Abstract
Background
Whereas street vended foods are readily available sources of meals for many people across the world, the microbial safety of such food is always uncertain. In developing countries the major sources of food-borne illnesses are street vended foods. The aim of this study was thus to assess the prevalence and antibiogram of bacteria from white lupin in Bahir Dar Town.
Methods
A total of 40 samples were processed for detection of indicator bacteria and pathogens from December, 2011 to February, 2012 using standard bacteriological techniques. Antimicrobial susceptibility test was performed using the Kirby-Bauer disk diffusion method.
Results
The total coliform counts were 954.2±385 and 756.2±447.3 at the surface and the core of white lupin, respectively. On the other hand, the fecal coliform counts were 880.9±396.6 and 662.1±461.9 at surface and the core, respectively. There was a statistically significant difference in total colifoms and fecal coliform counts between the surface and core of white lupin (p <0.05). Escherichia coli 29 (72.5%), Salmonella spp. 23 (57.5%) and Shigella spp. 8 (20%) were the pathogens isolated. Most bacterial isolates were resistant to tetracycline, cotriamoxazole and erythromycin whereas many of them were sensitive to chloramphenicol, nalidixic acid, gentamicin and ciprofloxacin. The overall multiple antimicrobial resistances rate was 75%.
Conclusion
This study revealed contamination of white lupin and a potential health to consumers, and the bacteria isolated showed high rates of multiple drug resistance. Surveillance of antimicrobial resistance should be done on food borne pathogens. In addition, further studies should be conducted on the bacteriological quality of waters used for soaking white lupin.
Keywords: Coliforms, antimicrobial resistance, pathogens, white lupin
Introduction
Street foods are defined as ready to eat foods and beverages prepared and/or sold by vendors especially on streets and other public places for immediate consumption (1). These foods are well appreciated by consumers, mostly by urban workers because of their taste, low cost, nutrient value and ready availability for immediate consumption.
Street foods displayed on open work area can easily be contaminated by dust, exhaust smoke, insects, and hands of the buyers (2). In most cases, tap water is not available for washing hands and utensils at vending sites; hand and utensil washing are usually done in one or more buckets, and sometimes without soap. Toilets, waste disposal and refrigeration facilities are rarely available. Wastewater and garbage are therefore discarded nearby, providing nutrients for insects and other household rodents, which may carry food borne pathogens (3).
Contamination from raw materials and equipment, additional processing conditions, improper handling and unhygienic conditions contribute substantially to the entry of bacterial pathogens (4). Lack of awareness about food safety and hygiene among vendors also results in food contamination (5). The vendors can be carriers of pathogens like E. coli, Salmonella, Shigella, Campylobacter and S. aureus, who eventually transfer these food-borne pathogens to consumers.
Street food vendors are often poor, less aware of food safety and untrained on food preparation, handling and storage. As a result, ready-to-eat street foods are exposed to contamination by a variety of microorganisms. Moreover, antibiotic resistant bacteria have been isolated from different street vended foods (6, 7, 8). Consequently, street foods are perceived to be a major public health risk (1).
In Ethiopia, health risks associated with street foods are common. Salmonella, Shigella and other food-borne pathogens were isolated from different street foods (9, 10, and 11). Studies indicated that foods provided to the consumers were contaminated by pathogens and the sanitation conditions of the vending area were poor (12, 13). White lupin is considered as an economical and nutritious food for a very rapidly increasing world population. Lupin food products have a hypocholesterolemic effect potentially leading to reduced cardiovascular risk (14, 15). Lupinus albus flour is added to nutritive value and also provides functional properties in bakery and pastry products, protein concentrates and other industrial products, as well as the elaboration of lactose free milk and yoghurt analogues (16). Lupinus albus seeds are also sometimes used as a complete or partial substitute for soybeans in the production of milk, milk powder, and tofu (17). It is therefore widely consumed by people at home and in the groceries. However, there is no information on microbiological quality and safety of white lupin (Lupinus albus) which is the most common street food in Bahir Dar Town. The aim of this study was thus to assess the bacteriological safety of white lupin and determine the antimicrobial susceptibility of pathogens in Bahir Dar Town, Ethiopia.
