sEH inhibition (144, 145). EETs and sEH inhibition anti-inflammatory effects on endothelial cells have also been demonstrated to be mediated through PPARγ activity (185). In a more recent study, endothelial expression of the human CYP2J2 or CYP2C8 epoxygenases in mice attenuated endotoxin-induced inflammation in the lung (52). These CYP2J2 and CYP2C8 transgenic mice attenuated NFkB activation, cellular adhesion molecules, cytokine expression, and neutrophil infiltration (52). Inflammation associated with chronic cardiovascular diseases is also reduced by sEH inhibition (129, 136). Administration of sEH inhibitors to rodents with angiotensin-induced or deoxycorticosterone (DOCA) salt hypertension decreased infiltration of macrophages and inflammation in the kidney (194, 329). Likewise, Ephx2 −/− mice had decreased renal inflammation associated with DOCA-salt hypertension (194). Thus the potential for EET analogs or sEH inhibitors as an anti-inflammatory therapy for cardiovascular diseases such as atherosclerosis and metabolic syndrome is very high.
Another way that EETs appear to be important for the maintenance of vascular homeostasis is through actions on platelets (86, 116, 168, 219). Initial experimental studies by Fitzpatrick et al. (86) demonstrated that 8,9-EET and 14,15-EET inhibited thromboxane B2 (TxB2) formation in platelet suspensions; however, platelet aggregation induced by arachidonic acid was inhibited by all EET regioisomers. EET inhibition of arachidonic acid-induced platelet aggregation was not correlated with reduced TxB2 levels (86). 11,12-EET has also been demonstrated to activate the NOS pathway in human platelets (321). Additional studies determined that EETs inhibited thrombin-mediated Ca2+ entry into platelets (193). EETs also cause membrane hyperpolarization of human platelets (168). Platelet membrane hyperpolarization is mediated through EET activation of large-conductance KCa channels that ultimately inhibits platelet adhesion to endothelial cells (168). Likewise, 11,12-EET or CYP2J2 overexpression in endothelial cells increases the expression and activity of tissue-type plasminogen activator (t-PA) through activation of Gαs and PKA cell-signaling pathways (219). 5,6-EET has also been demonstrated to stimulate t-PA release from human microvascular endothelial cells (219). Thrombin-stimulated t-PA release from endothelial cells was inhibited by epoxygenase enzyme inhibitors or the EET antagonist 14,15-EEZE (219). On the whole, these studies demonstrate that EETs have potential to decrease thrombolytic events since EETs decrease platelet aggregation and enhance expression of endothelial cell fibrinolytic enzymes (FIG. 3).
Although the primary source for EETs appears to be generation by endothelial cells, there is increasing evidence that circulating cells can generate EETs and store EETs in the phospholipid cell membrane. Human platelets contain all four EET regioisomers, and the majority of these EETs were associated with phoshphatidylcholine (13, 280, 331). Incubation of human platelets with thrombin or platelet-activating factor released EETs (331). Platelet COX activity can also metabolize 5,6-EET that contributes to the vasoconstrictor activity (9). Red blood cells (erythrocytes) also store EETs in membrane phospholipids (148, 149). Erythrocytes have sEH protein expression and activity that regulates the hydrolysis and release of EETs in the circulation (154, 198). ATP stimulation of P2X7 receptors on red blood cells induces EET release (150). Lastly, macrophages appear to have the capacity to generate epoxygenase metabolites (235). Human peritoneal macrophages incubated with arachidonic acid were demonstrated to generate EETs and DHETs (292). CYP2J2 has been identified as an epoxygenase enzyme expressed in human monocytes, and this expression increased in response to phorbol 12-myristate 13-acetate, macrophage-colony stimulating factor, and granulocyte/macrophage-colony stimulating factor (212). These studies provide emerging evidence that EETs and sEH in circulating cells could impact cardiovascular function; however, future studies are required to determine the physiological relevance of these findings.
VIII. EETs and sEH Regulation of Coronary Vascular and Cardiac Function
EETs increase coronary blood flow and improve cardiac function when investigated under normal physiological conditions (105, 169). Even with this being the case, there are surprisingly very few publications that have investigated EETs and sEH cell signaling mechanism and interactions with hormonal and paracrine factors with regards to cardiac function. On the other hand, there are numerous investigations related to cardiac pathophysiology that will be covered in a later section.
The cell signaling mechanisms by which EETs relax coronary artery vascular smooth muscle cells have been detailed in previous sections. Information concerning how EETs and sEH interact with hormonal paracrine factors to influence coronary blood flow are lacking (169). EETs do act as EDHFs through activation of vascular smooth muscle cell KCa channel in the coronary microcirculation (19, 101, 171). Epoxygenase metabolites of docosahexaenoate also dilate coronary arterioles by activating KCa channels (310). It has become increasingly apparent that in addition to actions on the coronary circulation, epoxygenase metabolites can influence cardiac myocytes (105, 130). Interestingly, EETs utilize different cell membrane channels and signaling mechanisms to impact cardiac myocyte function (105).
In an early study to determine the actions of EETs on heart function, administration of EETs into an isolated guinea pig heart had no effect on contractility or coronary pressure (205). However, in cardiac myocytes, 5,6-EET and 11,12-EET increased cell shortening and intracellular calcium concentrations, whereas 8,9-EET or 14,15-EET had no effect on myocytes (205). On the other hand, 14,15-EET reduced developed left ventricular pressure in isolated rat hearts (237). These findings suggest that EETs have complex actions on cardiac cells that could involve multiple cell membrane channels. Subsequent studies have provided evidence that EETs acting on cardiac myocyte Na+ channels, L-type Ca2+ channels, and KATP channels are potential mechanisms by which EETs can alter heart contractility (38, 175, 188).
Cardiac Na+ channels are necessary for action potentials in cardiac myocytes. 8,9-EET has been demonstrated to modulate Na+ channel gating behavior to act as a voltage-dependent inhibitor of cardiac Na+ channels (175). Other EETs also inhibited the Na+ current (INa) in cardiac cells, whereas 8,9-DHET had a small effect on cardiac INa (175). Additional evidence demonstrated that cardiac myocytes rapidly incorporate 8,9-EET into phospholipid membranes (175). This effect of EETs inhibiting cardiac Na+ channels by modulating gating behavior could be a potential modulator in pathological states such as cardiac ischemia.
In the heart, KATP channels are important modulators of the resting membrane potential and the cardiac action potential. In isolated rat cardiac ventricular myocytes, 11,12-EET but not 11,12-DHET increased KATP channel open probability by reducing channel sensitivity to ATP (188). 11,12-EET also hyperpolarized membrane potential of isolated cardiac myocytes, and this response was inhibited by the KATP channel blocker glyburide (188). Likewise, cardiac specific overexpression of the epoxygenase enzyme CYP2J2 shortened cardiac myocyte action potentials most likely due to enhanced maximal peak transient outward K+ currents (217, 249, 252). Experiments in Kir6.2 mutants expressed in HEK293 cells found that the region in the COOH terminus from Lys-185 to Arg-201 was necessary for EET activation of the channel (188, 252). This EET-Kir6.2 interaction was computer modeled and determined that the ATP binding site on Kir6.2 would be allosterically changed by this EET interaction. Interestingly, EET activation of KATP channels appears to be a mechanism responsible for EET cardioprotective effects against ischemia reperfusion injury.
Cardiac myocyte L-type Ca2+ channels are the major pathways for Ca2+ entry during excitation and the L-type Ca2+ current (ICa) in cardiac cells results in intracellular Ca2+ release to initiate and regulate heart muscle contraction. The cardiac myocyte L-type Ca2+ channel responses have not been consistent. Porcine L-type Ca2+ channels reconstituted into a lipid bilayer were inhibited by administration of EETs (38). On the other hand, inhibitors of epoxygenase enzymes suppressed ICa when administered to rat ventricular myocytes (298). In support of EETs activating L-type Ca2+ channels, 11,12-EET enhanced ICa currents and cardiac myocyte contraction (298). Cardiac myocyte intracellular cAMP levels were also increased by the addition of 11,12-EET (298). Experimental studies in transgenic mice provide additional support to the notion that EETs enhance ICa currents in cardiac cells (299). Cardiac-specific overexpression of CYP2J2 significantly enhanced cardiac ICa, and epoxygenase inhibition reduced ICa in transgenic heart cells (299). This enhanced ICa in CYP2J2 cardiac-specific transgenic mice is likely due to Cav1.12 channel phosphorylation, since the channel protein levels were not altered whereas the phosphorylated portion was increased (299). These findings suggest that EETs act via cAMP/PKA-dependent phosphorylation of the L-type Ca2+ channel to regulate heart contraction.
The actions of EETs to cardiac function have concentrated primarily on the cardiac myocyte ion channels. These findings have not been coupled to investigations of EETs on normal cardiac function. The fact that EETs also increase coronary blood flow has made it difficult to separate cardiac contractile actions from the actions on the coronary microcirculation. Thus the actions of EETs on cardiac function remain an area that is largely unexplored.
IX. EET Analogs and EET Receptors: Do EET Receptors Exist?
EET analogs and antagonists have been a pharmacological means to evaluate cardiovascular actions. These pharmacological tools have also been instrumental in the initial characterization of EET binding sites and receptors (102, 227, 270). On the other hand, the loss of the epoxygenase pathway in cell culture systems has been an impediment to identifying EET receptors (18, 90, 270). Even with this being the case, synthetic EET analogs that resist metabolism and have improved solubility have facilitated the identification of the structure-function relationships required for EET activity (17, 227). Overall, analogs for 5,6-EET, 11,12-EET, and 14,15-EET, as well as antagonists, have been synthesized and evaluated for vascular and cardiac actions and EET structural requirements.
The first generation of 11,12-EET and 14,15-EET analogs were methyl esters and sulfonimide substitutions of the carboxylic acid to improve stability and resist β-oxidation (102, 270). Subsequently, newer generations involved elimination of specific olefin bonds and substitution of the epoxide with sulfur or ether moieties to resist metabolism by sEH (102, 270). Other changes included changing the carbon chain length and combinational changes. Based on the findings of three independent groups using these 11,12-EET and 14,15-EET analogs, a structure-activity relationship emerged (70, 102, 132). 11,12-EET analogs were demonstrated to inhibit vascular smooth muscle cell TNF-α-induced VCAM-1 expression (70). Afferent arteriolar and mesenteric resistance artery dilation to a similar series of 11,12-EET analogs also provided additional evidence supporting a structure-activity relationship for 11,12-EET (54, 132). The third evaluation involved the bovine coronary artery dilator actions of 14,15-EET analogs (68, 69, 102, 308). These studies resulted in the following structural requirements for full EET activity, an acidic carboxyl group, Δ8 olefin bond, 20-carbon chain length, and a cis-epoxide. Experimental studies with 5,6-EET analogs in bovine coronary arteries have provided initial evidence for its structure-activity relationship (307). The methyl derivative of 5,6-EET, 5,6-EET-Me, or the stable analog 5-[pentadeca-3(Z),6(Z),9(Z)-trienuyloxy]pentanoic acid (PTPA) induces vasorelaxation through KCa activation (307). Various 5,6-EET analogs determined that the presence of an 8,9 or 11,12 olefin bond contributed to the 5,6-EET-induced vasorelaxation (307). PTPA vasorelaxation was also decreased by COX inhibition providing further support to previous studies that COX metabolism contributes to the vascular actions of 5,6-EET (307). In contrast, the vascular actions of 11,12-EET and 14,15-EET are independent of COX metabolism (19, 138). Overall, EET analogs have provided additional valuable information on vascular actions of EETs, and on the basis of EET structure activity relationships, more recent studies have attempted to search for EET binding sites/receptors.
There is mounting evidence that EETs act through receptors, and some of these studies suggest that EETs have affinity to already known binding sites and receptors. Previous experimental findings have demonstrated that EETs bind to fatty acid binding proteins (264, 294). There is also evidence that 14,15-EET in high concentrations inhibits vascular thromboxane TP receptors and that 14,15-EET elicits relaxation in mesenteric resistance arteries by activating PGE2 (EP2) receptors (11, 303). In addition, the cardiac protective properties of 11,12-EET appear to be mediated by δ- and/or κ-opioid receptors (109). However, these previous studies have failed to demonstrate high-affinity binding for EETs. An approach to characterizing high-affinity EET binding sites/receptors has been developed using EET analogs, agonists, and antagonists.
