Microbial elicitors and the plant defense hormone jasmonic acid differentially modulates the plant’s innate immune response.
Abstract
The endogenous Arabidopsis (Arabidopsis thaliana) peptides, AtPeps, elicit an innate immune response reminiscent of pattern-triggered immunity. Detection of various danger signals, including microbe-associated molecular patterns (MAMPs), leads to elevated transcription of PROPEPs, the AtPep precursors, and PEPRs, the AtPep receptors. It has been hypothesized that AtPeps are involved in enhancing pattern-triggered immunity. Following this idea, we analyzed the relationship between MAMP- and AtPep-elicited signaling. We found that the perception of MAMPs enhanced a subsequent AtPep-triggered production of reactive oxygen species (ROS). Intriguingly, other components of AtPep-triggered immunity like Ca2+ influx, mitogen-activated protein kinase phosphorylation, ethylene production, and expression of early defense genes, as well as ROS-activated genes, remained unchanged. By contrast, treatment with methyl jasmonate promoted an increase of all analyzed AtPep-triggered responses. We positively correlated the intensities of generic AtPep-triggered responses with the abundance of the two AtPep receptors by generating constitutively expressing PEPR1 and PEPR2 transgenic lines and by analyzing pepr1 and pepr2 mutants. Further, we show that enhanced, as well as basal, ROS production triggered by AtPeps is absent in the double mutant of the respiratory burst oxidase homologs D and F (rbohD rbohF). We present evidence that the enhancement of AtPep-triggered ROS is not based on changes in the ROS detoxification machinery and is independent of mitogen-activated protein kinase and Ca2+ signaling pathways. Taken together, these results indicate an additional level of regulation besides receptor abundance for the RbohD/RbohF-dependent production of AtPep-elicited ROS, which is specifically operated by MAMP-triggered pathways.
Plant immune response is triggered by the recognition of potential danger: Specialized plasma membrane receptors monitor the cellular environment to detect specific danger signals (Boller and Felix, 2009). These danger signals can originate from exogenous sources, such as pathogen-associated molecular patterns and microbe-associated molecular patterns (MAMPs) connected to the presence of potentially harmful microbes, and herbivore-associated molecular patterns formed during herbivore feeding. In addition, danger signals can have an endogenous origin; damage-associated molecular patterns (DAMPs) are host molecules modified and/or released to the apoplast by cellular damage (Boller and Felix, 2009).
The Arabidopsis (Arabidopsis thaliana) genome encodes seven PROPEPs, the precursors of the so-called AtPeps (Huffaker et al., 2006; Huffaker and Ryan, 2007). This family of peptides has the ability to trigger immune responses reminiscent of pattern-triggered immunity (PTI) and were thus characterized as potential DAMPs (Boller and Felix, 2009). AtPeps are sensed by two pattern recognition receptors of the receptor-like kinase family (PEPR1 and PEPR2), which seem to share structural and functional similarity to the flagellin-receptor FLAGELLIN-SENSING2 (FLS2) and the elongation factor Tu (EF-Tu)-receptor EFR (Yamaguchi et al., 2006, 2010; Krol et al., 2010). Recently, responses triggered by the conserved epitopes of flagellin (flg22) and EF-Tu (elf18) as well as AtPeps have been shown to be dependent on the presence of BRI1-ASSOCIATED KINASE1 and BAK1-LIKE1, indicating convergence of signaling pathways (Chinchilla et al., 2007; Heese et al., 2007; Schulze et al., 2010; Roux et al., 2011).
One of the early responses triggered by MAMPs and DAMPs is the production of apoplastic reactive oxygen species (ROS) by the Arabidopsis NADPH oxidases RbohD and RbohF (Torres et al., 2006). In recent years, multiple functions have been assigned to this so-called “oxidative burst.” ROS are supposed to be directly toxic for invading pathogens, thus blocking their further proliferation, but also to act indirectly in defense by cross linking plant cell wall components (Torres, 2010). In addition, ROS have been shown to be involved in various intra- and intercellular signaling events. Elevated levels of ROS lead to activation of mitogen-activated protein kinases (MAPKs), ROS-mediated changes of redox conditions facilitate activation of redox-controlled transcription factors, and ROS-based modifications of lipids can generate signaling molecules like cyclic oxylipins (Torres, 2010). In addition to the intracellular signaling activity, ROS has also been shown to spread systemically by subsequently inducing ROS production in neighboring cells. In this way, a ROS wave is assumed to spread out from the local area of stress throughout the whole plant body (Miller et al., 2009).
Perception of MAMPs and DAMPs ultimately leads to induced resistance against subsequent microbial infections (Zipfel et al, 2004; Yamaguchi et al., 2010). One aspect of induced resistance is the so-called priming effect that is thought to be based on a more sensitive detection system in combination with faster and stronger responses to newly approaching threats (Conrath et al., 2006). This seems to be facilitated by the accumulation of dormant signaling components like MAPKs and a persistent change in histone modification patterns adjacent to defense-related genes (Beckers et al., 2009; Jaskiewicz et al., 2011).
Since MAMP perception also induces the expression of some PROPEPs and both PEPRs, it has been hypothesized that these components might enhance PTI (Huffaker and Ryan, 2007).
Here, we show that recognition of the MAMP flg22 massively enhances a subsequent AtPep-triggered oxidative burst. This enhancement seems to be exclusive for ROS because all other investigated generic AtPep-elicited responses, including medium alkalinization, MAPK activation, expression of early defense and ROS marker genes, and ethylene production, remained unchanged. By contrast, a pretreatment of leaf tissue with methyl jasmonate (MeJA), but not methyl salicylate (MeSA), led to a slight but general enhancement of AtPep-triggered responses, probably related to changes in receptor abundance. It has been shown before (Yamaguchi et al., 2010), and we present further evidence here, that a manipulation of the expression levels of PEPR genes affects all of the typical AtPep-elicited responses. However, the MAMP-triggered enhancement of AtPep ROS is still detectable in pepr1 or pepr2 single mutants, as well as in transgenic lines constitutively expressing either PEPR1 or PEPR2, and is thus independent of the number of receptors per cell. We present further evidence that the AtPep ROS response and its MAMP-dependent enhancement depends on the presence of RbohD and RbohF and that the enhancement is not the result of a reduced ROS detoxification capacity. Thus, we propose a second level of regulation for the AtPep-elicited oxidative burst that is modified by previous MAMP perception, which might be involved in induced resistance and systemic signaling.
RESULTS
MAMP Pretreatment Leads to an Enhanced AtPep-Triggered Production of ROS
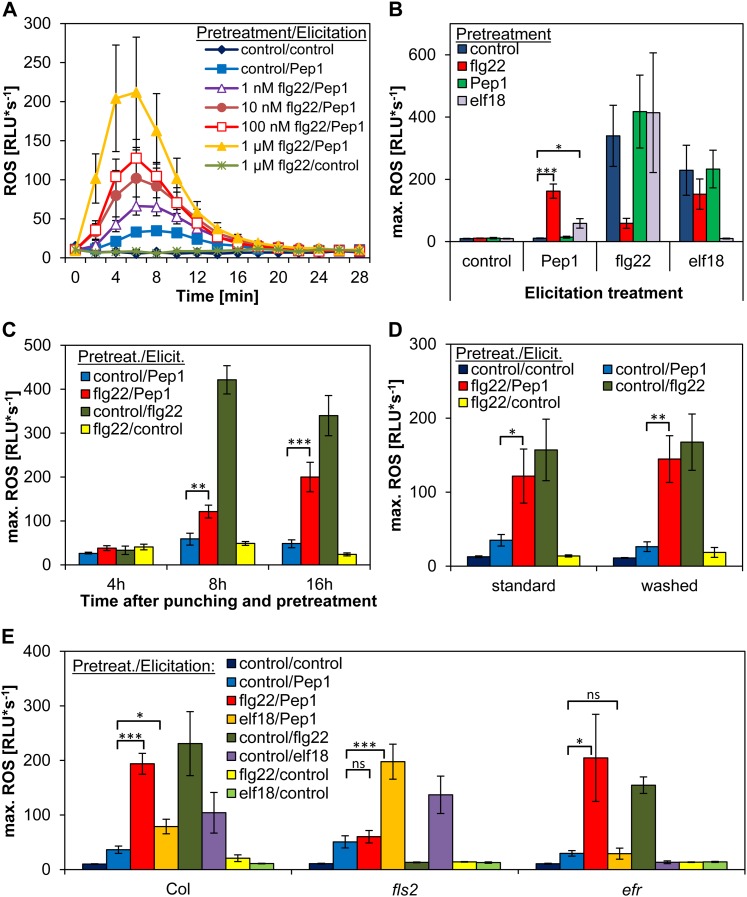
Detection of MAMPs like flg22 or elf18 rapidly induces the expression of PROPEPs and PEPRs (Zipfel et al., 2004; Huffaker et al., 2006; Zipfel et al., 2006; Denoux et al., 2008). Thus, we were wondering about the impact of MAMP perception on the various responses triggered by AtPeps (Huffaker et al., 2006; Huffaker and Ryan, 2007; Krol et al., 2010; Yamaguchi et al., 2010; Ranf et al., 2011). First, we analyzed ROS production in response to AtPep1, which is rather low in leaf discs punched from adult leaves (Krol et al., 2010). We found a strong increase of ROS production upon AtPep1 perception after pretreatment with flg22 (Fig. 1). This observed increase in ROS was positively correlated to the flg22 concentration used for the pretreatment (Fig. 1A) and independent of the MAMP used, although it was less pronounced with elf18 than with flg22 (Fig. 1B; Supplemental Fig. S1). Control treatments of flg22- or elf18-pretreated leaf discs did not induce ROS production (Fig. 1B). Surprisingly, the pretreatment of leaf discs with flg22 did not enhance the elf18-triggered ROS or vice versa (Fig. 1B). The final ROS production was independent of the presence of the pretreatment solution since a washing step between pretreatment and final treatment did not change the detectable ROS pattern (Fig. 1D). Lack of the flg22 receptor fls2 or the elf18 receptor efr impaired the enhancement of AtPep1-triggered ROS by flg22 or elf18 pretreatment, respectively (Fig. 1E). The ROS enhancing effect of a flg22 pretreatment could be observed already at 8 h after punching and pretreatment, but it was most robust after 16 h (Fig. 1C); thus, we used this time point for further analyses.