Materials and Methods
Study design and location: A cross-sectional study was conducted to assess the bacteriological safety of ready-to-eat white lupin in Bahir Dar Town from December 2011 to February 2012. Bahir Dar Town is located at 11°38'N, 37° 10'E on the southern side of Lake Tana. The town has a total population of 256,999 (18). Street vended foods such as sambusa, fried fish are common around the main roads, bus station, groceries, taxi ranks.
Sampling: A total of 40 ready-to-eat white lupin samples were analyzed. Two hundred grams of white lupin was collected using sterile glass containers and immediately transported to the post graduate microbiology laboratory of Bahir Dar University. Samples were stored in refrigerator until microbiological analysis was carried out. Bacteriological analysis was done within a maximum of four hours of collection. The sample was divided into two. One set was used for bacteriological analysis on the surface and the other half was used for bacteriological analysis in the core after removal of the seed coat because the seed coat of white lupin is removed with the teeth and the core part is consumed. Therefore, microbiological analysis was done on the surface and core components. For microbiological analysis of the core part, the seed coat was aseptically removed and the core of the seed was crushed with sterile mortar and piston. Twenty-five gms of the core was mixed with 225 ml of sterilize peptone water in a flask. Another twenty-five grams of white lupin was mixed with 225 ml of sterilized peptone water in a flask for the microbial analysis of the surface. The samples prepared were used for enumeration of total and fecal coliforms on the surface and the core. For isolation of Salmonella and Shigella, the flasks were incubated at 37°C for 24 hours in aerobic atmosphere for primary enrichment.
Enumeration of total and fecal coliforms: The total coliform counts on the surface and the core were determined with the MPN method. Lauryl tryptose broth (Oxoid, England) and brilliant green lactose bile broth (Oxoid, England) were used for the presumptive and confirmatory tests, respectively. The tubes were incubated at 35°C for a maximum of 48 hrs and observed for gas production. The number of positive tubes and negative tubes on brilliant green lactose bile broth tubes were used for calculation of the most probable number (MPN/g) using the MPN tables as provided in the standardized procedure (19). For enumeration of fecal coliforms, a loop full of culture from all presumptive positive lauryl tryptose broth tubes was aseptically transferred into tubes of E. coli (EC) broth tubes. Following incubation at 45°C for 48 hrs, EC tubes were observed for gas accumulation in the Durham tubes. MPN for fecal coliforms was obtained using the MPN table (19).
Isolation of E. coli: From positive EC broth tubes, loop full of culture was streaked on Eosin Methylene Blue agar (Oxoid, England) and incubated at 35°C for 24–48 hrs. Escherichia coli colonies were differentiated by their characteristic green metallic sheen. Presumptive E. coli colonies were sub-cultured, purified and preserved on nutrient agar slants for biochemical characterization (19).
Isolation of Salmonella and Shigella: Twenty-five grams of white lupin samples were enriched on Selenite broth (Oxoid, England) prior to inoculation on to salmonella-shigella agar (Oxoid, England) plates. The plates were incubated at 370C under aerobic atmosphere and examined after 24hrs. Typical colonies of Salmonella as (color less colonies with or without black centers) and Shigella colonies (colorless colonies 1 to 2 mm in diameter) were picked and further characterized through a series of biochemical tests (19).
Antimicrobial susceptibility testing: Antimicrobial susceptibility tests of Salmonella spp., Shigella spp. and E. coli were performed on Mueller-Hinton agar (Oxoid, England) following the disc diffusion technique. The antimicrobials tested include: cefoxitin (30 µg), tetracycline (30 µg), cotrimoxazole (25 µg), gentamicin (10 µg), chloramphenicol (30 µg), nalidixic acid (30 µg), erythromycin (15 µg) and ciprofloxacin (5 µg) (Oxoid, England) (20). Morphologically identical 4–6 bacterial colonies from overnight culture were suspended in 5ml nutrient broth and incubated for 4 hours at 37°C. Turbidity of the broth culture was equilibrated to match 0.5 McFarland standards. The surface of Mueller Hinton agar (Oxoid, England) plate was evenly inoculated with the cultures using a sterile cotton swab. The antibiotic discs were applied on the surface of the inoculated agar. After 18–24 hours of incubation, the diameter of growth inhibition around the discs was measured and interpreted as sensitive, intermediate or resistant according to Clinical and Laboratory Standards Institute (21). Reference strain of E. coli ATCC 25922 was used as quality control for antimicrobial susceptibility tests.