It is apparent that EETs activate vascular smooth muscle cell KCa channels through G protein (Gs)-dependent mechanism and that cAMP and PKA are key signaling molecules required for KCa channel activation (18, 270). These data strongly support the notion that EETs act through a G protein-coupled receptor (GPCR). Initial evidence for a cell membrane EET receptor was determined by measuring cAMP-induced aromatase enzyme activity in vascular smooth muscle cells. 14,15-EET or the 14–15-EET sulfonimide analog (14,15-EET-SI) inhibited aromatase activity in vascular smooth muscle cells (262). The corresponding diol, 14,15-DHET, did not alter aromatase activity (262). Inhibition of aromatase activity persisted when 14,15-EET-SI was covalently tethered to silica beads that restricted it from entering the cell (262). These findings suggest that 14,15-EET acted on the cell surface and not intra-cellularly.
Further evidence for a GPCR EET receptor has been gathered in guinea pig mononuclear cells and human U937 cells. Experimental studies in U937 macrophage cells demonstrated that 11,12-EET was three times more potent than 14,15-EET in stimulating cAMP accumulation, and the Ki of 11,12-EET for binding to U937 cell membranes was three times lower than 14,15-EET (309). Specific and saturable binding with a Kd of 5.7 nM in guinea pig mononuclear cells and a Kd of 13.9 nM in human U937 cells was determined using the radioligand 14,15-[3H]EET (309). Yang et al. utilized 20-125I-14,15-EE5ZE and 20-125I-14,15-EE8ZE binding to U937 macrophages to demonstrate that EET analogs but not PGs or LOX metabolites displaced the radioligands from their binding site (309). Binding of 20-125I-14,15-EE8ZE in U937 cells was also inhibited by GTPγS, suggesting G protein coupling. Additionally, various EETs competed for 20-125I-14,15-EE5ZE and 20-125I-14,15-EE8ZE binding to U937 macrophages with an order of potency of 11,12-EET ≥ 14,15-EET > 8,9-EET (309). Intriguingly, there are differences in potency and activity when comparing EETs such as 11,12-EET and 14,15-EET in various vascular tissues. Many of these apparent differences could be resolved with the identification of multiple vascular EET binding sites/receptors.
The identification of EET receptors would greatly enhance the ability to target the epoxygenase pathway for the treatment of cardiovascular diseases. EET selectivity will be one roadblock that could be overcome by determining the identity of EET receptors. This would expedite high throughput library screening for the development of selective pharmaceuticals. Even with this being the case, EET analogs have been evaluated in vivo and have been demonstrated to have beneficial cardiovascular actions.
X. EETs and sEH in Cardiovascular Diseases
The importance of EETs and sEH in cardiovascular disease has been driven by experimental findings demonstrating that the epoxygenase pathway is a key component in the regulation of vascular and cardiac function that can be altered in disease states (136, 251, 327, 333). Genetic based evidence in humans has also found that the epoxygenase pathway could importantly contribute to cardiovascular diseases. EPHX2 genetic polymorphisms that result in amino acid substitutions can influence sEH activity (66, 267). These genetic polymorphisms have been linked to cardiovascular disease incidence. The Atherosclerosis Risk in Communities (ARIC) study found the EPHX2 K55R polymorphism was associated with an increase in the risk for the incidence of coronary artery disease (173). Likewise, the Coronary Artery Risk Development in young adults (CARDIA) study determined that African Americans having one allele that results in EPHX2 R287Q substitution increased the risk for coronary artery calcification (92). Ischemic stroke and hypercholesterolemia are two other cardiovascular diseases that are associated with genetic variation in EPHX2 (94, 165, 246, 290). This finding is consistent with experimental evidence that sEH can regulate cholesterol levels (67). Interestingly, EPHX2 polymorphisms have also been associated with graft function associated with kidney transplantations (176). Variations in the CYP2C and CYP2J genes have also been associated with cardiovascular risk and diseases (56, 195, 265). A single nucleotide polymorphism in the CYP2J2 gene is associated with hypertension in the male Caucasian population (163). Myocardial infarction and cardiovascular disease have also been associated with EPHX2, CYP2C8, and CYP2C9 genetic variants (112, 241). More recently, genetic variations in EPHX2 were linked to vasodilator responses in humans (174). EPHX2 R55 variant allele carriers had increased forearm vasodilator responses, and Q287 carriers also demonstrated changes in vasodilator responses (174). Intriguingly, genetic variation in EPHX2 has been associated with heart failure and vascular disease risk in rat models of cardiovascular disease (93). Thus there is increasing evidence that genetic variation in the epoxygenase enzymatic pathways are associated with cardiovascular diseases and can impact on cardiovascular function.
The contribution of the epoxygenase pathway to cardiovascular diseases has progressed to a stage where it is being targeted for therapeutic potential. Although the development of EET agonists and antagonists for use in vivo is limited, sEH inhibitors have advanced to the point where they have been tested in humans (37, 136, 255). What has emerged from in vitro and in vivo studies with EET and EET analogs and sEH inhibitors is that the epoxygenase pathway has an important role in hypertension, cardiac hypertrophy, ischemic injury, and vascular inflammation and atherosclerosis.
The possible contribution of EETs to blood pressure control and hypertension was first noted in studies where rats treated with an epoxygenase enzyme inhibitor and administered high salt became hypertensive (26, 119, 192). A number of subsequent studies determined that increased renal epoxygenase enzymes and EET generation in response to a high-salt diet was required for the proper vascular and renal tubular function to maintain cardiovascular homeostasis (25, 128, 242, 266). Further experimental studies determined that renal vascular sEH expression was increased in angiotensin-dependent hypertension (141). Administration of sEH inhibitors to increase EETs lowered blood pressure in a number of experimental animal models of hypertension (107, 141, 154, 186, 194, 314). Although sEH inhibition or Ephx2 gene deletion lowered blood pressure, it did not return values down to normal (194). The lowering of blood pressure did not occur in some SHR strains and was variable in different Ephx2 −/− mice colonies (48,93, 190, 196, 259). One consistent finding in these hypertension studies was that sEH inhibition resulted in improved vascular function and decreased inflammation and end organ damage (130, 194, 329).
Kidney, vascular, heart, and brain damage associated with hypertension is reduced with sEH inhibition or EET analog administration. Renal vascular and glomerular injury is reduced by sEH inhibition in angiotensin-dependent and DOCA-salt hypertension (194, 329). Hypertension-induced vascular hypertrophy was decreased and afferent arteriolar constriction to angiotensin normalized by sEH inhibition (329). Decreased renal glomerular injury was observed when sEH inhibitors were administered prior to or after the establishment of hypertension (120, 194, 329). This was associated with decreased damage to the glomerular capillary barrier and is consistent with EETs protecting this barrier (194, 254). Renal macrophage infiltration during hypertension was also decreased by sEH inhibition (194, 329). Angiotensin-induced hypertension in Goto-Kakizaki rats did not have a decrease in blood pressure in response to sEH inhibitor treatment but did have decreased renal vascular and glomerular injury, and inflammation (220). These and a number of other studies have demonstrated vascular and organ protection by sEH inhibitors irrespective of changes in blood pressure (130, 136, 165). Cardiac hypertrophy associated with DOCA-salt hypertension is also decreased by sEH inhibitor administration (186). Likewise, cerebral ischemic injury in hypertensive rats is greatly reduced by sEH inhibition (55, 258). Inhibition of sEH appears to protect the brain in stroke-prone SHR by its dual action to improve vascular function and remodeling and protecting the neurons from apoptosis (258). More recent studies have used newly developed EET analogs and determined their beneficial actions in cardiovascular diseases. An 11,12-EET analog, NUDSA, has been evaluated in two cardiovascular studies (133, 262). NUDSA dilated afferent arterioles to a similar extent as 11,12-EET and lowered blood pressure in SHR and angiotensin-dependent hypertension (133). Administration of NUDSA reversed the metabolic syndrome phenotype in heme-oxygenase-2 deficient mice (262). NUDSA treatment for 2 wk decreased body weight, improved glucose regulation, and improved endothelial-dependent relaxation (262). Interestingly, renal Cyp2c44 expression was increased by NUDSA administration, suggesting that induction of the epoxygenase enzyme could contribute to the biological actions of this EET analog (262). These studies have provided significant evidence that EET analogs and sEH inhibitors have the ability to improve vascular function and protect organs from hypertensive injury through actions on multiple cell types.
Cardioprotection is another area where therapeutic targeting of the epoxygenase pathway has demonstrated promise. These experimental studies have focused on cardiac hypertrophy and ischemia reperfusion injury (130, 136). Data have been generated utilizing both pharmacological and genetic means. As with the beneficial effects in hypertension, cardiac protective effects attributed to increasing EETs appear to be due to actions on multiple cell types within the heart.
The beneficial effects of EETs and sEH inhibition to prevent the development of left ventricular hypertrophy have been assessed in various animal models (3, 186, 252, 300). The importance of Ephx2 was further demonstrated in that an allelic variation of Ephx2 that increases sEH expression and activity was associated with heart failure in a rat model of heart failure (206). Isoproterenol-induced cardiac hypertrophy is also associated with induction of sEH and decreased CYP2C11 and EET levels (332). As mentioned previously, sEH inhibition decreases cardiac hypertrophy in hypertension (186); however, the decrease in cardiac size could have been a consequence of decreased blood pressure in sEH inhibitor-treated animals. Subsequent studies evaluated sEH inhibition in mice with pressure overload. Mice were treated with sEH inhibitors prior to or following the establishment of left ventricular hypertrophy (3, 300). The sEH inhibitors prevented the development of and even reversed established left ventricular hypertrophy (3, 300). Decreased cardiac hypertrophy was associated with decreased NFκB activation in cardiac myocytes in mice administered sEH inhibitors (300). Interestingly, sEH inhibition also had an antiarrhythmic effect in pressure overload mice (300). Ephx2 −/− mice hearts are also protected from pressure overload-induced heart failure and cardiac arrhythmias (300). These findings suggest that sEH inhibitors are a potential therapeutic approach for heart failure treatment.
Improved cardiac function following ischemia reperfusion injury has also been observed with EET and EET analogs, cardiac myocyte overexpression of CYP epoxygenase enzymes, sEH inhibition, and Ephx2 gene-deficient mice (130, 217). EETs administered to guinea pig hearts and isolated ventricular myocytes had no observed effects on cardiac contractility or coronary perfusion pressure (205). Likewise, EETs did not improve cardiac function in hearts subjected to low-flow ischemia for 1 h (205). This negative study administered a mixture of EETs and only tested one dose (205). A subsequent study found that in an in vivo rat model intravenous infusion of racemic 14,15-EET prior to occlusion of the left anterior descending coronary artery resulted in decreased infarct size (110). 11,12-EET and 14,15-EET reduced infarct size in dogs when administered 15 min before ischemia or 5 min before reperfusion (110). Interestingly, the sEH metabolite 14,15-DHET had no cardiac protective effect in ischemia reperfusion in the dogs (110). Intracoronary administration of the sEH inhibitor AUDA produced a dose-related reduction in infarct size in dogs (110). AUDA also enhanced the cardioprotective actions of 14,15-EET when coadministered (110). More recently, cardioprotective effects of a compound with dual EET agonist and sEH inhibitor properties, UA-8 significantly improved left ventricular developed pressure (LVDP) and reduced infarct size following ischemia reperfusion (10).
Experimental studies in genetically manipulated mice have also demonstrated a cardioprotective role for EETs (250). Ephx2 −/− mice had improved LVDP following ischemia reperfusion as determined in the Langendorff-perfused heart preparation (250). There was also a reduction of infarct size and decreased lactate dehydrogenase release in Ephx2 −/− mice (250). Cardiac myocyte overexpression of human CYP2J2 in mice also improved cardiac function in hearts subjected to global ischemia followed by reperfusion (325). Postischemic electrocardiogram abnormalities were also prevented by cardiomyocyte-specific overexpression of CYP2J2 (325). Administration of epoxygenase enzyme inhibitors or the EET antagonist 14,15-EEZE prevented the beneficial cardiac effects observed in the Ephx2 −/− and CYP2J2 transgenic mice (111, 250). These studies clearly demonstrate that pharmacological or genetic manipulation of EETs can protect the heart from ischemia reperfusion injury.
Additional mechanistic studies have determined that a key component to the cardiac protection afforded by EETs is KATP channel activation (249, 250). Experimental studies in dogs and mice have demonstrated that the cardioprotective actions of EETs can be abolished by the nonselective KATP channel antagonist glibenclamide (111, 250). Inhibition of KATP and KCa channels can block the beneficial effects on cardiac function in Ephx2 −/− mice (111, 250). There are multiple cell signaling pathways that also contribute to EET-mediated cardioprotection. A cell signaling pathway known to be increased during reperfusion in hearts of CYP transgenic overexpressors is the ERK/MAPK pathway (10, 249). Administration of the ERK precursor MEK1/2 inhibitor PD980459 blocked the cardioprotection observed in CYP transgenic mice (249). JAK/STAT signaling also appears to contribute to EET-mediated protection from cell death following ischemia reperfusion (202). The reduced infarct size in response to 14,15-EET is abolished by STAT3 inhibition (202). Furthermore, in cultured cardiomyocytes, STAT3 siRNA abolished cytoprotection provided by 14,15-EET or sEH inhibition (202). These findings demonstrate that EET actions on cardiomyocytes involve JAK/STAT cell-signaling events that reduce apoptosis associated with ischemia reperfusion (FIG. 4).