Figure 1.
Elevated AtPep1-triggered ROS production after pretreatment with flg22. A, Leaf discs were pretreated with indicated flg22 concentrations or without any peptide (control) for 16 h and then treated with 1 µm AtPep1 or without any peptide (control). Graphs represent mean values of ROS production in at least eight replicates. B and E, Leaf discs of Col-0 plants (B) and fls2 and efr mutants (E) were pretreated with the indicated elicitors (1 µm) or without any peptide (control) for 16 h and then treated with 1 µm of the indicated elicitor or without any peptide (control). C, Same as in B, but the pretreatment was performed for the indicated period of time. D, “Standard” was performed as in B, but in “washed,” leaf discs were washed before the final treatment. Columns represent averages of the peak values of ROS production of at least six biological replicates. Error bars show se of the mean. Asterisks represent Student’s t test results (*P < 0.05, **P < 0.01, ***P < 0.001). RLU, Relative light units; ns, not significant.
Taken together, we observed a strong and robust enhancement of the ROS production triggered by AtPep1 when leaf discs were pretreated for at least 8 h with MAMPs like flg22 and elf18. This enhancement is based on the perception of these MAMPs by their receptors, but it is independent of the presence of the pretreatment solution at the time when ROS is triggered by AtPep1.
flg22 Perception Does Not Enhance Other AtPep1-Triggered Responses
The spectrum of responses elicited by AtPep1 perception is reminiscent of the one triggered by flg22 (Boller and Felix, 2009; Krol et al., 2010; Yamaguchi et al., 2010). Thus, we further analyzed a selection of early and late responses to investigate if the MAMP-mediated enhancement of AtPep1-triggered responses is a global phenomenon or specific for ROS.
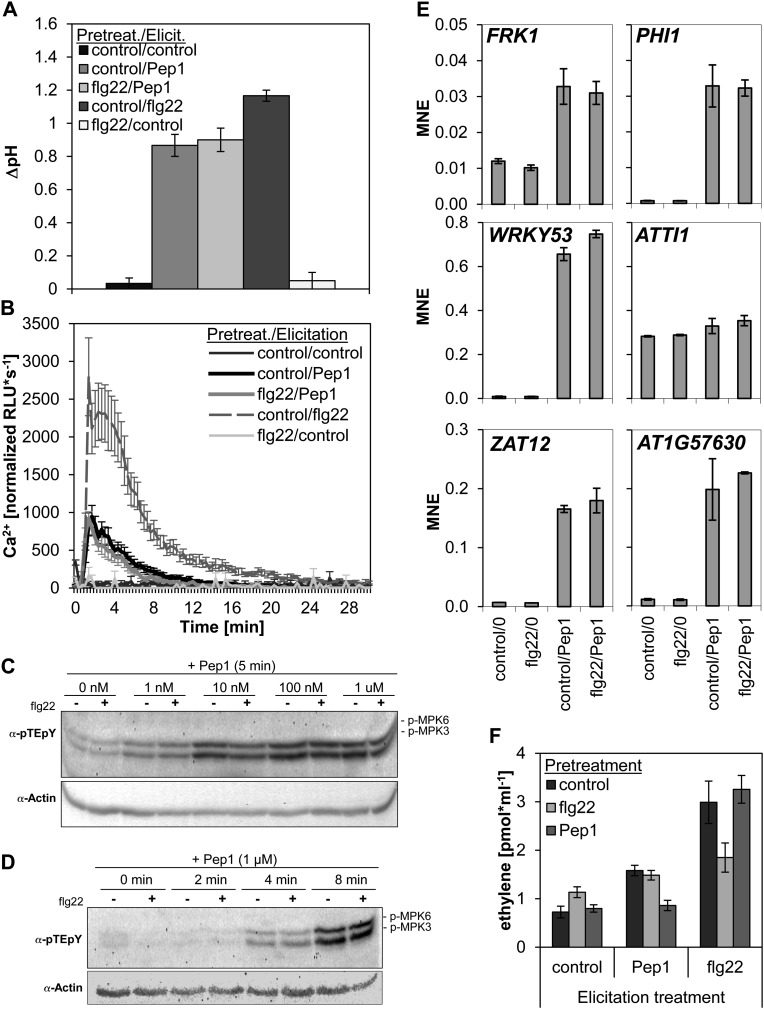
One of the first cellular responses to MAMP or AtPep detection is the alkalinization of the surrounding medium (Huffaker et al., 2006). We used liquid cell cultures, either pretreated with 1 µm flg22 or a control solution, and elicited the alkalinization response 16 h after pretreatment. As shown in Figure 2A, despite a clear response to the addition of AtPep1, we did not detect any difference between the flg22-pretreated and the control-pretreated cell cultures.
Figure 2.
flg22 pretreatment does not enhance other AtPep1-triggered responses. A, Medium alkalinization assay. Cell cultures were either pretreated with 1 µm flg22 or without any peptide (control) for 16 h and then treated with 1 µm of the indicated elicitor or mock treated (control). Bars represent mean pH shift values of five biological replicates. Error bars show se of the mean. B, Measurement of cytosolic calcium concentrations. Leaf discs were pretreated with 1 µm flg22 or without any peptide (control) for 16 h and then treated with the indicated elicitor (1 µm) or mock treated (control). Graphs represent normalized mean values of 12 biological replicates. Error bars show se of the mean. C and D, MAPK phosphorylation. Leaf discs were pretreated with 1 µm flg22 (+) or without any peptide (−) for 16 h and then treated for 5 min with the indicated concentrations of AtPep1 or a mock treatment (0 nm; C) and treated with 1 µm AtPep1 for the indicated period of time (D). MAPK phosphorylation was detected by immunoblotting using an antiphospho-p44/42-MAPK antibody detecting the pTE-pY motif of MPK6 and MPK3. The immunoblot was reprobed with anti-actin antibody to determine equal loading. E, Induction of marker gene transcription. Leaf discs were pretreated with 1 µm flg22 or without any peptide (control) for 16 h and then directly flash frozen (0) or treated with 1 µm AtPep1 for 30 min before freezing (Pep1). Transcript levels of indicated genes were first normalized to the reference gene UBQ10 before calculating the mean of three biological replicates. Error bars show se of the mean. F, Ethylene production. Leaf strips of Col-0 plants were pretreated with either 1 µm flg22 or 1 µm AtPep1 or without any peptide (control) for 16 h and then treated (elicitation treatment) with either 1 µm flg22, 1 µm AtPep1, or without any peptide (control) for 4 h. Columns represent averages of detected ethylene values of six biological replicates. Error bars show se of the mean. MNE, Mean normalized expression.