Data analysis: Data were analyzed using SPSS software (version 16.0). Descriptive statistics, percentile, was used to analyze values and an independent sample t-test was used to compare means of total coliforms and fecal coliforms between the core and surface. Significance was determined at p <0.05 level.
Results
Description of preparation of white lupin: Street vended white lupin undergoes some stages of preparation before consumption. The farmers roast the seeds and soak them in river water for 35 consecutive days. They put and transport the seed in bags and sell them in open market. The vendors purchase white lupin, wash 2–4 times with water, add different additives such as garlic, green pepper, ginger and salt. Then they put the seeds in open plastic containers (Figure 1).
Figure1.
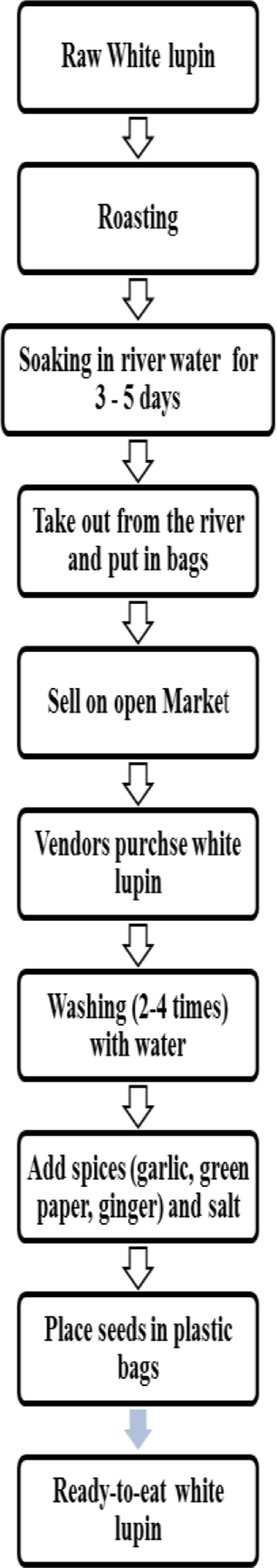
Flow chart for preparation of ready-toeat white lupin (Lupinus albus)
Total and fecal coliform counts of white lupin: The total coliform counts on the surface of the white lupin ranged from 15 to 1100/g with a mean value of 954.24/g. However, the total coliform counts from the core ranged from 11 to 1100 /g with a mean value of 756.16 MPN/g. Out of 40 samples, 37 (92.5%) on the surface and the core were contaminated with fecal coliforms. Fecal coliform counts ranging from 15 to 1100 MPN/g with a mean value of 880.95 MPN/g and 11 to 1100 with a mean value of 662.10 MPN/g were obtained at the surface and core, respectively. However, fecal coliforms were not detected in 3 (7.5%) of samples in both cases (Table 1).
Table 1.
Mean total and fecal coliform counts (MPN/g) on the surface and core of white lupin, Bahir Dar, Ethiopia, 2012.
| Indicators | Mean count and standard deviation on surface of white lupin (MPN/g) |
Mean counts and standard deviation in the core of white lupin (MPN/g) |
| Total coliforms | 954.2 ±358.5 | 756.2 ±447.3 |
| Fecal coliform | 880.9±398.6 | 662.1± 461.9 |
Pathogens isolated from white lupin: The total number of bacteria isolated from the core and the surface of white lupin samples is shown in Table 2. Salmonella, Shigella and E. coli were detected from both the surface and inside of the ready to eat white lupin samples. Twelve (30%) Salmonella, 18 (45%) E. coli and 5 (12.5%) Shigella was isolated from the surface. Eleven (27.5%) Salmonella, 11(27.5%) E. coli and 3 (7.5%) Shigella were isolated from the core.
Table 2.