Figure 4.
EETs cardioprotection and protection from ischemic injury. EETs have been postulated to directly activate sarcolemmal KATP, mitochondrial KATP (mitoKATP), and mitochondrial KCa (mitoKCa) to limit the loss of mitochondrial membrane potential (Δψm) to slow opening of the mitochondrial permeability transition pore (mPTP) resulting in decreased apoptosis (157). Other intracellular signaling mechanisms include PI3K/Akt inhibition of GSK3β, MAPK- and PKA/CRBP-mediated inhibition of JNK activation, and STAT-3 tyrosine phosphorylation-mediated nuclear translocation.
Although glibenclamide administration provided initial evidence for a contribution of the sarcolemmal KATP channel, there is also significant evidence the EETs also act through cell signaling mechanisms on the mitochondrial membrane. Inhibitors of PI3K significantly reduced the protective effects on contractile function in Ephx2 −/− and sEH inhibited mice (250). In addition, B-type natriuretic peptide could be a possible link between EETs and activation of the PI3K pathway (34). The downstream cell signaling events including Akt phosphorylation and subsequent increases in phospho-glycogen kinase 3β (p-GSK3β) that is cardioprotective by delaying or inhibiting opening of the mitochondrial permeability transition pore (mPTP) (34). Recent studies have demonstrated that EETs can minimize adverse effects of stress on mitochondrial structure and function (157). Mitochondrial fragmentation and t-tubule swelling in cardiomyocytes following ischemia reperfusion was significantly reduced in hearts overexpressing CYP2J2 (157). 14,15-EET also reduced laser-induced loss of mitochondrial membrane potential (ΔΨm) and mPTP opening (157). EET protective effects on the mitochondria are blocked by paxillin, a KCa channel inhibitor, or 5-hydroxydecanoate (5-HD), a mitochondrial KATP channel inhibitor (157). EETs also reduce mitochondrial damage associated with doxorubicin-induced cardiotoxicity (157). Doxorubicin decreases EET generation in hearts, and overexpression of CYP2J2 provides cardioprotection and decreases cardiomyocyte apoptosis following administration of doxorubicin (325). Thus EETs can result in changes in mitochondria that can prevent apoptosis associated with ischemia reperfusion in the heart (FIG. 4).
Increasing EETs also has the potential to protect the brain from ischemic damage. Cerebral infarct size is reduced in stroke-prone SHR and WKY rats administered sEH inhibitors or with CYP2C11 induction prior to the ischemic event (5, 55, 258). Cerebral ischemia reperfusion injury in mice is also decreased by sEH inhibition or Ephx2 gene deletion (324). Administration of sEH inhibitors hours prior to ischemia or at the time of reperfusion also results in decreased cerebral injury (324). This cerebral protection afforded by sEH inhibitor appears to be mediated by EETs since epoxygenase inhibition negates this protection (258, 324). The mechanism for cerebral protection is multifactorial and involves the cerebral blood vessels and neurons.
Alterations in cerebral blood flow, inflammation, and neuronal cell apoptosis in response to cerebral ischemia appear to impart protection from the insult (55, 166, 258, 324). Similar to cardiac ischemia, the MAPK and PI3K/Akt cell signaling pathways likely contribute to neuronal and endothelial cell cytoprotection (258, 324). These findings provide further evidence that EETs and sEH inhibition targets several cell types that contribute to protection against cell death following ischemia reperfusion.
Another cardiovascular disease area of emerging investigation for therapeutic targeting of EETs and sEH inhibition is vascular remodeling and atherosclerosis (257, 278, 323). Experimental studies have demonstrated that sEH inhibition protects against pathological vascular remodeling (257). These findings are in agreement with the fact that EETs can influence vascular smooth muscle cell migration and proliferation. Renal vascular hypertrophy and collagen deposition associated with angiotensin-dependent hypertension was decreased by sEH inhibitor treatment (329). Inhibition of sEH also attenuated vascular hypertrophic remodeling and collagen deposition in the middle cerebral artery of stroke-prone SHR (258). Carotid artery ligation flow-induced inward remodeling in stroke-prone SHR was reduced to a level comparable to WKY rats by sEH inhibitor administration (257). The hyperplastic response to carotid artery ligation was decreased by sEH inhibition of Ephx2 gene deletion in mice (257). Interestingly, sEH inhibition of Ephx2 gene deletion in mice did not attenuate neointimal formation in the femoral artery in response to wire-induced endothelial vascular injury (257). This finding is consistent with the notion that EETs are produced by the endothelium and that endothelial-derived EET generation is required for sEH inhibition to have a beneficial vascular effect. Atherosclerosis in apolipoprotein E gene deleted mice is also reduced by sEH inhibition (278, 323). In another study, inhibition of sEH also attenuated atherosclerosis, aortic aneurysm formation, and dyslipidemia in apolipoprotein E gene-deleted mice (323). Interestingly, the attenuation of atherosclerosis was associated with a reduction in plasma inflammatory cytokines and down-regulation of ICAM-1, VCAM-1, and IL-6 in the vascular wall (323). These findings suggest that anti-inflammation in response to sEH inhibition is an additional factor that provides vascular protection.
There are areas of investigation where the potential cardiovascular benefit of EETs and sEH inhibitors is questionable. Administration of sEH inhibitors enhances hypoxic-induced pulmonary vasoconstriction and vascular remodeling. Pulmonary hypertension and vascular remodeling are associated with increased EET generation. Likewise, Ephx2 −/− mice demonstrate an increased pulmonary vasoconstriction in response to hypoxia (159). On the other hand, monocrotoline-induced pulmonary hypertension and vascular remodeling as well as tobacco-mediated inflammation are reduced by sEH inhibition or increased CYP2C excpression (239,260, 330). EETs can also increase endothelial cell permeability that can potentially result in increased alveolar fluid volume (5, 146). Increased glomerular capillary permeability may account for increased albuminuria in 5/6-nephrectomized mice administered a sEH inhibitor (155). In contrast, cisplatin-induced acute nephrotoxicity is attenuated by sEH inhibition (82, 224). 11,12-EET added to transplant preservation solution improves kidney function (304). As a final point, Ephx2 gene deletion reduced survival after cardiac arrest and cardiopulmonary resuscitation (124). Therefore, future studies determining the cardiovascular benefits of sEH inhibitors and EET analogs must be approached with cautious optimism.
XI. Conclusions
EETs have many actions that contribute importantly to cardiac and vascular physiology to maintain cardiovascular homeostasis. The angiogenic actions of EETs have resulted in investigations of this pathway in cancer and tumor biology. Increased epoxygenase enzyme expression and increasing EETs do appear to be associated with increased tumor size (152, 153). Additional studies in the area of angiogenesis and cancer are needed to determine the contribution of this pathway to this disease. Although many EET cell-signaling mechanisms have been determined, there is still significant opportunity for exploration in this area. PPAR-α and PPAR-γ can be activated by EETs, DHETs, and sEH inhibitors (71, 72, 215, 216, 272, 296); however, the exact nature of the interaction between the epoxygenase pathway and PPARs requires further examination. Experimental evidence suggests that EETs could act via receptor-dependent and receptor-independent mechanisms and function in a paracrine or autocrine manner. Identifying EET receptors or molecules that bind EETs will further elucidate and define cell-signaling mechanisms. The development of novel pharmacological and molecular biological tools such as EET agonists and antagonists will be necessary to properly define the cardiovascular actions of EETs. The ability to define the unique cardiovascular cell signaling mechanisms and receptors for EETs in future studies will provide novel EET targets for developing treatments for cardiovascular diseases.
Acknowledgments
Grants: This work was supported by National Institutes of Health Grants HL-59699 and DK-38226.
Footnotes
Disclosures: Conflict of interest statement: United States Patent No. 7, 732, 470: Compositions and methods for the treatment of renal and cardiovascular disease, J. D. Imig and J. R. Falck, issued June 8, 2010; United States Patent No. 7, 550,617: Compositions and methods for the treatment of renal and cardiovascular disease, J. D. Imig and J. R. Falck, issued June 23, 2009; and United States Patent Application No. 61472410: Epoxyeicosatrienoic acid analogs and methods of making and using the same, J. D. Imig, J. R. Falck, and W. B. Campbell; April 6, 2011.
References
- 1.Adeagbo ASO, Oyekan AO, Joshua IG, Falkner KC, Prough RA, Pierce WM. Epoxyeicosatrienoic acid release mediates nitric oxide-independent dilation of rat mesenteric vessels. In: Vanhoutte PM, editor. EDHF. London: Taylor & Francis; 2001. pp. 195–206. [Google Scholar]
- 2.Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JY, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:9018–9023. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai D, Pang W, Li N, Xu M, Jones PD, Yang J, Zhang Y, Chiamvimonvat N, Shyy JY, Hammock BD, Zhu Y. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106:564–569. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- 5.Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, Hurn PJ. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke. 2002;33:1677–1684. doi: 10.1161/01.str.0000016332.37292.59. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez DF, Gjerde EA, Townsley MI. Role of EETs in regulation of endothelial permeability in rat lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L445–L451. doi: 10.1152/ajplung.00150.2003. [DOI] [PubMed] [Google Scholar]
- 7.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 8.Athirakul K, Bradbury JA, Graves JP, DeGraff LM, Ma J, Zhao Y, Couse JF, Quigley R, Harder DR, Zhao X, Imig JD, Pedersen TL, Newman JW, Hammock BD, Conley AJ, Korach KS, Coffman TM, Zeldin DC. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J. 2008;22:4096–4108. doi: 10.1096/fj.08-114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balazy M. Metabolism of 5,6-epoxyeicosatrienoic acid by the human platelet. Formation of novel thromboxane analogs. J Biol Chem. 1991;266:23561–23567. [PubMed] [Google Scholar]
- 10.Batchu SN, Lee SB, Qadhi RS, Chaudhary KR, El-Sikhry H, Kodela R, Falck JR, Seubert JM. Cardioprotective effect of a dual acting epoxyeicosatrienoic acid analogue towards ischaemia reperfusion injury. Br J Pharmacol. 2011;162:897–907. doi: 10.1111/j.1476-5381.2010.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther. 2009;328:231–239. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- 12.Bellien J, Favre J, Iacob M, Gao J, Thuillez C, Richard V, Joannides R. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension. 2010;55:674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190. [DOI] [PubMed] [Google Scholar]
- 13.Bernstrom K, Kayganich K, Murphy RC, Fitzpatrick FA. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. J Biol Chem. 1992;267:3686–3690. [PubMed] [Google Scholar]
- 14.Bolz SS, Fisslthaler B, Pieperhoff S, De Wit C, Fleming I, Busse R, Pohl U. Antisense oligonucleotides against cytochrome P450 2C8 attenuate EDHF-mediated Ca2+ changes and dilation in isolated resistance arteries. FASEB J. 2000;14:255–260. doi: 10.1096/fasebj.14.2.255. [DOI] [PubMed] [Google Scholar]
- 15.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 16.Bukhari IA, Gauthier KM, Jagadeesh SG, Sangras B, Falck JR, Campbell WB. 14,15-Dihydroxy-eicosa-5(Z)-enoic acid selectively inhibits 14,15-epoxyeicosatrienoic acid-induced relaxations in bovine coronary arteries. J Pharmacol Exp Ther. 2011;336:47–55. doi: 10.1124/jpet.110.169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell WB, Deeter C, Gauthier KM, Ingraham RH, Falck JR, Li PL. 14,15-Dihy-droxyeicosatrienoic acid relaxes bovine coronary arteries by activation of KCa channels. Am J Physiol Heart Circ Physiol. 2002;282:H1656–H1664. doi: 10.1152/ajpheart.00597.2001. [DOI] [PubMed] [Google Scholar]
- 18.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflügers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 20.Campbell WB, Holmes BB, Falck JR, Capdevila JH, Gauthier KM. Regulation of potassium channels in coronary smooth muscle by adenoviral expression of cytochrome P-450 epoxygenase. Am J Physiol Heart Circ Physiol. 2006;290:H64–H71. doi: 10.1152/ajpheart.00516.2005. [DOI] [PubMed] [Google Scholar]