Influx of extracellular Ca2+ ions into the cytosol is another quick response to AtPep perception (Ranf et al., 2011). To detect changes in cytosolic Ca2+ concentrations, we made use of the aequorin luminescence-based Ca2+ detection method that has been used for leaf discs previously (Krol et al., 2010). We also could not detect an enhancement of the AtPep1-triggered Ca2+ influx in the flg22-pretreated leaf discs compared with the control (Fig. 2B) in this assay.
Next, we assessed the phosphorylation kinetics and intensities of the stress-activated MAPKs, MPK3 and MPK6. As displayed in Figure 2C, we did not detect a stronger MPK3 or MPK6 phosphorylation in flg22-pretreated leaf discs after a 5 min treatment with AtPep1. Similarly, we did not detect a more rapid phosphorylation of MPK3 and MPK6 after addition of AtPep1 in flg22-pretreated leaf discs compared with the control-pretreated ones (Fig. 2D).
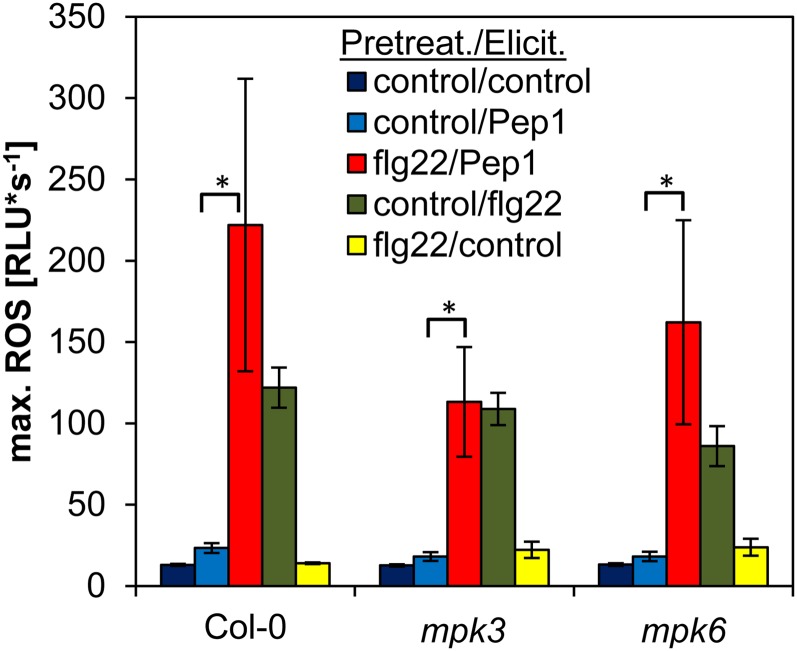
MPK3 has been connected to basal pathogen resistance, whereas MPK6 plays a role in elicitor-induced resistance (Galletti et al., 2011). Thus, we also tested whether mutants lacking either MPK3 or MPK6 are compromised for the enhancing effect of flg22 on the subsequent AtPep-triggered ROS production. Figure 3 shows that the enhancement of the AtPep1-triggered ROS by flg22 pretreatment in mpk3 and mpk6 mutant plants was comparable to wild-type plants.
Figure 3.
Lack of MPK3 or MPK6 does not impair flg22-mediated elevation of AtPep1-triggered ROS production. A, Leaf discs of Col-0, mpk3, and mpk6 mutant plants were pretreated with either 1 µm flg22 or without any peptide (control) for 16 h and then treated with either 1 µm AtPep1, 1 µm flg22, or without any peptide (control) as indicated. Columns represent averages of the peak values of ROS production of 12 biological replicates. Error bars show se of the mean. Asterisks represent Student’s t test results (*P < 0.05). RLU, Relative light units.
We then studied the effect of the flg22 pretreatment on the subsequent induction of defense-related genes in response to AtPep1. We selected a set of genes covering distinct signaling pathways. FLG22-INDUCED RECEPTOR-LIKE KINASE1 (FRK1) is induced via MAPK-mediated signaling, whereas PHOSPHATE-INDUCED1 (PHI1) transcription is activated by calcium-dependent protein kinases (CDPKs; Boudsocq et al., 2010). WRKY53 has been shown to change its transcription profile due to stress-mediated long-term modifications of the methylation pattern of the adjacent histone (Jaskiewicz et al., 2011). Zat12 is known to be ROS responsive and has been used to monitor the rapid spread of ROS in plants after local wounding (Miller et al., 2009). Finally, we picked TRYPSIN INHIBITOR PROTEIN1 (ATTI1) and At1g57630, which have been identified to respond to a variety of treatments that trigger the production of ROS (singlet oxygen, superoxide, or hydrogen peroxide) in diverse subcellular compartments (Gadjev et al., 2006).
Our data show that the pretreatment with flg22, despite of its strong induction of FRK1 in the short term, had no effect on the expression of these genes after 16 h (Fig. 2E, compare control/0 and flg22/0). A subsequent treatment with AtPep1 strongly stimulated expression of all genes investigated within 30 min, except ATTI1, but this stimulation was independent of the pretreatment (Fig. 2E, compare control/Pep1 and flg22/Pep1).
Finally, we measured the release of ethylene in control-, flg22-, and AtPep1-pretreated leaf strips. The pretreatments had little effect on the release of ethylene 16 h later in the control-treated samples (Fig. 2F, control). When stimulated with flg22, the leaf discs that had not been pretreated responded in the same way as leaf discs pretreated with AtPep1. As before, the flg22-pretreated leaf discs responded much less to a second stimulation by flg22 (Fig. 2F, flg22). The AtPep1-triggered ethylene response was lower than that elicited by flg22; nevertheless, it was clear that it could not be enhanced by flg22 pretreatment (Fig. 2F, AtPep1). Again, as before, the AtPep1-pretreated leaf strips did not respond to a second stimulation by AtPep1.
Lack of RbohD and RbohF Impairs AtPep1-Triggered ROS Production
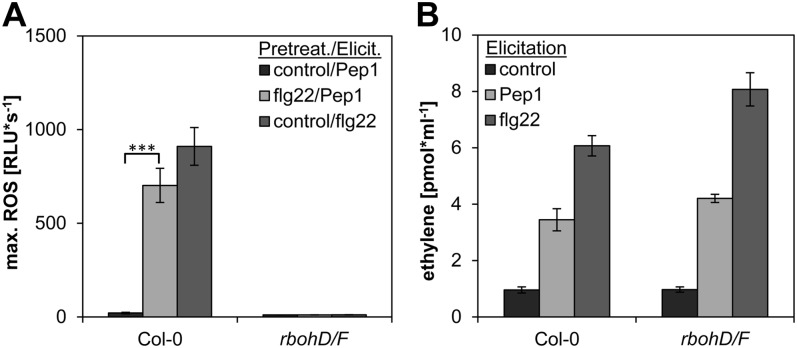
Because ROS production seems to be the only flg22-enhanced AtPep-elicited response, we analyzed this in more detail. The NADPH oxidases RbohD and RbohF are the main producers of apoplastic ROS in response to elicitors and the presence of pathogens (Torres et al., 2002). However, cell wall peroxidases and polyamine oxidases can also be sources of ROS (Bolwell et al., 2002; Yoda et al., 2009). Thus, we determined the ROS-response of rbohD rbohF double mutant plants after treatment with AtPep1. As shown in Figure 4A, ROS could be detected neither in the flg22-pretreated leaf discs nor in the control-pretreated discs. To exclude a general insensitivity of the rbohD rbohF double mutant toward AtPep1, we additionally measured the release of ethylene. Since the mutant plants were similar to the wild type in this regard (Fig. 4B), we concluded that the initial and the flg22-enhanced ROS production upon AtPep1 perception is mediated by the enzymatic activities of RbohD and RbohF.
Figure 4.
Lack of functional RbohD and RbohF blocks AtPep1-triggered ROS with and without pretreatment, but does not impair the production of ethylene. A, ROS production. Leaf discs of Col-0 and rbohD rbohF double knockout mutants were either pretreated with 1 µm flg22 or without any peptide (control) for 16 h and then treated with either 1 µm AtPep1 or 1 µm flg22 as indicated. Columns represent averages of the peak values of ROS production of 12 biological replicates. Error bars show se of the mean. RLU, Relative light units. B, Ethylene production. Leaf strips were incubated in water for 16 h and then treated with either 1 µm AtPep1, 1 µm flg22, or without any peptide (control). Ethylene production was measured after 4 h of incubation. Error bars show se of the mean of six biological replicates. Asterisks represent Student’s t test results (***P < 0.001).