The frequency and percentage of pathogenic bacteria from core and surface of white lupin, Bahir Dar Ethiopia, 2012.
| Section of white lupin | Frequency of Isolation n (%) | ||
| Salmonella spp. | Shigella spp. | E. coli | |
| Core | 11 (27.5%) | 3 (7.5%) | 11 (27.5%) |
| Surface | 12 (30%) | 5 (12.5%) | 18 (45%) |
| Total | 23 (57.5%) | 8 (20%) | 29 (72.5%) |
Antimicrobial susceptibility of the bacterial isolates: Analysis of species specific resistance rates indicated that all E. coli isolates were resistant to tetracycline. On the other hand, all E. coli isolates were susceptible to chloramphenicol, cotrimoxazole, erythromycin, nalidixic acid and ciprofloxacin. Salmonella spp. showed the highest resistance to erythromycin and erythromycin (86.9 %) while all isolates were sensitive to ciprofloxacin, chloramphenicol and gentamicin. Shigella spp. had resistant rates of 100% to tetracycline and cotrimoxazole whereas all were sensitive to ciprofloxacin and cefoxitin (Table 3).
Table 3.
Antimicrobial resistance patterns of bacteria isolated from street vended white lupin, Bahir Dar Ethiopia, 2012.
| Antimicrobials tested | E.coli (n=29) | Salmonella spp. (n=23) | Shigella spp. (n=8) |
| Tetracycline | 100% | 13% | 100% |
| Gentamicin | 58.6% | 0 | 0 |
| Cefoxitin | 20.7% | 13% | 0 |
| Chloramphenicol | 0 | 0 | 12.5% |
| Ciprofloxacin | 0 | 0 | 37.5% |
| Cotrimoxazole | 0 | 86.9% | 100% |
| Erythromycin | 0 | 86.9% | 62.5% |
| Nalidixic acid | 0 | 21.9% | 12.5% |
The overall multiple antimicrobial resistance rate was 75.2%. The resistances against two or more antimicrobial agents were All Shigella spp. and 86.9% of Salmonella spp. and 58.6% of E. coli showed multiple drug resistance (Table 4). Fifteen, 2, and 3 of Salmonella spp. were resistant to (erythromycin and cotrimoxazole), (erythromycin, nalidixic acid, cotrimoxazole), (cefoxitin, erythromycin, nalidixic acid, cotrimoxazole, tetracycline). In the case of E. coli, 11 were resistant to gentamicin and tetracycline. Of the eight Shigella isolates, three were resistant to (cotrimoxazole and tetracycline), two were resistant to (erythromycin, cotrimoxazole tetracycline), two were resistant to (erythromycin, cotrimoxazole, tetracycline, ciprofloxacin) and one was resistant to chloramphenicol, erythromycin, nalidixic acid, cotrimoxazole, tetracycline and ciprofloxacin.
Table 4.
Multiple antimicrobial resistance of Salmonella spp., Shigella spp. and E. coli in Bahir Dar town, 2012.
| Bacterial isolates | Antibiogram | ||||||
| R0 | R1 | R2 | R3 | R4 | R5 | R6 | |
| E. coli (n=29) | 0 | 12 (41.4%) | 11 (37.9%) | 6 (20.7%) | 0 | 0 | 0 |
|
Salmonella spp (n=23) |
3 (13%) | 0 | 15 (65.2%) | 2 (8.7%) | 0 | 3 (13%) | 0 |
|
Shigella spp (n=8) |
0 | 0 | 3 (37.5%) | 2 (25%) | 2 (25%) | 0 | 1 (12.5%) |
| Total=60 | 3 (5%) | 12 (20%) | 29 (48.3%) | 10 (16.7%) | 2 (3.3%) | 3 (5%) | 1 (1.7%) |
*R0= Sensitive to all tested antimicrobials; R1, R2, R3, R4, R5, R6 -Resistant to one, two, three, four, five, six antimicrobials, respectively.
Discussion
The presence of coliform bacteria in ready-to-eat foods indicates unhygienic conditions during processing, handling and distribution or post processing contamination. The source of coliforms in white lupin could be the river water in which the white lupin is soaked by the farmers for 3 to 7 days to remove the bitter substance inside the seed and also it could be from the water that is used by the street vendors to wash the white lupin before it is ready for the consumers. One major source of contamination of foods sold by street vendors is the water used for washing and processing (22). In Onitsha-Owerri, south east Nigeria, a study on microbial safety of the ready-to-eat food wall nut (Tetracarpidium conophorum) showed high coliform counts. The shell of wall nut is normally removed with the teeth possibly resulting in contaminants being easily swallowed. Poor handling of wall nut and the natural microflora could thus have contributed to the high level of contamination (23).