- 21.Capdevila JH, Falck JR. Biochemical and molecular characteristics of the cytochrome P450 arachidonic acid monooxygenase. Prostaglandins Other Lipid Mediat. 2000;62:271–292. doi: 10.1016/s0090-6980(00)00085-x. [DOI] [PubMed] [Google Scholar]
- 22.Capdevila JH, Falck JR. The CYP P450 arachidonic acid monooxygenases: from cell signaling to blood pressure regulation. Biochem Biophys Res Commun. 2001;285:571–576. doi: 10.1006/bbrc.2001.5167. [DOI] [PubMed] [Google Scholar]
- 23.Capdevila JH, Falck JR. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. 2002:68–69. 325–344. doi: 10.1016/s0090-6980(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 24.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- 25.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid mono-oxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 2007;72:683–689. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- 26.Capdevila JH, Wei S, Yan J, Karara A, Jacobson HR, Falck JR, Guengerich FP, DuBois RN. Cytochrome P-450 arachidonic acid epoxygenase. Regulatory control of the renal epoxygenase by dietary salt loading. J Biol Chem. 1992;267:21720–21726. [PubMed] [Google Scholar]
- 27.Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med Res Rev. 2007;27:469–527. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- 28.Carroll MA, Balazy M, Margiotta P, Falck JR, McGiff JC. Renal vasodilator activity of 5,6-epoxyeicosatrienoic acid depends upon conversion by cyclooxygenase and release of prostaglandins. J Biol Chem. 1993;268:12260–12266. [PubMed] [Google Scholar]
- 29.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol. 2006;291:F155–F161. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- 30.Carroll MA, Garcia MP, Falck JR, McGiff JC. 5,6-Epoxyeicosatrienoic acid, a novel arachidonate metabolite. Mechanism of vasoactivity in the rat. Circ Res. 1990;67:1082–1088. doi: 10.1161/01.res.67.5.1082. [DOI] [PubMed] [Google Scholar]
- 31.Certikova Chabova V, Walkowska A, Kompanowska-Jezierska E, Sadowski J, Kujal P, Vernerova Z, Vanourkova Z, Kopkan L, Kramer HJ, Falck JR, Imig JD, Hammock BD, Vaneckova I, Cervenka L. Combined inhibition of 20-hydroxyeicosatetraenoic acid formation and of epoxyeicosatrienoic acids degradation attenuates hypertension and hypertension-induced end-organ damage in Ren-2 transgenic rats. Clin Sci. 2010;118:617–632. doi: 10.1042/CS20090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, Gill SS, Hammock BD, Estabrook RW. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223:639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- 33.Chataigneau T, Feletou M, Duhault J, Vanhoutte PM. Epoxyeicosatrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br J Pharmacol. 1998;123:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhary KR, Batchu SN, Das D, Suresh MR, Falck JR, Graves JP, Zeldin DC, Seubert JM. Role of B-type natriuretic peptide in epoxyeicosatrienoic acid-mediated improved post-ischaemic recovery of heart contractile function. Cardiovasc Res. 2009;83:362–370. doi: 10.1093/cvr/cvp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;297:H495–H507. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chawengsub Y, Gauthier KM, Nithipatikom K, Hammock BD, Falck JR, Narsimhaswamy D, Campbell WB. Identification of 13-hydroxy-14,15-epoxyeicosatrienoic acid as an acid-stable endothelium-derived hyperpolarizing factor in rabbit arteries. J Biol Chem. 2009;284:31280–31290. doi: 10.1074/jbc.M109.025627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen D, Whitcomb R, Mcintyre E, Tran V, Do ZN, Sabry J, Patel DV, Anandan SK, Gless R, Webb HK. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin Pharmacol. doi: 10.1177/0091270010397049. In press. [DOI] [PubMed] [Google Scholar]
- 38.Chen G, Cheung DW. Modulation of endothelium-dependent hyperpolarization and relaxation to acetylcholine in rat mesenteric artery by cytochrome P450 enzyme activity. Circ Res. 1996;79:827–833. doi: 10.1161/01.res.79.4.827. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Capdevila JH, Zeldin DC, Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol Pharmacol. 1999;55:288–295. doi: 10.1124/mol.55.2.288. [DOI] [PubMed] [Google Scholar]
- 40.Chen JK, Capdevila J, Harris RC. Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells. Proc Natl Acad Sci USA. 2002;99:6029–6034. doi: 10.1073/pnas.092671899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen JK, Chen J, Imig JD, Wei S, Hachey DL, Guthi JS, Falck JR, Capdevila JH, Harris RC. Identification of novel endogenous cytochrome P450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem. 2008;283:24514–24524. doi: 10.1074/jbc.M709873200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen YJ, Jiang H, Quilley J. The nitric oxide- and prostaglandin-independent component of the renal vasodilator effect of thimerosal is mediated by epoxyeicosatrienoic acids. J Pharmacol Exp Ther. 2003;304:1292–1298. doi: 10.1124/jpet.102.042671. [DOI] [PubMed] [Google Scholar]
- 43.Cheng HF, Harris RC. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525–530. doi: 10.1161/01.HYP.0000116221.27079.ea. [DOI] [PubMed] [Google Scholar]
- 44.Cheng LM, Jiang JG, Sun ZY, Chen C, Dackor RT, Zeldin DC, Wang DW. The epoxyeicosatrienoic acid-stimulated phosphorylation of EGF-R involves the activation of metalloproteinases and the release of HB-EGF in cancer cells. Acta Pharmacol Sin. 2010;31:211–218. doi: 10.1038/aps.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141:441–448. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheranov SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN. An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood. 2008;111:5581–5591. doi: 10.1182/blood-2007-11-126680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 48.Corenblum MJ, Wise VE, Georgi K, Hammock BD, Doris PA, Fornage M. Altered soluble epoxide hydrolase gene expression and function and vascular disease risk in the stroke-prone spontaneously hypertensive rat. Hypertension. 2008;51:567–573. doi: 10.1161/HYPERTENSIONAHA.107.102160. [DOI] [PubMed] [Google Scholar]
- 49.Davis BB, Morisseau C, Newman JW, Pedersen TL, Hammock BD, Weiss RH. Attenuation of vascular smooth muscle cell proliferation by 1-cyclohexyl-3-dodecyl urea is independent of soluble epoxide hydrolase inhibition. J Pharmacol Exp Ther. 2006;316:815–821. doi: 10.1124/jpet.105.091876. [DOI] [PubMed] [Google Scholar]
- 50.Davis BB, Thompson DA, Howard LL, Morisseau C, Hammock BD, Weiss RH. Inhibitors of soluble epoxide hydrolase attenuate vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2002;99:2222–2227. doi: 10.1073/pnas.261710799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeLozier TC, Tsao CC, Coulter SJ, Foley J, Bradbury JA, Zeldin DC, Goldstein JA. CYP2C44, a new murine CYP2C that metabolizes arachidonic acid to unique stereospecific products. J Pharmacol Exp Ther. 2004;310:845–854. doi: 10.1124/jpet.104.067819. [DOI] [PubMed] [Google Scholar]
- 52.Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, Degraff LM, Lih FB, Foley J, Bradbury JA, Graves JP, Tomer KB, Falck JR, Zeldin DC, Lee CR. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291:H517–H531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 54.Dimitropoulou C, West L, Field MB, White RE, Reddy LM, Falck JR, Imig JD. Protein phosphatase 2A and Ca2+-activated K+ channels contribute to 11,12-epoxyeicosatrienoic acid analog mediated mesenteric arterial relaxation. Prostaglandins Other Lipid Mediat. 2007;83:50–61. doi: 10.1016/j.prostaglandins.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46:842–848. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dreisbach AW, Japa S, Sigel A, Parenti MB, Hess AE, Srinouanprachanh SL, Rettie AE, Kim H, Farin FM, Hamm LL, Lertora JJ. The Prevalence of CYP2C8, 2C9, 2J2, and soluble epoxide hydrolase polymorphisms in African Americans with hypertension. Am J Hypertens. 2005;18:1276–1281. doi: 10.1016/j.amjhyper.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 57.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 58.Earley S, Pastuszyn A, Walker BR. Cytochrome p-450 epoxygenase products contribute to attenuated vasoconstriction after chronic hypoxia. Am J Physiol Heart Circ Physiol. 2003;285:H127–H136. doi: 10.1152/ajpheart.01052.2002. [DOI] [PubMed] [Google Scholar]
- 59.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol. 2009;297:H1096–H1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol Heart Circ Physiol. 1990;259:H1171–H1177. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- 61.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II: salt hypertension. Hypertension. 2007;50:1069–1076. doi: 10.1161/HYPERTENSIONAHA.107.098806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension. 2006;47:557–562. doi: 10.1161/01.HYP.0000198545.01860.90. [DOI] [PubMed] [Google Scholar]
- 63.Enayetallah AE, French RA, Barber M, Grant DF. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J Histochem Cytochem. 2006;54:329–335. doi: 10.1369/jhc.5A6808.2005. [DOI] [PubMed] [Google Scholar]
- 64.Enayetallah AE, French RA, Grant DF. Distribution of soluble epoxide hydrolase, cytochrome P450 2C8, 2C9 and 2J2 in human malignant neoplasms. J Mol Histol. 2006;37:133–141. doi: 10.1007/s10735-006-9050-9. [DOI] [PubMed] [Google Scholar]
- 65.Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem. 2004;52:447–454. doi: 10.1177/002215540405200403. [DOI] [PubMed] [Google Scholar]
- 66.Enayetallah AE, Grant DF. Effects of human soluble epoxide hydrolase polymorphisms on isoprenoid phosphate hydrolysis. Biochem Biophys Res Commun. 2006;341:254–260. doi: 10.1016/j.bbrc.2005.12.180. [DOI] [PubMed] [Google Scholar]
- 67.Enayetallah AE, Luria A, Luo B, Tsai HJ, Sura P, Hammock BD, Grant DF. Opposite regulation of cholesterol levels by the phosphatase and hydrolase domains of soluble epoxide hydrolase. J Biol Chem. 2008;283:36592–36598. doi: 10.1074/jbc.M806315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falck JR, Kodela R, Manne R, Atcha KR, Puli N, Dubasi N, Manthati VL, Capdevila JH, Yi XY, Goldman DH, Morisseau C, Hammock BD, Campbell WB. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–5075. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, Deeter C, Sharma KK, Gauthier KM, Campbell WB. Comparison of vasodilatory properties of 14,15-EET analogs: structural requirements for dilation. Am J Physiol Heart Circ Physiol. 2003;284:H337–H349. doi: 10.1152/ajpheart.00831.2001. [DOI] [PubMed] [Google Scholar]
- 70.Falck JR, Reddy LM, Reddy YK, Bondlela M, Krishna UM, Ji Y, Sun J, Liao JK. 11,12-Epoxyeicosatrienoic acid (11,12-EET): structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorg Med Chem Lett. 2003;13:4011–4014. doi: 10.1016/j.bmcl.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 71.Fang X, Hu S, Watanabe T, Weintraub NL, Snyder GD, Yao J, Liu Y, Shyy JY, Hammock BD, Spector AA. Activation of peroxisome proliferator-activated receptor alpha by substituted urea-derived soluble epoxide hydrolase inhibitors. J Pharmacol Exp Ther. 2005;314:260–270. doi: 10.1124/jpet.105.085605. [DOI] [PubMed] [Google Scholar]
- 72.Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J, Liu Y, Sangras B, Falck JR, Weintraub NL, Spector AA. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H55–H63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- 73.Fang X, Kaduce TL, VanRollins M, Weintraub NL, Spector AA. Conversion of epoxyeicosatrienoic acids (EETs) to chain-shortened epoxy fatty acids by human skin fibroblasts. J Lipid Res. 2000;41:66–74. [PubMed] [Google Scholar]
- 74.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 75.Fang X, Kaduce TL, Weintraub NL, Spector AA. Cytochrome P450 metabolites of arachidonic acid: rapid incorporation and hydration of 14,15-epoxyeicosatrienoic acid in arterial smooth muscle cells. Prostaglandins Leukot Essent Fatty Acids. 1997;57:367–371. doi: 10.1016/s0952-3278(97)90412-9. [DOI] [PubMed] [Google Scholar]
- 76.Fang X, Kaduce TL, Weintraub NL, VanRollins M, Spector AA. Functional implications of a newly characterized pathway of 11,12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle. Circ Res. 1996;79:784–793. doi: 10.1161/01.res.79.4.784. [DOI] [PubMed] [Google Scholar]
- 77.Fang X, Moore SA, Stoll LL, Rich G, Kaduce TL, Weintraub NL, Spector AA. 14,15-Epoxyeicosatrienoic acid inhibits prostaglandin E2 production in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 1998;275:H2113–H2121. doi: 10.1152/ajpheart.1998.275.6.H2113. [DOI] [PubMed] [Google Scholar]