MAMP-Induced Enhancement of AtPep-Triggered ROS Is Mostly Independent of the Abundance of the Two PEPRs and the Applied AtPep
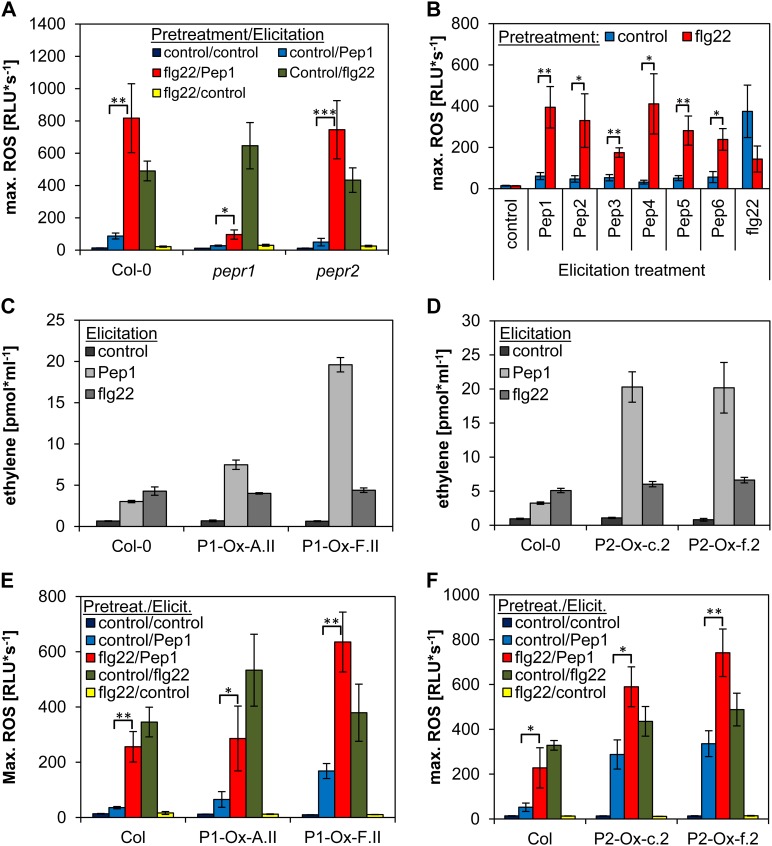
The two AtPep receptors, PEPR1 and PEPR2, differ in their specificity. PEPR1 is able to detect all known AtPeps, whereas PEPR2 mainly binds AtPep1 and AtPep2 (Yamaguchi et al., 2010). What is the role of the two receptors in the observed phenomena? To answer this question, we first assessed the enhanced ROS production upon AtPep1 treatment in flg22-pretreated pepr1 and pepr2 single mutants. Despite the known reduced intensity of AtPep1-triggered responses, both single mutants showed a stronger AtPep1 ROS response when pretreated with flg22 (Fig. 5A). Likewise, in wild-type plants, all of the AtPep peptides stimulated ROS production after pretreatment with flg22 in a similar way as AtPep1 (Fig. 5B).
Figure 5.
flg22-dependent enhancement of AtPep-triggered ROS is independent of the abundance of PEPR1 or PEPR2 and the added AtPep peptide. A, B, E, and F, ROS production. Leaf discs of the indicated genotypes (A, E, and F) or Col-0 (B) were either pretreated with 1 µm flg22 or without any peptide (control) for 16 h and then treated with 1 µm of the indicated peptide or without any peptide (control). Columns represent averages of the peak values of ROS production of 12 biological replicates. Error bars show se of the mean. C and D, Ethylene production. Leaf discs of Col-0 and two independent transgenic lines expressing either 35S::PEPR1::yellow fluorescent protein (P1-Ox-A.II and P1-Ox-F.II) or 35S::PEPR2::yellow fluorescent protein (P2-Ox-c.2 and P2-Ox-f.2) were floated on water for 16 h, then treated with the indicated elicitors and incubated for 4 h before measurement. Columns represent the mean of six independent replicates. Error bars show se of the mean. Asterisks represent Student’s t test results (*P < 0.05, **P < 0.01, ***P < 0.001). RLU, Relative light units.
To examine the possible role of receptor abundance in the enhanced ROS production, we generated transgenic Arabidopsis plants constitutively expressing either PEPR1 or PEPR2 in the pepr1 pepr2 double mutant background (Supplemental Fig. S2). These plants showed a much stronger ethylene response compared with wild-type plants when treated with AtPep1, suggesting that the higher levels of PEPR1 or PEPR2 caused an enhancement of AtPep-triggered responses (Fig. 5, C and D). Interestingly, most of the transgenic plants overexpressing PEPRs displayed a more pronounced oxidative burst even in the absence of a pretreatment with flg22 (Fig. 5, E and F). However, in all these plants, the AtPep1-triggered ROS was still enhanced when leaf discs were pretreated with flg22. Since the PEPR transcription was driven by the constitutive Cauliflower mosaic virus 35S promoter in the pepr1 pepr2 double mutant background, we assume that PEPR transcription is not further induced upon flg22 pretreatment; thus, the flg22-mediated enhancement of AtPep1-triggered ROS is independent of induced PEPR transcription. Moreover, additional AtPep-triggered responses beside ROS are enhanced in the constitutive PEPR-expressing plants (Fig. 5, C and D), indicating that an elevated receptor abundance enhances all AtPep-triggered responses. Thus, a change in PEPR abundance cannot be the cause for the observed enhanced AtPep-triggered ROS after flg22 pretreatment.
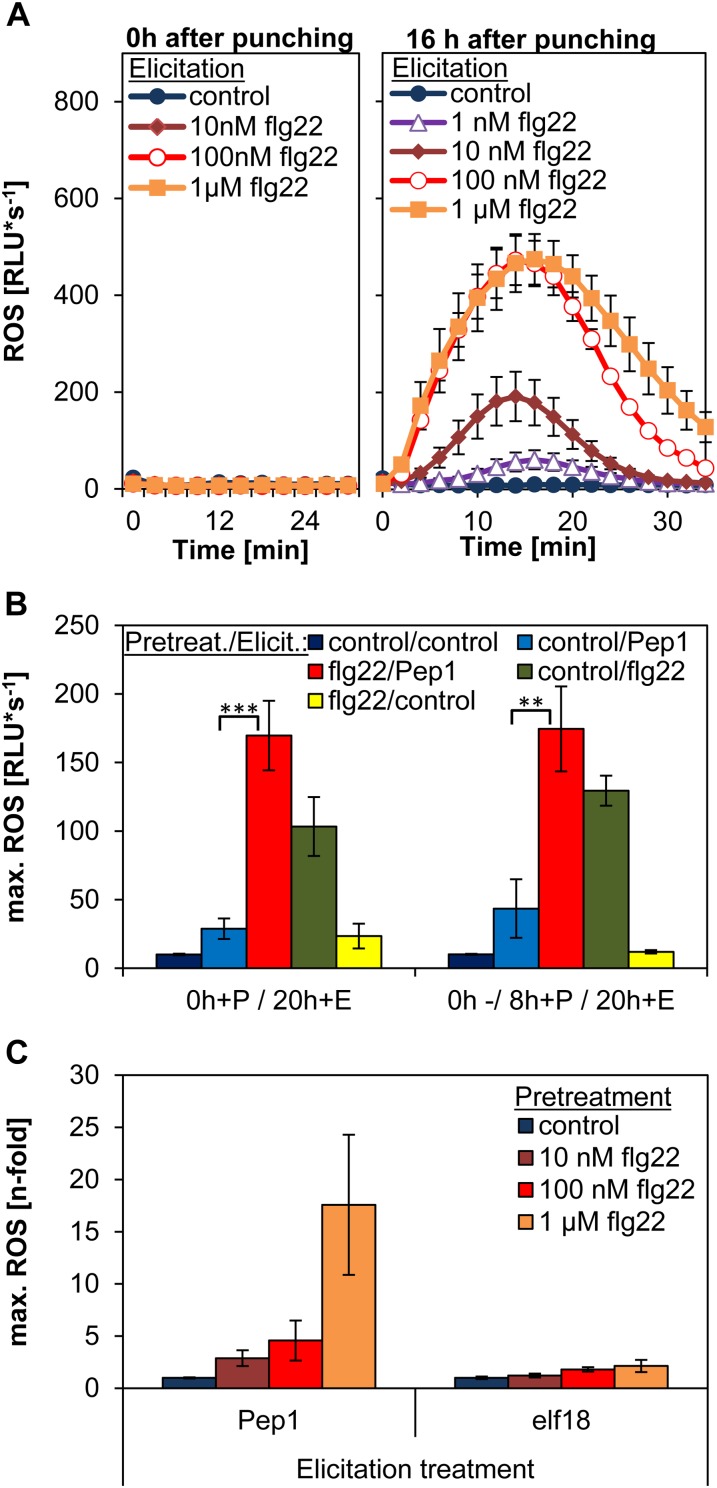
Enhancement of AtPep1-Triggered ROS Is Not Based on an Altered ROS Detoxification Machinery Elicited by the flg22 Pretreatment
To assess the possibility that the increased AtPep ROS could be a consequence of the depletion of ROS detoxifying compounds due to previous bursts, we assessed the initial flg22 treatment for ROS. Intriguingly, after directly applying flg22 to freshly cut leaf tissue, we could not detect any increased production of ROS with our luminol-based assay. This might indicate that the initial flg22 treatment directly after wounding the leaf tissue does not induce apoplastic ROS production (Fig. 6A, left). However, when flg22 is added to plant tissue after the usual 16-h lag phase, it induces a strong ROS production (Fig. 6A, right). Notably, the flg22-induced ROS burst is already saturated at around 100 nm, whereas the AtPep-triggered ROS in pretreated samples can still be enhanced by further increasing the concentration of flg22 up to 1 µm for pretreatment (Figs. 1A and 6B). In addition, including a waiting period of 8 h before adding the pretreatment solution, which will enable a detectable flg22-elicited ROS burst (Fig. 1C), did not change the enhancement of the ROS production triggered by subsequent AtPep1 treatment (Fig. 6B). Finally, comparing AtPep1-triggered ROS and elf18-triggered ROS in flg22-pretreated samples shows that when the flg22 pretreatment strongly increases the AtPep1-triggered ROS, it has only a very small impact on the ROS production elicited by elf18 (Fig. 6B).