There are potential health risks associated with initial contamination of foods by pathogenic bacteria as well as subsequent contamination by vendors during preparation and handling and cross contamination as well (24). The presence of Salmonella, Shigella and E. coli O157:H7 in 25 gms of a sample examined is regarded as potentially hazardous to consumers, and is unacceptable for consumption (25). The vendors can be carriers of pathogens like E. coli, Salmonella, Shigella, Campylobacter and S. aureus and can eventually transfer these food-borne hazards to consumers. From a study done in Onitsha-Owerri, south east Nigeria, Salmonella, Shigella and E. coli, were isolated from ready-toeat foods, indicating poor sanitary control and practice (23).
Salmonella was isolated from the inside and surface of white lupin samples. The presence of Salmonella was also reported in another study from ready-to-eat spaghetti in an open market in Ethiopia (26). Other studies also isolated pathogens including Salmonella spp. on street foods (24, 27). The presence of Salmonella in these foods is attributed to inadequate sanitation and poor personal hygiene.
For pathogens with no animal reservoir such as Shigella spp., human feces provide the primary source of contamination, and infected food handlers have frequently been implicated as the cause of disease outbreaks of food borne diseases. The source of Shigella in the present study may be river water in which white lupin is soaked and washed. Several studies of shigellosis have shown that various water sources contaminated with human feces can contribute to indirect fecal-oral transmission (28). Presence of E. coli could only be attributed to fecal contamination from the hands of food handlers and/or from contaminated working surfaces and utensils (29). In this study, E. coli was isolated in the majority of white lupin samples, which indicates the existence of unhygienic food processing and handling.
The antimicrobial resistance of bacteria isolated from food and other sources, against commonly used antibiotics has increased from time to time (30). Antimicrobial resistance rates of Salmonella spp. and E. coli in this study are higher than the study conducted Nigeria (31). Salmonella spp. was sensitive to ciprofloxacin, gentamicin and cotrimoxazole which is comparable to the findings of different studies (6, 32). Gentamicin and cotrimoxazole were also effective against E. coli. This result is supported by results of other studies (33, 34). The resistance rates of Shigella spp. in this study are higher than the findings of a study conducted in Ethiopia (7) but are comparable to another study (6). Multiple drug resistance of E. coli, Salmonella and Shigella has been reported from various studies and the rates recorded from this study are higher than most of the results reported by other researchers (8, 31).
The present study revealed bacterial contamination in white lupin as indicated by high counts of total and fecal colifoms and isolation of pathogens. This suggests a potential health risk due to consumption of white lupin. Such risks can be minimized by avoiding poor handling and raising the awareness of food handlers. The study also indicated that the pathogens isolated showed high rates of resistance against commonly used antibiotics. Therefore, surveillance of antimicrobial resistance should be done on food-borne pathogens. In addition, further studies should be conducted on the bacteriological quality of waters used for soaking white lupin.