- 78.Fang X, Weintraub NL, McCaw RB, Hu S, Harmon SD, Rice JB, Hammock BD, Spector AA. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–H2420. doi: 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- 79.Fang X, Weintraub NL, Oltman CL, Stoll LL, Kaduce TL, Harmon S, Dellsperger KC, Morisseau C, Hammock BD, Spector AA. Human coronary endothelial cells convert 14,15-EET to a biologically active chain-shortened epoxide. Am J Physiol Heart Circ Physiol. 2002;283:H2306–H2314. doi: 10.1152/ajpheart.00448.2002. [DOI] [PubMed] [Google Scholar]
- 80.Fang X, Weintraub NL, Stoll LL, Spector AA. Epoxyeicosatrienoic acids increase intracellular calcium concentration in vascular smooth muscle cells. Hypertension. 1999;34:1242–1246. doi: 10.1161/01.hyp.34.6.1242. [DOI] [PubMed] [Google Scholar]
- 81.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 82.Fife KL, Liu Y, Schmelzer KR, Tsai HJ, Kim IH, Morisseau C, Hammock BD, Kroetz DL. Inhibition of soluble epoxide hydrolase does not protect against endotoxin-mediated hepatic inflammation. J Pharmacol Exp Ther. 2008;327:707–715. doi: 10.1124/jpet.108.142398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Filosa JA, Blanco VM. Neurovascular coupling in the mammalian brain. Exp Physiol. 2007;92:641–646. doi: 10.1113/expphysiol.2006.036368. [DOI] [PubMed] [Google Scholar]
- 84.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 85.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 86.Fitzpatrick FA, Ennis MD, Baze ME, Wynalda MA, McGee JE, Liggett WF. Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. Influence of stereochemistry. J Biol Chem. 1986;261:15334–15338. [PubMed] [Google Scholar]
- 87.Fleming I. Cytochrome P450 and vascular homeostasis. Circ Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 88.Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s) Pharmacol Res. 2004;49:525–533. doi: 10.1016/j.phrs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 89.Fleming I. DiscrEET regulators of homeostasis: epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharmacol Sci. 2007;28:448–452. doi: 10.1016/j.tips.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Fleming I. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat. 2007;82:60–67. doi: 10.1016/j.prostaglandins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 92.Fornage M, Boerwinkle E, Doris PA, Jacobs D, Liu K, Wong ND. Polymorphism of the soluble epoxide hydrolase is associated with coronary artery calcification in African-American subjects: The Coronary Artery Risk Development in Young Adults (CAR-DIA) study. Circulation. 2004;109:335–339. doi: 10.1161/01.CIR.0000109487.46725.02. [DOI] [PubMed] [Google Scholar]
- 93.Fornage M, Hinojos CA, Nurowska BW, Boerwinkle E, Hammock BD, Morisseau CH, Doris PA. Polymorphism in soluble epoxide hydrolase and blood pressure in spontaneously hypertensive rats. Hypertension. 2002;40:485–490. doi: 10.1161/01.hyp.0000032278.75806.68. [DOI] [PubMed] [Google Scholar]
- 94.Fornage M, Lee CR, Doris PA, Bray MS, Heiss G, Zeldin DC, Boerwinkle E. The soluble epoxide hydrolase gene harbors sequence variation associated with susceptibility to and protection from incident ischemic stroke. Hum Mol Genet. 2005;14:2829–2837. doi: 10.1093/hmg/ddi315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fries S, Grosser T. The cardiovascular pharmacology of COX-2 inhibition. Hematology Am Soc Hematol Educ Program. 2005:445–451. doi: 10.1182/asheducation-2005.1.445. [DOI] [PubMed] [Google Scholar]
- 96.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Evidence against a role of cytochrome P450-derived arachidonic acid metabolites in endothelium-dependent hyperpolarization by acetylcholine in rat isolated mesenteric artery. Br J Pharmacol. 1997;120:439–446. doi: 10.1038/sj.bjp.0700932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuloria M, Smith TK, Aschner JL. Role of 5,6-epoxyeicosatrienoic acid in the regulation of newborn piglet pulmonary vasculartone. Am J Physiol Lung Cell Mol Physiol. 2002;283:L383–L389. doi: 10.1152/ajplung.00444.2001. [DOI] [PubMed] [Google Scholar]
- 98.Fulton D, McGiff JC, Quilley J. Role of phospholipase C and phospholipase A2 in the nitric oxide-independent vasodilator effect of bradykinin in the rat perfused heart. J Pharmacol Exp Ther. 1996;278:518–526. [PubMed] [Google Scholar]
- 99.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 100.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 101.Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-Epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension. 2005;45:666–671. doi: 10.1161/01.HYP.0000153462.06604.5d. [DOI] [PubMed] [Google Scholar]
- 102.Gauthier KM, Falck JR, Reddy LM, Campbell WB. 14,15-EET analogs: characterization of structural requirements for agonist and antagonist activity in bovine coronary arteries. Pharmacol Res. 2004;49:515–524. doi: 10.1016/j.phrs.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 103.Gauthier KM, Goldman DH, Aggarwal NT, Chawengsub Y, Falck JR, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in acetylcholine-induced relaxations of mouse arteries. Am J Physiol Heart Circ Physiol. 2011;300:H725–H735. doi: 10.1152/ajpheart.00696.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoicmSI: a 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003;42:555–561. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- 105.Gauthier KM, Yang W, Gross GJ, Campbell WB. Roles of epoxyeicosatrienoic acids in vascular regulation and cardiac preconditioning. J Cardiovasc Pharmacol. 2007;50:601–608. doi: 10.1097/FJC.0b013e318159cbe3. [DOI] [PubMed] [Google Scholar]
- 106.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol. 1992;263:H519–H525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 107.Ghosh S, Chiang PC, Wahlstrom JL, Fujiwara H, Selbo JG, Roberds SL. Oral delivery of 1,3-dicyclohexylurea nanosuspension enhances exposure and lowers blood pressure in hypertensive rats. Basic Clin Pharmacol Toxicol. 2008;102:453–458. doi: 10.1111/j.1742-7843.2008.00213.x. [DOI] [PubMed] [Google Scholar]
- 108.Goto K, Fujii K, Onaka U, Abe I, Fujishima M. Angiotensin-converting enzyme inhibitor prevents age-related endothelial dysfunction. Hypertension. 2000;36:581–587. doi: 10.1161/01.hyp.36.4.581. [DOI] [PubMed] [Google Scholar]
- 109.Gross GJ, Baker JE, Hsu A, Wu HE, Falck JR, Nithipatikom K. Evidence for a role of opioids in epoxyeicosatrienoic acid-induced cardioprotection in rat hearts. Am J Physiol Heart Circ Physiol. 2010;298:H2201–H2207. doi: 10.1152/ajpheart.00815.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol. 2008;294:H2838–H2844. doi: 10.1152/ajpheart.00186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gross GJ, Hsu A, Falck JR, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol. 2007;42:687–691. doi: 10.1016/j.yjmcc.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gschwendtner A, Ripke S, Freilinger T, Lichtner P, Muller-Myhsok B, Wichmann HE, Meitinger T, Dichgans M. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with an increased risk of ischemic stroke in white Europeans. Stroke. 2008;39:1593–1596. doi: 10.1161/STROKEAHA.107.502179. [DOI] [PubMed] [Google Scholar]
- 113.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 114.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 115.Harder DR, Lange AR, Gebremedhin D, Birks EK, Roman RJ. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J Vasc Res. 1997;34:237–243. doi: 10.1159/000159228. [DOI] [PubMed] [Google Scholar]
- 116.Heizer ML, McKinney JS, Ellis EF. 14,15-Epoxyeicosatrienoic acid inhibits platelet aggregation in mouse cerebral arterioles. Stroke. 1991;22:1389–1393. doi: 10.1161/01.str.22.11.1389. [DOI] [PubMed] [Google Scholar]
- 117.Hoebel BG, Graier WF. 11,12-Epoxyeicosatrienoic acid stimulates tyrosine kinase activity in porcine aortic endothelial cells. Eur J Pharmacol. 1998;346:115–117. doi: 10.1016/s0014-2999(98)00118-6. [DOI] [PubMed] [Google Scholar]
- 118.Hoepfl B, Rodenwaldt B, Pohl U, De Wit C. EDHF, but not NO or prostaglandins, is critical to evoke a conducted dilation upon ACh in hamster arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H996–H1004. doi: 10.1152/ajpheart.01082.2001. [DOI] [PubMed] [Google Scholar]
- 119.Holla VR, Makita K, Zaphiropoulos PG, Capdevila JH. The kidney cytochrome P-450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest. 1999;104:751–760. doi: 10.1172/JCI7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Honetschlagerova Z, ZH Z, Vanourkova Z, Sporkova A, Kramer HJ, Hwang SH, Tsai HJ, Hammock BD, Imig JD, Cervenka L, Kopkan L. Renal mechanisms contributing to the antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats with inducible hypertension. J Physiol. 2010;589:207–219. doi: 10.1113/jphysiol.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2001;280:H2462–H2469. doi: 10.1152/ajpheart.2001.280.6.H2462. [DOI] [PubMed] [Google Scholar]
- 122.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005;96:376–383. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang A, Sun D, Smith CJ, Connetta JA, Shesely EG, Koller A, Kaley G. In eNOS knockout mice skeletal muscle arteriolar dilation to acetylcholine is mediated by EDHF. Am J Physiol Heart Circ Physiol. 2000;278:H762–H768. doi: 10.1152/ajpheart.2000.278.3.H762. [DOI] [PubMed] [Google Scholar]
- 124.Hutchens MP, Nakano T, Dunlap J, Traystman RJ, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase gene deletion reduces survival after cardiac arrest and cardiopulmonary resuscitation. Resuscitation. 2008;76:89–94. doi: 10.1016/j.resuscitation.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iber H, Chen Q, Cheng PY, Morgan ET. Suppression of CYP2C11 gene transcription by interleukin-1 mediated by NF-kappaB binding at the transcription start site. Arch Biochem Biophys. 2000;377:187–194. doi: 10.1006/abbi.2000.1772. [DOI] [PubMed] [Google Scholar]
- 126.Imig JD. Afferent arteriolar reactivity to angiotensin II is enhanced during the early phase of angiotensin II hypertension. Am J Hypertens. 2000;13:810–818. doi: 10.1016/s0895-7061(00)00264-8. [DOI] [PubMed] [Google Scholar]
- 127.Imig JD. Eicosanoid regulation of the renal vasculature. Am J Physiol Renal Physiol. 2000;279:F965–F981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 128.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 129.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24:169–188. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 130.Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol. 2010;56:329–335. doi: 10.1097/FJC.0b013e3181e96e0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Imig JD, Deichmann PC. Afferent arteriolar responses to ANG II involve activation of PLA2 and modulation by lipoxygenase and P-450 pathways. Am J Physiol Renal Physiol. 1997;273:F274–F282. doi: 10.1152/ajprenal.1997.273.2.F274. [DOI] [PubMed] [Google Scholar]
- 132.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11,12-EET analogs involves PP2A activity and Ca2+ -activated K+ channels. Microcirculation. 2008;15:137–150. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Imig JD, Elmarakby A, Nithipatikom K, Wei S, Capdevila JH, Tuniki VR, Sangras B, Anjaiah S, Manthati VL, Sudarshan Reddy D, Falck JR. Development of epoxyeicosatrienoic acid analogs with in vivo anti-hypertensive actions. Front Physiol. 2010;1:157. doi: 10.3389/fphys.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Imig JD, Falck JR, Inscho EW. Contribution of cytochrome P450 epoxygenase and hydroxylase pathways to afferent arteriolar autoregulatory responsiveness. Br J Pharmacol. 1999;127:1399–1405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- 136.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Imig JD, Inscho EW, Deichmann PC, Reddy KM, Falck JR. Afferent arteriolar vasodilation to the sulfonimide analog of 11,12-epoxyeicosatrienoic acid involves protein kinase A. Hypertension. 1999;33:408–413. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- 138.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 139.Imig JD, Pham BT, LeBlanc EA, Reddy KM, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension. 2000;35:307–312. doi: 10.1161/01.hyp.35.1.307. [DOI] [PubMed] [Google Scholar]
- 140.Imig JD, Simpkins AN, Renic M, Harder DR. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev Mol Med. 2011;13:e7. doi: 10.1017/S1462399411001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 142.Imig JD, Zhao X, Falck JR, Wei S, Capdevila JH. Enhanced renal microvascular reactivity to angiotensin II in hypertension is ameliorated by the sulfonimide analog of 11,12-epoxyeicosatrienoic acid. J Hypertens. 2001;19:983–992. doi: 10.1097/00004872-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 143.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci USA. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jian MY, King JA, Al-Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol. 2008;38:386–392. doi: 10.1165/rcmb.2007-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang H, Anderson GD, McGiff JC. Red blood cells (RBCs), epoxyeicosatrienoic acids (EETs) and adenosine triphosphate (ATP) Pharmacol Rep. 2010;62:468–474. doi: 10.1016/s1734-1140(10)70302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang H, McGiff JC, Quilley J, Sacerdoti D, Reddy LM, Falck JR, Zhang F, Lerea KM, Wong PY. Identification of 5,6-trans-epoxyeicosatrienoic acid in the phospholipids of red blood cells. J Biol Chem. 2004;279:36412–36418. doi: 10.1074/jbc.M403962200. [DOI] [PubMed] [Google Scholar]