Figure 6.
Enhanced AtPep ROS is independent of previous ROS production. A, Leaf discs were treated with the indicated concentrations of flg22 either directly after punching (left) or after the standard 16-h incubation time floating on water (right). Graphs display averages of 12 replicates. Error bars show se of the mean. B, Indicated pretreatment (1 µm flg22 or without any peptide [control]) was performed either directly after punching (0h+P/20h+E) or at 8 h after punching (0h-/8h+P/20h+E). Twenty hours after punching, all leaf discs were treated with indicated elicitors or without any peptide (control). Columns represent averages of the peak values of ROS production of eight biological replicates. Error bars show se of the mean. C, Relative effect of pretreatments on ROS production. Leaf discs were pretreated as indicated for 16 h and then treated with either 1 µm AtPep1 or 1 µm elf18 (elicitation). Columns represent relative averages of the peak values of ROS production normalized to the respective control treatment. Error bars show se of the mean of 12 biological replicates. Asterisks represent Student’s t test results (**P < 0.01, ***P < 0.001). RLU, Relative light units.
Jasmonate Seems to Enhance All AtPep-Triggered Responses
In addition to MAMPs, other molecules have been reported to induce the expression of PROPEPs and PEPRs (Huffaker et al., 2006; Huffaker and Ryan, 2007; Yamaguchi et al., 2010). Among these, MeJA was especially effective (Yamaguchi et al., 2010). Thus, we wondered whether a pretreatment with MeJA or MeSA would enhance ROS production in a similar way as the flg22 pretreatment.
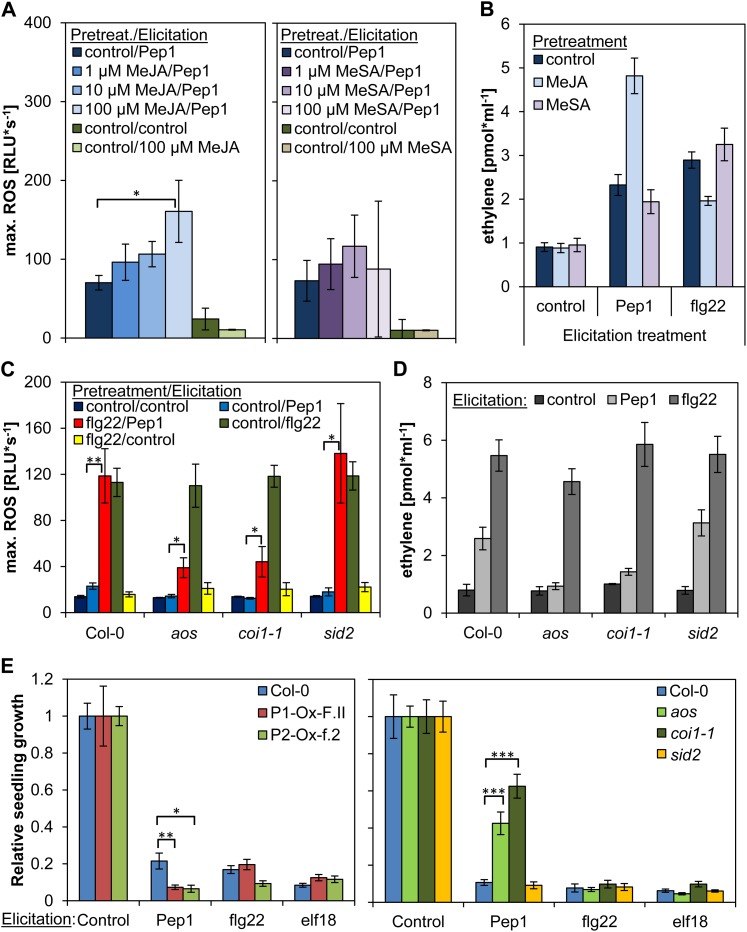
Indeed, pretreatment with MeJA (but not with MeSA) induced a slight enhancement of subsequent AtPep1-triggered ROS production (Fig. 7A). Accordingly, the jasmonate (JA) synthesis mutant allene oxide synthase (aos) and JA-insensitive mutant coronatine-insensitive1 (coi1-1), but not the salicylate synthesis mutant SA-induction deficient2 (sid2), exhibited a reduced ROS release upon AtPep1 treatment (Fig. 7C). Neither MeJA nor MeSA triggered a detectable ROS production (Fig. 7A). Thus, we hypothesized that the flg22 pretreatment might constitutively elevate endogenous JA and JA-Ile levels, which in turn would specifically enhance AtPep-triggered ROS production. JA measurements revealed 4-fold increased levels of JA and JA-Ile levels in flg22-pretreated samples compared with control-pretreated samples (Supplemental Fig. S3). Therefore, we assessed the specificity of JA on AtPep-triggered responses, but in contrast to a pretreatment with flg22, pretreatment with MeJA led to a strong increase of AtPep1-triggered ethylene production (Fig. 7B). We consistently observed a reduction of AtPep1-induced ethylene production in the aos and coi1-1 mutants, as well as in opr3, another JA synthesis mutant, but not in the sid2 mutant (Fig. 7D; Supplemental Fig. S4). In addition, the inhibition of seedling growth by AtPep1 is significantly reduced in aos and coi1-1 mutants, whereas it is strongly enhanced in transgenic plants constitutively expressing PEPR1 or PEPR2 (Fig. 7E).
Figure 7.