- 149.Jiang H, Quilley J, Reddy LM, Falck JR, Wong PY, McGiff JC. Red blood cells: reservoirs of cis- and trans-epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2005;75:65–78. doi: 10.1016/j.prostaglandins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 150.Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151:1033–1040. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jiang H, Zhu AG, Mamczur M, Morisseau C, Hammock BD, Falck JR, McGiff JC. Hydrolysis of cis- and trans-epoxyeicosatrienoic acids by rat red blood cells. J Pharmacol Exp Ther. 2008;326:330–337. doi: 10.1124/jpet.107.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 153.Jiang JG, Ning YG, Chen C, Ma D, Liu ZJ, Yang S, Zhou J, Xiao X, Zhang XA, Edin ML, Card JW, Wang J, Zeldin DC, Wang DW. Cytochrome P450 epoxygenase promotes human cancer metastasis. Cancer Res. 2007;67:6665–6674. doi: 10.1158/0008-5472.CAN-06-3643. [DOI] [PubMed] [Google Scholar]
- 154.Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension. 2005;45:759–765. doi: 10.1161/01.HYP.0000153792.29478.1d. [DOI] [PubMed] [Google Scholar]
- 155.Jung O, Jansen F, Mieth A, Barbosa-Sicard E, Pliquett RU, Babelova A, Morisseau C, Hwang SH, Tsai C, Hammock BD, Schaefer L, Geisslinger G, Amann K, Brandes RP. Inhibition of the soluble epoxide hydrolase promotes albuminuria in mice with progressive renal disease. PLoS One. 2010;5:e11979. doi: 10.1371/journal.pone.0011979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Karara A, Makita K, Jacobson HR, Falck JR, Guengerich FP, DuBois RN, Capdevila JH. Molecular cloning, expression, and enzymatic characterization of the rat kidney cytochrome P-450 arachidonic acid epoxygenase. J Biol Chem. 1993;268:13565–13570. [PubMed] [Google Scholar]
- 157.Katragadda D, Batchu SN, Cho WJ, Chaudhary KR, Falck JR, Seubert JM. Epoxyeico-satrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J Mol Cell Cardiol. 2009;46:867–875. doi: 10.1016/j.yjmcc.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 158.Keseru B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, Hammock BD, Falck JR, Weissmann N, Busse R, Fleming I. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J. 2008;22:4306–4315. doi: 10.1096/fj.08-112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Keseru B, Barbosa-Sicard E, Schermuly RT, Tanaka H, Hammock BD, Weissmann N, Fisslthaler B, Fleming I. Hypoxia-induced pulmonary hypertension: comparison of soluble epoxide hydrolase deletion vs. inhibition. Cardiovasc Res. 2010;85:232–240. doi: 10.1093/cvr/cvp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kessler P, Popp R, Busse R, Schini-Kerth VB. Proinflammatory mediators chronically downregulate the formation of the endothelium-derived hyperpolarizing factor in arteries via a nitric oxide/cyclic GMP-dependent mechanism. Circulation. 1999;99:1878–1884. doi: 10.1161/01.cir.99.14.1878. [DOI] [PubMed] [Google Scholar]
- 161.Kim IH, Heirtzler FR, Morisseau C, Nishi K, Tsai HJ, Hammock BD. Optimization of amide-based inhibitors of soluble epoxide hydrolase with improved water solubility. J Med Chem. 2005;48:3621–3629. doi: 10.1021/jm0500929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim IH, Morisseau C, Watanabe T, Hammock BD. Design, synthesis, and biological activity of 1,3-disubstituted ureasas potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J Med Chem. 2004;47:2110–2122. doi: 10.1021/jm030514j. [DOI] [PubMed] [Google Scholar]
- 163.King LM, Gainer JV, David GL, Dai D, Goldstein JA, Brown NJ, Zeldin DC. Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenet Genomics. 2005;15:7–13. doi: 10.1097/01213011-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 164.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci. 2007;27:4642–4649. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia. Front Biosci. 2008;13:2833–2841. doi: 10.2741/2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kohagura K, Endo Y, Ito O, Arima S, Omata K, Ito S. Endogenous nitric oxide and epoxyeicosatrienoic acids modulate angiotensin II-induced constriction in the rabbit afferent arteriole. Acta Physiol Scand. 2000;168:107–112. doi: 10.1046/j.1365-201X.2000.00638.x. [DOI] [PubMed] [Google Scholar]
- 168.Krotz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn HY, Campbell WB, Pohl U. Membrane-potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol. 2004;24:595–600. doi: 10.1161/01.ATV.0000116219.09040.8c. [DOI] [PubMed] [Google Scholar]
- 169.Larsen BT, Gutterman DD, Hatoum OA. Emerging role of epoxyeicosatrienoic acids in coronary vascular function. Eur J Clin Invest. 2006;36:293–300. doi: 10.1111/j.1365-2362.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 170.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome P450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, Hammock BD, Couper DJ, Heiss G, Zeldin DC. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet. 2006;15:1640–1649. doi: 10.1093/hmg/ddl085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee CR, Pretorius M, Schuck RN, Burch LH, Bartlett J, Williams SM, Zeldin DC, Brown NJ. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with forearm vasodilator responses in humans. Hypertension. 2011;57:116–122. doi: 10.1161/HYPERTENSIONAHA.110.161695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee HC, Lu T, Weintraub NL, VanRollins M, Spector AA, Shibata EF. Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes. J Physiol. 1999;519:153–168. doi: 10.1111/j.1469-7793.1999.0153o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee SH, Lee J, Cha R, Park MH, Ha JW, Kim S, Kim YS. Genetic variations in soluble epoxide hydrolase and graft function in kidney transplantation. Transplant Proc. 2008;40:1353–1356. doi: 10.1016/j.transproceed.2008.03.137. [DOI] [PubMed] [Google Scholar]
- 177.Leffler CW, Fedinec AL. Newborn piglet cerebral microvascular responses to epoxyeicosatrienoic acids. Am J Physiol Heart Circ Physiol. 1997;273:H333–H338. doi: 10.1152/ajpheart.1997.273.1.H333. [DOI] [PubMed] [Google Scholar]
- 178.Li J, Carroll MA, Chander PN, Falck JR, Sangras B, Stier CT. Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension. Front Biosci. 2008;13:3480–3487. doi: 10.2741/2942. [DOI] [PubMed] [Google Scholar]
- 179.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 180.Li PL, Chen CL, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle. Circ Res. 1999;85:349–356. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- 181.Li PL, Zhang DX, Ge ZD, Campbell WB. Role of ADP-ribose in 11,12-EET-induced activation of K(Ca) channels in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;282:H1229–H1236. doi: 10.1152/ajpheart.00736.2001. [DOI] [PubMed] [Google Scholar]
- 182.Li PL, Zou AP, Campbell WB. Regulation of potassium channels in coronary arterial smooth muscle by endothelium-derived vasodilators. Hypertension. 1997;29:262–267. doi: 10.1161/01.hyp.29.1.262. [DOI] [PubMed] [Google Scholar]
- 183.Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25:939–948. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- 184.Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol. 2008;295:H619–H631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The anti-inflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Loch D, Hoey A, Morisseau C, Hammock BO, Brown L. Prevention of hypertension in DOCA-salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem Biophys. 2007;47:87–98. doi: 10.1385/cbb:47:1:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 188.Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, Zeldin DC, Lee HC. Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J Physiol. 2006;575:627–644. doi: 10.1113/jphysiol.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108:9038–9043. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang H, Gill R, Morisseau C, Newman JW, Hammock BD. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem. 2007;282:2891–2898. doi: 10.1074/jbc.M608057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ma J, Zhang L, Li S, Liu S, MaC, Li W, Falck JR, Manthati VL, Reddy DS, Medhora M, Jacobs ER, Zhu D. 8,9-Epoxyeicosatrienoic acid analog protects pulmonary artery smooth muscle cells from apoptosis via ROCK pathway. Exp Cell Res. 2010;316:2340–2353. doi: 10.1016/j.yexcr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest. 1994;94:2414–2420. doi: 10.1172/JCI117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Malcolm KC, Fitzpatrick FA. Epoxyeicosatrienoic acids inhibit Ca2+ entry into platelets stimulated by thapsigargin and thrombin. J Biol Chem. 1992;267:19854–19858. [PubMed] [Google Scholar]
- 194.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–F748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marciante KD, Totah RA, Heckbert SR, Smith NL, Lemaitre RN, Lumley T, Rice KM, Hindorff LA, Bis JC, Hartman B, Psaty BM. Common variation in cytochrome P450 epoxygenase genes and the risk of incident nonfatal myocardial infarction and ischemic stroke. Pharmacogenet Genomics. 2008;18:535–543. doi: 10.1097/FPC.0b013e3282fd1287. [DOI] [PubMed] [Google Scholar]
- 196.Marino JP., Jr Soluble epoxide hydrolase, a target with multiple opportunities for cardiovascular drug discovery. Curr Top Med Chem. 2009;9:452–463. doi: 10.2174/156802609788340805. [DOI] [PubMed] [Google Scholar]
- 197.Matsuda H, Hayashi K, Wakino S, Kubota E, Honda M, Tokuyama H, Takamatsu I, Tatematsu S, Saruta T. Role of endothelium-derived hyperpolarizing factor in ACE inhibitor-induced renal vasodilation in vivo. Hypertension. 2004;43:603–609. doi: 10.1161/01.HYP.0000118053.42262.71. [DOI] [PubMed] [Google Scholar]
- 198.McGee J, Fitzpatrick F. Enzymatic hydration of leukotriene A4. Purification and characterization of a novel epoxide hydrolase from human erythrocytes. J Biol Chem. 1985;260:12832–12837. [PubMed] [Google Scholar]
- 199.McSherry IN, Sandow SL, Campbell WB, Falck JR, Hill MA, Dora KA. A role for heterocellular coupling and EETs in dilation of rat cremaster arteries. Microcirculation. 2006;13:119–130. doi: 10.1080/10739680500466400. [DOI] [PubMed] [Google Scholar]
- 200.Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, Harder DR. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H215–H224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- 201.Medhora M, Dhanasekaran A, Gruenloh SK, Dunn LK, Gabrilovich M, Falck JR, Harder DR, Jacobs ER, Pratt PF. Emerging mechanisms for growth and protection of the vasculature by cytochrome P450-derived products of arachidonic acid and other eicosanoids. Prostaglandins Other Lipid Mediat. 2007;82:19–29. doi: 10.1016/j.prostaglandins.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 202.Merkel MJ, Liu L, Cao Z, Packwood W, Young J, Alkayed NJ, Van Winkle DM. Inhibition of soluble epoxide hydrolase preserves cardiomyocytes: role of STAT3 signaling. Am J Physiol Heart Circ Physiol. 2010;298:H679–H687. doi: 10.1152/ajpheart.00533.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR) FASEB J. 2003;17:770–772. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- 204.Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther. 2006;111:584–595. doi: 10.1016/j.pharmthera.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 205.Moffat MP, Ward CA, Bend JR, Mock T, Farhangkhoee P, Karmazyn M. Effects of epoxyeicosatrienoic acids on isolated hearts and ventricular myocytes. Am J Physiol Heart Circ Physiol. 1993;264:H1154–H1160. doi: 10.1152/ajpheart.1993.264.4.H1154. [DOI] [PubMed] [Google Scholar]
- 206.Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gosele C, Heuser A, Fischer R, Schmidt C, Schirdewan A, Gross V, Hummel O, Maatz H, Patone G, Saar K, Vingron M, Weldon SM, Lindpaintner K, Hammock BD, Rohde K, Dietz R, Cook SA, Schunck WH, Luft FC, Hubner N. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet. 2008;40:529–537. doi: 10.1038/ng.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA. 1999;96:8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 209.Morisseau C, Hammock BD. Gerry Brooks and epoxide hydrolases: four decades to a pharmaceutical. Pest Manag Sci. 2008;64:594–609. doi: 10.1002/ps.1583. [DOI] [PubMed] [Google Scholar]
- 210.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H2128–H2134. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–H1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 212.Nakayama K, Nitto T, Inoue T, Node K. Expression of the cytochrome P450 epoxygenase CYP2J2 in human monocytic leukocytes. Life Sci. 2008;83:339–345. doi: 10.1016/j.lfs.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 213.Nayeem MA, Zeldin DC, Boegehold MA, Morisseau C, Marowsky A, Ponnoth DS, Roush KP, Falck JR. Modulation by salt intake of the vascular response mediated through adenosine A(2A) receptor: role of CYP epoxygenase and soluble epoxide hydrolase. Am J Physiol Regul Integr Comp Physiol. 2010;299:R325–R333. doi: 10.1152/ajpregu.00823.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 215.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Dispos. 2007;35:1126–1134. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 216.Ng VY, Morisseau C, Falck JR, Hammock BD, Kroetz DL. Inhibition ofsmooth muscle proliferation by urea-based alkanoic acids via peroxisome proliferator-activated receptor alpha-dependent repression of cyclin D1. Arterioscler Thromb Vasc Biol. 2006;26:2462–2468. doi: 10.1161/01.ATV.0000242013.29441.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nithipatikom K, Gross GJ. Epoxyeicosatrienoic acids: novel mediators of cardioprotection. J Cardiovasc Pharmacol Ther. 2010;15:112–119. doi: 10.1177/1074248409358408. [DOI] [PubMed] [Google Scholar]
- 218.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Galphas mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 220.Olearczyk JJ, Quigley JE, Mitchell BC, Yamamoto T, Kim IH, Newman JW, Luria A, Hammock BD, Imig JD. Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci. 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Oliw EH, Lawson JA, Brash AR, Oates JA. Arachidonic acid metabolism in rabbit renal cortex. Formation of two novel dihydroxyeicosatrienoic acids. J Biol Chem. 1981;256:9924–9931. [PubMed] [Google Scholar]
- 222.Oliw EH, Oates JA. Oxygenation of arachidonic acid by hepatic microsomes of the rabbit. Mechanism of biosynthesis of two vicinal dihydroxyeicosatrienoic acids. Biochim Biophys Acta. 1981;666:327–340. doi: 10.1016/0005-2760(81)90291-5. [DOI] [PubMed] [Google Scholar]
- 223.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 224.Parrish AR, Chen G, Burghardt RC, Watanabe T, Morisseau C, Hammock BD. Attenuation of cisplatin nephrotoxicity by inhibition of soluble epoxide hydrolase. Cell Biol Toxicol. 2009;25:217–225. doi: 10.1007/s10565-008-9071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–H2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 226.Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 227.Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol. 2010;60:27–59. doi: 10.1016/B978-0-12-385061-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, Szelid Z, Vermeersch P, Gillijns H, Pellens M, Grimminger F, van Zonneveld AJ, Collen D, Busse R, Janssens S. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension. 2006;47:762–770. doi: 10.1161/01.HYP.0000208299.62535.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pomposiello SI, Carroll MA, Falck JR, McGiff JC. Epoxyeicosatrienoic acid-mediated renal vasodilation to arachidonic acid is enhanced in SHR. Hypertension. 2001;37:887–893. doi: 10.1161/01.hyp.37.3.887. [DOI] [PubMed] [Google Scholar]
- 230.Pomposiello SI, Quilley J, Carroll MA, Falck JR, McGiff JC. 5,6-Epoxyeicosatrienoic acid mediates the enhanced renal vasodilation to arachidonic acid in the SHR. Hypertension. 2003;42:548–554. doi: 10.1161/01.HYP.0000090095.87899.36. [DOI] [PubMed] [Google Scholar]
- 231.Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–29625. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- 232.Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J Biol Chem. 2002;277:15671–15676. doi: 10.1074/jbc.M110806200. [DOI] [PubMed] [Google Scholar]
- 233.Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- 234.Pozzi A, Popescu V, Yang S, Mei S, Shi M, Puolitaival SM, Caprioli RM, Capdevila JH. The anti-tumorigenic properties of peroxisomal proliferator-activated receptor alpha are arachidonic acid epoxygenase-mediated. J Biol Chem. 2010;285:12840–12850. doi: 10.1074/jbc.M109.081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pratt PF, Rosolowsky M, Campbell WB. Effects of epoxyeicosatrienoic acids on polymorphonuclear leukocyte function. Life Sci. 2002;70:2521–2533. doi: 10.1016/s0024-3205(02)01533-3. [DOI] [PubMed] [Google Scholar]
- 236.Proctor KG, Falck JR, Capdevila J. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res. 1987;60:50–59. doi: 10.1161/01.res.60.1.50. [DOI] [PubMed] [Google Scholar]
- 237.Rastaldo R, Paolocci N, Chiribiri A, Penna C, Gattullo D, Pagliaro P. Cytochrome P-450 metabolite of arachidonic acid mediates bradykinin-induced negative iontropic effect. Am J Physiol Heart Circ Physiol. 2001;280:H2823–H2832. doi: 10.1152/ajpheart.2001.280.6.H2823. [DOI] [PubMed] [Google Scholar]
- 238.Ren Y, Garvin JL, Falck JR, Renduchintala KV, Carretero OA. Glomerular autacoids stimulated by bradykinin regulate efferent arteriole tone. Kidney Int. 2003;63:987–993. doi: 10.1046/j.1523-1755.2003.00810.x. [DOI] [PubMed] [Google Scholar]
- 239.Revermann M, Barbosa-Sicard E, Dony E, Schermuly RT, Morisseau C, Geisslinger G, Fleming I, Hammock BD, Brandes RP. Inhibition of the soluble epoxide hydrolase attenuates monocrotaline-induced pulmonary hypertension in rats. J Hypertens. 2009;27:322–331. doi: 10.1097/hjh.0b013e32831aedfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ribeiro JD, Toro AA, Baracat EC. Antileukotrienes in the treatment of asthma and allergic rhinitis. J Pediatr. 2006;82:S213–S221. doi: 10.2223/JPED.1553. [DOI] [PubMed] [Google Scholar]
- 241.Rodenburg EM, Visser LE, Danser AH, Hofman A, van Noord C, Witteman JC, Uitterlinden AG, Stricker BH. Genetic variance in CYP2C8 and increased risk of myocardial infarction. Pharmacogenet Genomics. 2010;20:426–434. doi: 10.1097/FPC.0b013e32833a96d8. [DOI] [PubMed] [Google Scholar]
- 242.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 243.Rosolowsky M, Campbell WB. Role of PGI2 and epoxyeicosatrienoic acids in relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol Heart Circ Physiol. 1993;264:H327–H335. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- 244.Sacerdoti D, Bolognesi M, Di Pascoli M, Gatta A, McGiff JC, Schwartzman ML, Abraham NG. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H1999–H2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 245.Sacerdoti D, Colombrita C, Di Pascoli M, Schwartzman ML, Bolognesi M, Falck JR, Gatta A, Abraham NG. 11,12-Epoxyeicosatrienoic acid stimulates heme-oxygenase-1 in endothelial cells. Prostaglandins Other Lipid Mediat. 2007;82:155–161. doi: 10.1016/j.prostaglandins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 246.Sato K, Emi M, Ezura Y, Fujita Y, Takada D, Ishigami T, Umemura S, Xin Y, Wu LL, Larrinaga-Shum S, Stephenson SH, Hunt SC, Hopkins PN. Soluble epoxide hydrolase variant (Glu287Arg) modifies plasma total cholesterol and triglyceride phenotype in familial hypercholesterolemia: intrafamilial association study in an eight-generation hyperlipidemic kindred. J Hum Genet. 2004;49:29–34. doi: 10.1007/s10038-003-0103-6. [DOI] [PubMed] [Google Scholar]
- 247.Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 250.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, LuriaA, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Seubert JM, Xu F, Graves JP, Collins JB, Sieber SO, Paules RS, Kroetz DL, Zeldin DC. Differential renal gene expression in prehypertensive and hypertensive spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F552–F561. doi: 10.1152/ajprenal.00354.2004. [DOI] [PubMed] [Google Scholar]
- 252.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sharma JN, Mohammed LA. The role of leukotrienes in the pathophysiology of inflammatory disorders: is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology. 2006;14:10–16. doi: 10.1007/s10787-006-1496-6. [DOI] [PubMed] [Google Scholar]
- 254.Sharma M, McCarthy ET, Reddy DS, Patel PK, Savin VJ, Medhora M, Falck JR. 8,9-Epoxyeicosatrienoic acid protects the glomerular filtration barrier. Prostaglandins Other Lipid Mediat. 2009;89:43–51. doi: 10.1016/j.prostaglandins.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Shen HC, Ding FX, Wang S, Deng Q, Zhang X, Chen Y, Zhou G, Xu S, Chen HS, Tong X, Tong V, Mitra K, Kumar S, Tsai C, Stevenson AS, Pai LY, Alonso-Galicia M, Chen X, Soisson SM, Roy S, Zhang B, Tata JR, Berger JP, Colletti SL. Discovery of a highly potent, selective, bioavailable soluble epoxide hydrolase inhibitor with excellent ex vivo target engagement. J Med Chem. 2009;52:5009–5012. doi: 10.1021/jm900725r. [DOI] [PubMed] [Google Scholar]
- 256.Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab. 2008;28:111–125. doi: 10.1038/sj.jcbfm.9600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Simpkins AN, Rudic RD, Roy S, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide hydrolase inhibition modulates vascular remodeling. Am J Physiol Heart Circ Physiol. 2010;298:H795–H806. doi: 10.1152/ajpheart.00543.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174:2086–2095. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 260.Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA. 2005;102:2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Snyder GD, Krishna UM, Falck JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;283:H1936–H1942. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- 262.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 264.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 265.Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, Node K, Borgel J, Mugge A, Lindpaintner K, Huesing A, Maisch B, Zeldin DC, Liao JK. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110:2132–2136. doi: 10.1161/01.CIR.0000143832.91812.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sporkova A, Kopkan L, Varcabova S, Huskova Z, Hwang SH, Hammock BD, Imig JD, Kramer HJ, Cervenka L. Role of cytochrome P450 metabolites in the regulation of renal function and blood pressure in 2-kidney 1-clip hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1468–R1475. doi: 10.1152/ajpregu.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Srivastava PK, Sharma VK, Kalonia DS, Grant DF. Polymorphisms in human soluble epoxide hydrolase: effects on enzyme activity, enzyme stability, and quaternary structure. Arch Biochem Biophys. 2004;427:164–169. doi: 10.1016/j.abb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 268.Stephenson AH, Sprague RS, Lonigro AJ. 5,6-Epoxyeicosatrienoic acid reduces increases in pulmonary vascular resistance in the dog. Am J Physiol Heart Circ Physiol. 1998;275:H100–H109. doi: 10.1152/ajpheart.1998.275.1.H100. [DOI] [PubMed] [Google Scholar]
- 269.Stephenson AH, Sprague RS, Losapio JL, Lonigro AJ. Differential effects of 5,6-EET on segmental pulmonary vasoactivity in the rabbit. Am J Physiol Heart Circ Physiol. 2003;284:H2153–H2161. doi: 10.1152/ajpheart.00844.2002. [DOI] [PubMed] [Google Scholar]
- 270.Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic acid analogs and vascular function. Curr Med Chem. 2010;17:1181–1190. doi: 10.2174/092986710790827843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sun D, Yan C, Jacobson A, Jiang H, Carroll MA, Huang A. Contribution of epoxyei-cosatrienoic acids to flow-induced dilation in arteries of male ERalpha knockout mice: role of aromatase. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1239–R1246. doi: 10.1152/ajpregu.00185.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sun D, Yang YM, Jiang H, Wu H, Ojaimi C, Kaley G, Huang A. Roles of CYP2C29 and RXR gamma in vascular EET synthesis of female mice. Am J Physiol Regul Integr Comp Physiol. 2010;298:R862–R869. doi: 10.1152/ajpregu.00575.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome P450 epoxygenase-derived eicosanoids. Circ Res. 2002;90:1020–1027. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- 274.Takahashi K, Capdevila J, Karara A, Falck JR, Jacobson HR, Badr KF. Cytochrome P-450 arachidonate metabolites in rat kidney: characterization and hemodynamic responses. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;258:F781–F789. doi: 10.1152/ajprenal.1990.258.4.F781. [DOI] [PubMed] [Google Scholar]
- 275.Theken KN, Deng Y, Kannon MA, Miller TM, Plolyac SM, Lee CR. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metab Dispos. 2011;39:22–29. doi: 10.1124/dmd.110.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tran KL, Aronov PA, Tanaka H, Newman JW, Hammock BD, Morisseau C. Lipid sulfates and sulfonates are allosteric competitive inhibitors of the N-terminal phosphatase activity of the mammalian soluble epoxide hydrolase. Biochemistry. 2005;44:12179–12187. doi: 10.1021/bi050842g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tsao CC, Coulter SJ, Chien A, Luo G, Clayton NP, Maronpot R, Goldstein JA, Zeldin DC. Identification and localization of five CYP2Cs in murine extrahepatic tissues and their metabolism of arachidonic acid to regio- and stereoselective products. J Pharmacol Exp Ther. 2001;299:39–47. [PubMed] [Google Scholar]