JA regulates AtPep-triggered responses. A, ROS production in Col-0. Leaf discs were either pretreated with indicated concentrations of MeJA (left) or MeSA (right) or without any hormone (control) for 16 h and then either treated with 1 µm AtPep1, 100 µm MeJA (left), 100 µm MeSA (right), or mock (control) as indicated. Columns represent averages of the peak values of ROS production of 12 biological replicates. B, Ethylene production in Col-0. Leaf strips were pretreated with the indicated concentrations of either MeJA or MeSA or mock (control) for 16 h and then treated with either 1 µm of AtPep1 or flg22 or mock (control) as indicated (elicitation) and incubated for 4 h before measurement. Columns represent the mean of six independent replicates. C, ROS production in aos, coi1-1, and sid2. Leaf discs of Col-0, aos, coi1-1, and sid2 mutant plants were pretreated with 1 µm flg22 or without any peptide (control) for 16 h and then treated with or without 1 µm of elicitor as indicated. Columns represent averages of the peak values of ROS production of 12 biological replicates. D, Ethylene production in aos, coi1-1, and sid2. Leaf strips of Col-0, aos, coi1-1, and sid2 mutant plants were incubated in water for 16 h and then treated with either 1 µm of AtPep1 or flg22 or without any peptide (control) and incubated for 4 h before measurement. Columns represent the mean of six independent replicates. E, Seedling growth inhibition. Five-day-old seedlings of the indicated genotypes were treated for 10 d with 1 µm of the indicated elicitor or without any peptide. Columns represent the mean weight of 12 seedlings out of six biological replicates. Error bars show se of the mean. Asterisks represent Student’s t test results (*P < 0.05, **P < 0.01, ***P < 0.001). RLU, Relative light units.
JA levels have been shown to rapidly increase upon wounding (Glauser et al., 2009). To exclude the possibility that a rapid wave of JA triggered by the final AtPep treatment somehow modulates the ROS production, we measured JA and JA-Ile levels in control- and flg22-pretreated samples directly after addition of AtPep1. We found that, independent of the pretreatment, JA and JA-Ile levels did not change within the first 10 min after AtPep1 treatment (Supplemental Fig. S3). Additional measurements with later time points revealed that AtPep1 triggers a transient and comparably weak rise in JA and JA-Ile levels around 1 h after treatment (Supplemental Fig. S5).
Together, these results indicate that, in contrast to the specific effect of flg22, JA enhances all AtPep-elicited responses.
DISCUSSION
MAMPs such as flg22 are characteristic of whole classes of microbes, which might or might not be pathogenic for a specific plant. The perception of MAMPs triggers a defense response, which comprises both early and late responses and ultimately may stop the invasion of a microbe (Boller and Felix, 2009). Similarly, the Arabidopsis endogenous peptides, AtPeps, elicit a defense response and mediate an increased resistance to Pseudomonas syringae pv tomato DC3000 in a similar way as flg22 (Huffaker and Ryan, 2007; Yamaguchi et al., 2010). It has been hypothesized that AtPeps might act as an amplifier of PTI since PROPEP, as well as PEPR, expression is induced upon flg22 perception (Zipfel et al., 2004; Huffaker and Ryan, 2007). However, neither the release of AtPeps or PROPEPs nor an impact of pepr1 pepr2 knockout on plant defense has been reported yet, thus the biological function of PROPEPs and PEPRs remains elusive.
Here, we show that a previous stimulation by MAMPs such as flg22 or elf18 strongly increased the apoplastic ROS production upon subsequent AtPep perception. This effect was specific to ROS because no other AtPep response investigated was altered by MAMP pretreatment. Also, it was highly specific for AtPeps, since the subsequent perception of MAMPs did not lead to increased ROS production. A similarly specific enhancement of ROS production has been reported for grapevine (Vitis vinifera) cell suspensions that were pretreated with β-aminobutyric acid and subsequently elicited with oligogalacturonides (Dubreuil-Maurizi et al., 2010). In that case, the approximately 2-fold enhanced ROS production seemed to be connected to a slight increase in RbohD expression, but the impact of β-aminobutyric acid on ROS triggered by other elicitors had not been tested. However, enhanced RbohD expression suggests a general increase in the capacity to produce ROS, rather than an oligogalacturonide-specific enhancement of ROS production.
Our results show that induced ROS production in response to flg22 or AtPeps depends on functional RbohD/RbohF. Because MAMP-triggered ROS production did not change in MAMP-pretreated samples, we ruled out the possibility that an increase in RbohD/RbohF abundance is responsible for the observed specific enhancement of AtPep-triggered ROS. Due to the reported induction of PEPR expression upon flg22 perception (Zipfel et al., 2004), we hypothesized that an increase in PEPR abundance might be an explanation for this effect. However, analysis of plants constitutively expressing PEPR1 or PEPR2 in the pepr1 pepr2 double mutant background showed that the flg22-mediated enhancement of AtPep ROS was still present. Moreover, these plants also showed an enhanced basal ROS production, as well as a strong increase of ethylene release upon AtPep treatment, indicating a global enhancement of AtPep-triggered responses when PEPR levels increase.
MeJA has also been reported to induce PEPR expression (Yamaguchi et al., 2010). Mutants impaired in JA synthesis or detection consistently showed reduced AtPep-triggered responses, whereas pretreatment with MeJA led to elevated AtPep-triggered responses, mimicking plants constitutively expressing PEPRs. Pretreatment with flg22 caused an elevation of JA and JA-Ile levels, which could in principle promote the enhanced AtPep-triggered ROS production. On the other hand, flg22 pretreatment had no effect on other AtPep1-triggered responses, except ROS. Moreover, the JA-insensitive mutant coi1-1 showed, despite overall lower AtPep-triggered responses, a flg22-mediated enhancement of AtPep1-triggered ROS. Thus, we conclude that JA levels modulate AtPep-triggered responses most likely by regulating PEPR expression. By contrast, the enhancement of AtPep-triggered ROS by flg22 is largely independent of JA synthesis and perception.
Next, we hypothesized that the initial production of ROS triggered by flg22 or elf18 is mediating the enhancement of subsequent AtPep-triggered ROS by inhibiting the ROS degrading capacity. However, several lines of evidence contradict this hypothesis. First, depletion or modification of the ROS quenching capacity by a flg22-triggered oxidative burst should also affect the detectable ROS triggered by other elicitors such as elf18. However, the elf18-triggered ROS production essentially did not change in flg22-pretreated samples. Second, flg22 did not induce a detectable oxidative burst when added to freshly harvested leaf discs. The wounding response seems to suppress a flg22-mediated activation of RbohD/RbohF, and thus no apoplastic ROS is present to alter the ROS-degrading capacity. Finally, we found that increasing concentrations of flg22 positively correlated with the enhancement of AtPep-triggered ROS production, reaching 1 µm without signs of saturation. By contrast, flg22-triggered ROS production reached saturation as early as 100 nm flg22 concentration. Hence, we conclude that the MAMP-mediated enhancement of AtPep ROS production is not connected to a putative modification of the ROS quenching capacity.
Thus, we wondered if our observations could be explained by a posttranslational regulation of RbohD and RbohF. It has been shown that AtRbohD is synergistically activated by Ca2+ and direct phosphorylation (Nühse et al., 2007; Ogasawara et al., 2008). In addition, it was reported that Arabidopsis plants mutated for several CDPKs showed decreased oxidative burst upon flg22, suggesting a role for CDPKs in regulating ROS production (Boudsocq et al., 2010). By contrast, silencing of the stress-linked MAPKs SALICYLATE-INDUCED PROTEIN KINASE and WOUND-INDUCED PROTEIN KINASE in Nicotiana benthamiana did not impair MAMP-elicited ROS (Segonzac et al., 2011). However, neither Ca2+ influx nor MAPK phosphorylation kinetics or CDPK- and MAPK-dependent gene expression seemed to be enhanced in MAMP-pretreated samples, indicating that AtPep-triggered activation of RbohD and RbohF is not achieved via these pathways but might be linked to the PEPRs via different signaling routes. Another possibility would be a persistent modification of Rboh proteins rendering them much more sensitive to subsequent activating signals. In this case, even the comparably weaker AtPep-triggered Ca2+ influx might be sufficient to facilitate full RbohD and RbohF activation and respective ROS release. However, since this modification would be triggered by flg22 perception, which we usually did 16 h before the secondary treatment, this mechanism would require a very slow turnover of the RbohD and RbohF to keep the modification. Moreover, a flg22-mediated elevated sensitivity of RbohD/RbohF to activating signals should again lead to enhanced ROS production triggered by elf18, which we did not detect.
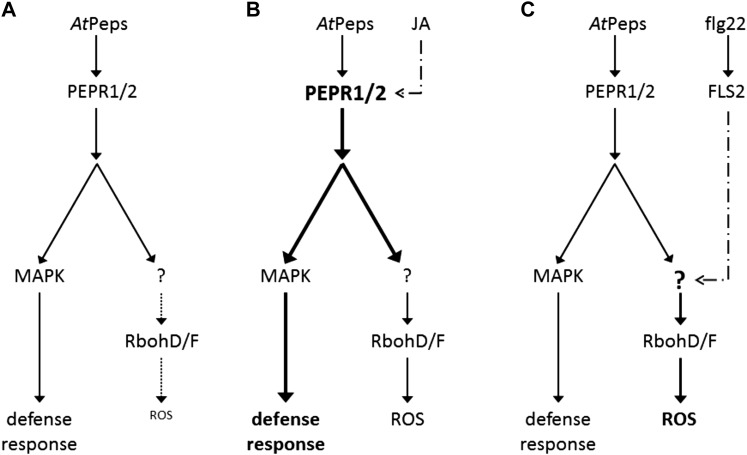
Taken together, we found a MAMP-triggered specific enhancement of AtPep-elicited ROS production that (1) depends on functional RbohD and RbohF, (2) is not solely based on an induced expression of PEPR1 and PEPR2, (3) appears to be independent of potential changes in the ROS detoxification machinery, (4) appears to be independent of Ca2+- and MAPK-mediated signaling pathways, and (5) is clearly distinct from the global enhancement of AtPep-triggered responses mediated by JA (Fig. 8). We thus propose a new layer of regulation for the RbohD/RbohF-dependent oxidative burst that connects signaling pathways triggered by exogenous and endogenous danger signals. Intriguingly, it has been shown that damage (wounding) led to a rapid cell-to-cell spreading of ROS throughout the whole plant, which was dependent on RbohD (Miller et al., 2009). However, they failed to induce this signal just by hydrogen peroxide treatment alone, indicating that additional compounds are needed for this signaling cascade to be initiated. AtPeps are endogenous elicitors that are supposed to be released upon damage or danger. Here, we showed that previous MAMP perception greatly enhances AtPep-triggered ROS production. Thus, we hypothesize that AtPeps might take part in this cell-to-cell signaling process for two reasons. First, microbes try to prevent the production of ROS by injecting effectors to block respective signaling pathways (Göhre et al., 2008; Gimenez-Ibanez et al., 2009). A release of AtPeps would thus make the spread of the ROS wave more robust. Second, the characteristics of the triggered oxidative burst might encode crucial information (Mittler et al., 2011). Thus, AtPeps might modulate the transduced information to distinguish between situations of potential danger and situations of actual danger. More research is needed to analyze this intriguing connection.
Figure 8.
Simplified model on the effect of JA and flg22 on AtPep-triggered responses. A, In untreated wild-type plants, AtPeps elicit a moderate defense response compared with MAMP-triggered responses (Krol et al., 2010; Ranf et al., 2011). A so-far-unknown PEPR-specific pathway leading to RbohD/RbohF activation is only weakly expressed; therefore, AtPeps trigger only low amounts of apoplastic ROS. B, JA positively regulates PEPR abundance. Increased levels of PEPRs trigger stronger responses upon AtPep perception. C, Previous perception of flg22 specifically triggers expression of the PEPR-specific ROS pathway. Thus, subsequent treatments with AtPeps lead to highly induced ROS production, but other AtPep-triggered responses remain unchanged.
MATERIALS AND METHODS
Plant Material
The Arabidopsis (Arabidopsis thaliana) plants used in this study were grown as one plant per pot at 21°C and an 8-h photoperiod for 4 to 5 weeks. All mutants used in this study are in the Columbia (Col-0) ecotype, except for opr3, which is in the Wassilewskija background. The transfer DNA insertion lines SALK_059281 (pepr1) and SALK_098161 (pepr2) were supplied by the Nottingham Arabidopsis Stock Centre. The mpk3-1 (SALK_151594) and mpk6-2 (SALK_073907) mutants were kindly provided by Roman Ulm (University of Geneva), sid2 was kindly provided by Jean-Pierre Métraux (University of Fribourg), the rbohD rbohF double mutant by Miguel Angel Torres (University of Madrid), the aos and coi1-1 mutants by Edward Farmer (University of Lausanne), and the opr3 mutant by Jürgen Zeier (University of Düsseldorf).
The Arabidopsis cell culture was maintained and used for experiments 4 to 8 d after subculture as described (Felix et al., 1999).
Generation of Transgenic Arabidopsis Lines
The PEPR1 and PEPR2 coding regions in pDONR/Zeo were obtained from the Arabidopsis Biological Resource Center based on the work of Gou et al. (2010). Gateway-based cloning was then used to insert PEPR1 and PEPR2 into the binary destination vector pEarley101 (Earley et al., 2006). Arabidopsis plants were transformed by Agrobacterium tumefaciens using the floral dip method (Clough and Bent, 1998).
Peptides
Peptides of flg22 (QRLSTGSRINSAKDDAAGLQIA), AtPep1 (ATKVKAKQRGKEKVSSGRPGQHN), AtPep2 (DNKAKSKKRDKEKPSSGRPGQTNSVPNAAIQVYKED), AtPep3 (EIKARGKNKTKPTPSSGKGGKHN), AtPep4 (GLPGKKNVLKKSRESSGKPGGTNKKPF), AtPep5 (SLNVMRKGIRKQPVSSGKRGGVNDYDM), AtPep6 (ITAVLRRRPRPPPYSSGRPGQNN), and elf18 (Ac-SKEKFERTKPHVNVGTIG) obtained from EZBiolabs were dissolved in a solution containing 1 mg mL–1 bovine serum albumin and 0.1 m NaCl.
Hormone Treatments
Analogously to the peptide treatments, MeSA and MeJA (Sigma-Aldrich) were diluted in dimethyl sulfoxide (DMSO; Sigma-Aldrich) to 10 mm. This stock solution was then diluted in water or the respective assay solutions to final concentrations between 1 and 100 µm. Additional DMSO was added to maintain equal amounts of DMSO in each dilution. As a negative control, similar amounts of DMSO were used.
Analysis of Plant Hormone Levels
Several leaf discs (90 mg fresh weight) were cut and floated for 16 h in darkness on 1 mL water with 1 µm flg22 or without any peptide (control). Leaf tissue samples were flash frozen in liquid nitrogen and stored at –80°C until hormone level quantification. Hormone extraction and analysis was then performed as described in Glauser et al. (2013).
Measurement of ROS Generation
ROS released by leaf tissue was assayed by hydrogen peroxide-dependent luminescence of luminol. Leaf discs of 5-mm diameter were cut and floated overnight in darkness in Lia White 96-well plates (Greiner Bio-One) on 0.1 mL water with or without elicitor peptides or hormones. For elicitation and ROS detection, horseradish peroxidase and luminol (Sigma-Aldrich) was added to a final concentration of 10 µg mL–1 and 100 µm, respectively. Luminescence was measured directly after addition of elicitor peptides or hormones in a MicroLumat LB96P plate reader (Berthold Technologies) for 30 min.
Alkalinization Assay
For medium alkalinization, aliquots of cell suspensions were assessed 5 d after subculturing. Pretreatment with flg22 or a negative control was performed 16 h before a second elicitor treatment, and the pH was measured before and 20 min after the second treatment using glass pH electrodes.
Cytoplasmic Calcium Measurements
Leaf discs of Arabidopsis Col-0 expressing apoaequorin were placed into 96-well microplates containing a solution supplemented with 5 µm coelenterazine (Synchem) and either 1 µm flg22 or without any peptide. The leaf discs were left under complete darkness for 16 h for reconstitution. Data were acquired using a MicroLumat LB96P 96-well microplate luminometer (Berthold Technologies). The substances were supplied to the wells via a computer-controlled dispensing system. Each experiment ended up with a discharge by adding 1 m calcium chloride in 10% (v/v) ethanol. The relative luminescence was determined from the ratio of the actual luminescence per second and the total luminescence that was emitted from the probe.
Measurement of Ethylene Production
For measurement of ethylene accumulation, leaf material was cut into strips of 10 mm2 in the evening. Three leaf strips were placed together in a 6-mL glass vial containing 0.2 mL of distilled, deionized water with or without elicitor peptides or hormones. Vials with leaf strips were incubated over night in the dark. After 16 h, elicitor peptide was added to the desired final concentration, and vials were closed with rubber septa. After 4 h of incubation on a shaker at room temperature, ethylene accumulating in the free air space was measured by a GC-14A gas chromatograph (Shimadzu).