- 278.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, Weiss RH. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–323. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Van de Voorde J, Vanheel B. Influence of cytochrome P-450 inhibitors on endothelium-dependent nitro-L-arginine-resistant relaxation and cromakalim-induced relaxation in rat mesenteric arteries. J Cardiovasc Pharmacol. 1997;29:827–832. doi: 10.1097/00005344-199706000-00018. [DOI] [PubMed] [Google Scholar]
- 280.VanRollins M, Kaduce TL, Knapp HR, Spector AA. 14,15-Epoxyeicosatrienoic acid metabolism in endothelial cells. J Lipid Res. 1993;34:1931–1942. [PubMed] [Google Scholar]
- 281.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 282.Wang D, Borrego-Conde LJ, Falck JR, Sharma KK, Wilcox CS, Umans JG. Contributions of nitric oxide, EDHF, and EETs to endothelium-dependent relaxation in renal afferent arterioles. Kidney Int. 2003;63:2187–2193. doi: 10.1046/j.1523-1755.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 283.Wang H, Lin L, Jiang J, Wang Y, Lu ZY, Bradbury JA, Lih FB, Wang DW, Zeldin DC. Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase C signaling pathways. J Pharmacol Exp Ther. 2003;307:753–764. doi: 10.1124/jpet.103.052787. [DOI] [PubMed] [Google Scholar]
- 284.Wang H, Zhao Y, Bradbury JA, Graves JP, Foley J, Blaisdell JA, Goldstein JA, Zeldin DC. Cloning, expression, and characterization of three new mouse cytochrome P450 enzymes and partial characterization of their fatty acid oxidation activities. Mol Pharmacol. 2004;65:1148–1158. doi: 10.1124/mol.65.5.1148. [DOI] [PubMed] [Google Scholar]
- 285.Wang X, Trottier G, Loutzenhiser R. Determinants of renal afferent arteriolar actions of bradykinin: evidence that multiple pathways mediate responses attributed to EDHF. Am J Physiol Renal Physiol. 2003;285:F540–F549. doi: 10.1152/ajprenal.00127.2003. [DOI] [PubMed] [Google Scholar]
- 286.Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, Wang DW, Zeldin DC. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther. 2005;314:522–532. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- 287.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 288.Webler AC, Michaelis UR, Popp R, Barbosa-Sicard E, Murugan A, Falck JR, Fisslthaler, Fleming Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol. 2008;295:C1292–C1301. doi: 10.1152/ajpcell.00230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DRJR, Siscovick DS, Fornage M. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190:26–34. doi: 10.1016/j.atherosclerosis.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 290.Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DRJR, Siscovick DS, Fornage M. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190:26–34. doi: 10.1016/j.atherosclerosis.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 291.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Epoxide hydrolases regulate epoxyeicosatrienoic acid incorporation into coronary endothelial phospholipids. Am J Physiol Heart Circ Physiol. 1999;277:H2098–H2108. doi: 10.1152/ajpheart.1999.277.5.H2098. [DOI] [PubMed] [Google Scholar]
- 292.Werner K, Schaefer WR, Schweer H, Deppert WR, Karck U, Zahradnik HP. Characterization and identification of cytochrome P450 metabolites of arachidonic acid released by human peritoneal macrophages obtained from the pouch of Douglas. Prostaglandins Leukot Essent Fatty Acids. 2002;67:397–404. doi: 10.1054/plef.2002.0449. [DOI] [PubMed] [Google Scholar]
- 293.Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol. 2005;145:775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Widstrom RL, Norris AW, Van Der Veer J, Spector AA. Fatty acid-binding proteins inhibit hydration of epoxyeicosatrienoic acids by soluble epoxide hydrolase. Biochemistry. 2003;42:11762–11767. doi: 10.1021/bi034971d. [DOI] [PubMed] [Google Scholar]
- 295.Williams JM, Murphy S, Burke M, Roman RJ. 20-Hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wray J, Bishop-Bailey D. Epoxygenases and peroxisome proliferator-activated receptors in mammalian vascular biology. Exp Physiol. 2008;93:148–154. doi: 10.1113/expphysiol.2007.038612. [DOI] [PubMed] [Google Scholar]
- 297.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- 298.Xiao YF, Huang L, Morgan JP. Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. J Physiol. 1998;508:777–792. doi: 10.1111/j.1469-7793.1998.777bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Xiao YF, Ke Q, Seubert JM, Bradbury JA, Graves J, Degraff LM, Falck JR, Krausz K, Gelboin HV, Morgan JP, Zeldin DC. Enhancement of cardiac L-type Ca2+ currents in transgenic mice with cardiac-specific overexpression of CYP2J2. Mol Pharmacol. 2004;66:1607–1616. doi: 10.1124/mol.104.004150. [DOI] [PubMed] [Google Scholar]
- 300.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, Davis BB, Low R, Hammock BD, Chiamvimonvat N. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yan G, Chen S, You B, Sun J. Activation of sphingosine kinase-1 mediates induction of endothelial cell proliferation and angiogenesis by epoxyeicosatrienoic acids. Cardiovasc Res. 2008;78:308–314. doi: 10.1093/cvr/cvn006. [DOI] [PubMed] [Google Scholar]
- 302.Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, Zeldin DC. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60:310–320. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]
- 303.Yang C, Kwan YW, Au AL, Poon CC, Zhang Q, Chan SW, Lee SM, Leung GP. 14,15-Epoxyeicosatrienoic acid induces vasorelaxation through the prostaglandin EP(2) receptors in rat mesenteric artery. Prostaglandins Other Lipid Mediat. 2010;93:44–51. doi: 10.1016/j.prostaglandins.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 304.Yang Q, Zhang RZ, Yim AP, He GW. Effect of 11,12-epoxyeicosatrienoic acid as an additive to St. Thomas& cardioplegia and University of Wisconsin solutions on endothelium-derived hyperpolarizing factor-mediated function in coronary microarteries: influence of temperature and time. Ann Thorac Surg. 2003;76:1623–1630. doi: 10.1016/s0003-4975(03)00735-5. [DOI] [PubMed] [Google Scholar]
- 305.Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, Lu ZY, Wang DW, Zeldin DC. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol. 2007;293:H142–H151. doi: 10.1152/ajpheart.00783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yang S, Wei S, Pozzi A, Capdevila JH. The arachidonic acid epoxygenase is a component of the signaling mechanisms responsible for VEGF-stimulated angiogenesis. Arch Biochem Biophys. 2009;489:82–91. doi: 10.1016/j.abb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yang W, Gauthier KM, Reddy LM, Sangras B, Sharma KK, Nithipatikom K, Falck JR, Campbell WB. Stable 5,6-epoxyeicosatrienoic acid analog relaxes coronary arteries through potassium channel activation. Hypertension. 2005;45:681–686. doi: 10.1161/01.HYP.0000153790.12735.f9. [DOI] [PubMed] [Google Scholar]
- 308.Yang W, Holmes BB, Gopal VR, Kishore RV, Sangras B, Yi XY, Falck JR, Campbell WB. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: use for studies of metabolism and ligand binding. J Pharmacol Exp Ther. 2007;321:1023–1031. doi: 10.1124/jpet.107.119651. [DOI] [PubMed] [Google Scholar]
- 309.Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20–125i-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther. 2008;324:1019–1027. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- 310.Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome P-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303:768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- 311.Ye D, Zhou W, Lee HC. Activation of rat mesenteric arterial KATP channels by 11,12-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol. 2005;288:H358–H364. doi: 10.1152/ajpheart.00423.2004. [DOI] [PubMed] [Google Scholar]
- 312.Ye D, Zhou W, Lu T, Jagadeesh SG, Falck JR, Lee HC. Mechanism of rat mesenteric arterial KATP channel activation by 14,15-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol. 2006;290:H1326–H1336. doi: 10.1152/ajpheart.00318.2005. [DOI] [PubMed] [Google Scholar]
- 313.Yu Z, Davis BB, Morisseau C, Hammock BD, Olson JL, Kroetz DL, Weiss RH. Vascular localization of soluble epoxide hydrolase in the human kidney. Am J Physiol Renal Physiol. 2004;286:F720–F726. doi: 10.1152/ajprenal.00165.2003. [DOI] [PubMed] [Google Scholar]
- 314.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 315.Zagorac D, Jakovcevic D, Gebremedhin D, Harder DR. Antiangiogenic effect of inhibitors of cytochrome P450 on rats with glioblastoma multiforme. J Cereb Blood Flow Metab. 2008;28:1431–1439. doi: 10.1038/jcbfm.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 317.Zeldin DC, DuBois RN, Falck JR, Capdevila JH. Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch Biochem Biophys. 1995;322:76–86. doi: 10.1006/abbi.1995.1438. [DOI] [PubMed] [Google Scholar]
- 318.Zeldin DC, Moomaw CR, Jesse N, Tomer KB, Beetham J, Hammock BD, Wu S. Biochemical characterization of the human liver cytochrome P450 arachidonic acid epoxygenase pathway. Arch Biochem Biophys. 1996;330:87–96. doi: 10.1006/abbi.1996.0229. [DOI] [PubMed] [Google Scholar]
- 319.Zhang B, Cao H, Rao GN. Fibroblast growth factor-2 is a downstream mediator of phosphatidylinositol 3-kinase-Akt signaling in 14,15-epoxyeicosatrienoic acid-induced angiogenesis. J Biol Chem. 2006;281:905–914. doi: 10.1074/jbc.M503945200. [DOI] [PubMed] [Google Scholar]
- 320.Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic acid. Stroke. 2002;33:2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- 321.Zhang L, Cui Y, Geng B, Zeng X, Tang C. 11,12-Epoxyeicosatrienoic acid activates the L-arginine/nitric oxide pathway in human platelets. Mol Cell Biochem. 2008;308:51–56. doi: 10.1007/s11010-007-9611-6. [DOI] [PubMed] [Google Scholar]
- 322.Zhang L, Ding H, Yan J, Hui R, Wang W, Kissling GE, Zeldin DC, Wang DW. Genetic variation in cytochrome P450 2J2 and soluble epoxide hydrolase and risk of ischemic stroke in a Chinese population. Pharmacogenet Genomics. 2008;18:45–51. doi: 10.1097/FPC.0b013e3282f313e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan SK, Gless R, Webb HK, Wang YX. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol. 2009;29:1265–1270. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- 324.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhang Y, El-Sikhry H, Chaudhary KR, Batchu SN, Shayeganpour A, Jukar TO, Bradbury JA, Graves JP, DeGraff LM, Myers P, Rouse DC, Foley J, Nyska A, Zeldin DC, Seubert JM. Overexpression of CYP2J2 provides protection against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2009;297:H37–H46. doi: 10.1152/ajpheart.00983.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhao X, Dey A, Romanko OP, Stepp DW, Wang MH, Zhou Y, Jin L, Pollock JS, Webb RC, Imig JD. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R188–R196. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- 327.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension. 2003;41:709–714. doi: 10.1161/01.HYP.0000047877.36743.FA. [DOI] [PubMed] [Google Scholar]
- 328.Zhao X, Quigley JE, Yuan J, Wang MH, Zhou Y, Imig JD. PPAR-alpha activator feno-fibrate increases renal CYP-derived eicosanoid synthesis and improves endothelial dilator function in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2006;290:H2187–H2195. doi: 10.1152/ajpheart.00937.2005. [DOI] [PubMed] [Google Scholar]
- 329.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 330.Zheng C, Wang L, Li R, Ma B, Tu L, Xu X, Dackor RT, Zeldin DC, Wang DW. Gene delivery of cytochrome P450 epoxygenase ameliorates monocrotaline-induced pulmonary artery hypertension in rats. Am J Respir Cell Mol Biol. 2010;43:740–749. doi: 10.1165/rcmb.2009-0161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zhu Y, Schieber EB, McGiff JC, Balazy M. Identification of arachidonate P-450 metabolites in human platelet phospholipids. Hypertension. 1995;25:854–859. doi: 10.1161/01.hyp.25.4.854. [DOI] [PubMed] [Google Scholar]
- 332.Zordoky BN, Aboutabl ME, El-Kadi AO. Modulation of cytochrome P450 gene expression and arachidonic acid metabolism during isoproterenol-induced cardiac hypertrophy in rats. Drug Metab Dispos. 2008;36:2277–2286. doi: 10.1124/dmd.108.023077. [DOI] [PubMed] [Google Scholar]
- 333.Zordoky BN, El-Kadi AO. Modulation of cardiac and hepatic cytochrome P450 enzymes during heart failure. Curr Drug Metab. 2008;9:122–128. doi: 10.2174/138920008783571792. [DOI] [PubMed] [Google Scholar]
- 334.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]