MAPK Phosphorylation
Leaf discs were cut in the evening and left overnight (16 h), floating on distilled, deionized water supplied with or without elicitor peptide. In the morning, the elicitor peptide of the secondary treatment was added. Leaf tissue (40 mg per sample) was shock frozen and ground to fine powder before addition of 80 µL extraction buffer (0.35 m Tris-HCl pH 6.8; 30% [v/v] glycerol; 10% [v/v] SDS; 0.6 m dithiothreitol; and 0.012% [w/v] bromphenol blue). Total cellular proteins (10 µg) were separated by electrophoresis in 12% SDS-polyacrylamide gel and electrophoretically transferred to a polyvinylidene fluoride membrane according to the manufacturer’s instructions (Bio-Rad). We used polyclonal primary antibodies against phospho-p44/42 MAPK (Cell Signaling Technologies) and actin (Sigma-Aldrich), with alkaline phosphatase-conjugated anti-rabbit and anti-mouse immunoglobulins (Sigma-Aldrich) as secondary antibodies, as required. Signal detection was performed using CDPstar (Roche).
Quantitative Reverse Transcription-PCR
Arabidopsis total RNA was extracted using the NucleoSpin RNA plant extraction kit (Macherey-Nagel) and treated with recombinant DNase according to the manufacturer’s specifications. Per PCR reaction, complementary DNA was synthesized from 10 ng of RNA with oligo(dT) primers using the Avian Myeloblastosis Virus reverse transcriptase according to the manufacturer’s instructions (Promega). Quantitative reverse transcription-PCR was performed in a 96-well format using a GeneAmp 7500 Sequence Detection System (Applied Biosystems). On the basis of the obtained threshold cycle values, normalized expression to the reference gene UBIQUITIN10 (UBQ10, AT4G05320) was calculated using the qGene protocol (Muller et al., 2002). The gene-specific primers used were as follows: UBQ10 (AT4G05320) with UBQ_fw (5′-GGCCTTGTATAATCCCTGATGAATAAG) and UBQ_rv (5′-AAAGAGATAACAGGAACGGAAACATAG), FRK1 (AT2G19190) with FRK1_fw (5′-TGCAGCGCAAGGACTAGAG) and FRK1_rv (5′-ATCTTCGCTTGGAGCTTCTC), PHI1 (AT1G35140) with PHI1_fw (5′-TTGGTTTAGACGGGATGGTG) and PHI1_rv (5′-ACTCCAGTACAAGCCGATCC), WRKY53 (AT4G23810) with WRKY53_fw (5′-TCACTTTTTCTGACCACTTTGG) and WRKY53_rv (5′-AAGGAAGAGATATGTTAAGTTGGG), ATTI1 (AT2G43510) with ATTI1_fw (5′-GTTGTCTTTTTCATCTTCTTCTTAGTC) and ATTI1_rv (5′-GCACAAAAGCCGAAACCAACATC), ZAT12 (AT5G59820) with Zat12_fw (5′-TGACTACGTTGAAGAAATCTAGCAG) and Zat12_rv (5′-GTTCTTCCAAGCTCCAACTTGAG), AT1G57630 with 57630_fw (5′-GGAAGGCCTTCAAAGAAACTTGTC) and 57630_rv (5′-GAACACGAACCAGTTGCTTGAATG), PEPR1 (AT1G73080) with PEPR1_qRT_fw (5′-GATTCCTATTGAGATATGGAAGAG) and PEPR1_qRT_rv (5′-CCTCTTCTAAGCTGCTGTTCAC), and PEPR2 (AT1G17750) with PEPR2_qRT_fw (5′-ACCAATAATTCACCGCGACATC) and PEPR2_qRT_rv (5′-CGCATTTTCTGGTGCAATGTAC).
Growth Inhibition Assays
Surface-sterilized seeds were sown on plates containing a Murashige and Skoog salts medium (Duchefa), 1% (w/v) Suc, and 0.8% (w/v) agar. Five days after germination and growth under continuous light, seedlings were transferred to a liquid Murashige and Skoog medium supplied with the elicitors indicated (two seedlings per 500 µL of medium in 24-well plates). The effect of treatment with different peptides on seedling growth was analyzed after 10 d by weighing fresh weight.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Elevated AtPep1-triggered ROS production after pretreatment with elf18.
Supplemental Figure S2. Expression of PEPR1 and PEPR2 in the generated transgenic lines.
Supplemental Figure S3. Increased JA levels in flg22-pretreated samples do not change upon AtPep1 treatment.
Supplemental Figure S4. The opr3 mutant shows reduced responsiveness to AtPep1 treatment.
Supplemental Figure S5. Treatment with AtPep1 triggers a slight increase of JA and JA-Ile levels.
Acknowledgments
We thank Jean-Pierre Métraux, Edward Farmer, and Jürgen Zeier for providing mutant seeds and Georg Felix and Thomas Nühse for helpful comments. In addition, we especially thank Gaetan Glauser and Armelle Vallat for performing the plant hormone measurements.
Glossary
- MAMP
microbe-associated molecular pattern
- DAMP
damage-associated molecular pattern
- ROS
reactive oxygen species
- PTI
pattern-triggered immunity
- MeJA
methyl jasmonate
- MeSA
methyl salicylate
- MAPK
mitogen-activated protein kinase
- CDPK
calcium-dependent protein kinase
- JA
jasmonate
- Col-0
Columbia
- DMSO
dimethyl sulfoxide
References
- Beckers GJM, Jaskiewicz M, Liu YD, Underwood WR, He SY, Zhang SQ, Conrath U. (2009) Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 21: 944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F. (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53: 1367–1376 [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan LB, He P, Bush J, Cheng SH, Sheen J. (2010) Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conrath U, Beckers GJM, Flors V, García-Agustín P, Jakab G, Mauch F, Newman MA, Pieterse CMJ, Poinssot B, Pozo MJ, et al. (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19: 1062–1071 [DOI] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol Plant 1: 423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil-Maurizi C, Trouvelot S, Frettinger P, Pugin A, Wendehenne D, Poinssot B. (2010) β-aminobutyric acid primes an NADPH oxidase-dependent reactive oxygen species production during grapevine-triggered immunity. Mol Plant Microbe Interact 23: 1012–1021 [DOI] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS. (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F. (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141: 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti R, Ferrari S, De Lorenzo G. (2011) Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide- or flagellin-induced resistance against Botrytis cinerea. Plant Physiol 157: 804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Hann DR, Ntoukakis V, Petutschnig E, Lipka V, Rathjen JP. (2009) AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol 19: 423–429 [DOI] [PubMed] [Google Scholar]
- Glauser G, Dubugnon L, Mousavi SAR, Rudaz S, Wolfender JL, Farmer EE. (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284: 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Vallat A, Balmer D. (2013) Hormone profiling. In Sanchez-Serrano JJ, Salinas J, eds, Arabidopsis Protocols, Ed 3 Humana Press, New York [Google Scholar]
- Göhre V, Spallek T, Häweker H, Mersmann S, Mentzel T, Boller T, de Torres M, Mansfield JW, Robatzek S. (2008) Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol 18: 1824–1832 [DOI] [PubMed] [Google Scholar]
- Gou X, He K, Yang H, Yuan T, Lin H, Clouse SD, Li J. (2010) Genome-wide cloning and sequence analysis of leucine-rich repeat receptor-like protein kinase genes in Arabidopsis thaliana. BMC Genomics 11: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A, Pearce G, Ryan CA. (2006) An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA 103: 10098–10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A, Ryan CA. (2007) Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proc Natl Acad Sci USA 104: 10732–10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskiewicz M, Conrath U, Peterhänsel C. (2011) Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep 12: 50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, Postel S, Arents M, Jeworutzki E, Al-Rasheid KAS, et al. (2010) Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem 285: 13471–13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: 1–10 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. (2011) ROS signaling: the new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. (2002) Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32: 1372–1379 [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AME, Peck SC. (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, et al. (2008) Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 283: 8885–8892 [DOI] [PubMed] [Google Scholar]
- Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. (2011) Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J 68: 100–113 [DOI] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C. (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. (2010) Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 285: 9444–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP. (2011) Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol 156: 687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA. (2010) ROS in biotic interactions. Physiol Plant 138: 414–429 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. (2010) PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 22: 508–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Pearce G, Ryan CA. (2006) The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc Natl Acad Sci USA 103: 10104–10109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda H, Fujimura K, Takahashi H, Munemura I, Uchimiya H, Sano H. (2009) Polyamines as a common source of hydrogen peroxide in host- and nonhost hypersensitive response during pathogen infection. Plant Mol Biol 70: 103–112 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]