References
- 1.WHO, author. Essential safety requirements for street-vended foods. Geneva: WHO; 1996. [Google Scholar]
- 2.FAO, author. Agriculture food and nutrition for Africa. A resource book for teachers of Agriculture. Rome: FAO; 1997. [Google Scholar]
- 3.Tambekar DH, Jaiswal VJ, Dhanorkar DV, Gulhane PB, Dudhane MN. Microbial quality and safety of street vended fruit juices. A Case Study of Amravati City. Inter J Food Safety. 2009;10:72–76. [Google Scholar]
- 4.Toit LD du, Venter I. Food practices associated with increased risk of bacterial food-borne disease of female students in self-catering residences at the Cape Peninsula University of Technology. J Fam Ecol Consum Sci. 2005;33:73–88. [Google Scholar]
- 5.Canet C, N'diaye C. Foods, Nutrition and Agriculture. Rome: FAO; 1996. Street foods in Africa. [Google Scholar]
- 6.Guchi B, Ashenafi M. Microbial load, prevalence and ntibiograms of Salmonella and Shigella in lettuce and green peppers from Addis Ababa. Ethiop J Health Sci. 2010;20:41–48. doi: 10.4314/ejhs.v20i1.69431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tassew H, Abdissa A, Beyene G, Gebre-Selassie S. Microbial flora and food borne pathogens on minced meat and their susceptibility to antimicrobial agents. Ethiop J Health Sci. 2010;20:137–143. doi: 10.4314/ejhs.v20i3.69442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoeder CM, White DG, Meng J. Retail meat and poultry as a reservoir of antimicrobial-resistant Escherichia coli. Food Microbiol. 2004;21:244–255. [Google Scholar]
- 9.Muleta D, Ashenafi M. Bacteriological profile and holding temperatures of street-vended foods from Addis Ababa. Inter J Environ Health Res. 2001;11:95–105. doi: 10.1080/09603120020019683. [DOI] [PubMed] [Google Scholar]
- 10.Ejeta G, Molla B, Alemayehu D, Muckle A. Salmonella serotype isolated from minced meat beef, mutton and pork in Addis Ababa, Ethiopia. Revue Med Vet. 2004;155:547–551. [Google Scholar]
- 11.Alemayehu D, Molla B, Muckle A. Prevalence and antimicrobial resistance of Salmonella isolated from apparently healthy slaughtered cattle in Ethiopia. Trop Anim Health Prod. 2002;35:309–316. doi: 10.1023/a:1025189204496. [DOI] [PubMed] [Google Scholar]
- 12.Hailesilassie M, Taddele H, Adhana K. Sources of contamination of ‘raw’ and ‘ready-to-eat’ foods and their public health risks in Mekelle City. ISABB Ethiop. J Food and Agri Sci. 2012;2:20–29. [Google Scholar]
- 13.Kibret M, Abera B. The sanitary conditions of food service establishments and food safety knowledge and practices of food handlers in Bahir Dar town. Ethiop J Health Sci. 2012;22:27–35. [PMC free article] [PubMed] [Google Scholar]
- 14.Erbas M, Certel M, Uslu MK. Some chemical properties of white lupin seeds (Lupinus albus L.) J Food Chem. 2005;89:341–345. [Google Scholar]
- 15.Martins JM, Riottot M, de Abreu MC, Viegas-Crespo AM, Lanca MJ, Almeida JA, Freire JB, Bento OP. Cholesterol-lowering effects of dietary blue lupin (Lupinus angustifolius L.) in intact and ileorectal anastomosed pigs. J Lipid Res. 2005;46:1539–1547. doi: 10.1194/jlr.M500129-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez MC, Altares P, Pedrosa MM, Burbano C, Cuadrado C, Goyoaga C, Muzquiz M, Jimenez-Martinez C, Davila-Ortiz G. Alkaloid variation during germination in different Lupin Species. J Food Chem. 2004;90:347–355. [Google Scholar]
- 17.Yeheyis L, Kijora C, Melaku S, Girma A, Peters K J. White lupin (Lupinus albus L.), the neglected multipurpose crop: Its production and utilization in the mixed crop-livestock farming system of Ethiopia. Livestock Res Rural Develop. 2010;22 Article# 74. Retrieved November 1, 2011, from http://www.lrrd.org/lrrd22/4/yehe22074.htm. [Google Scholar]
- 18.Central Statistical Agency (Ethiopia) and ICF International, author. Ethiopia Demographic and health Survey. Ethiopia and Calverton, Maryland, USA: Addis Ababa; 2010. [Google Scholar]
- 19.Collins CH, Lyne PM, Grange JM, Falkinham JO., III . Collins and Lyne's Microbiological Methods. 8th Ed. London: Arnold Publisher; 2004. pp. 168–186. [Google Scholar]
- 20.Bauer AW, Kirby WMM, Sherirs JC, Turck M. Antibiotic susceptibility testing by standard single disk method. Am J Clin Path. 1966;45:433–496. [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute, author. Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Information Supplement. CLSI document M100-S17. Wayne Pennsylvania: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- 22.Khalil K, Lindblom GB, Mazhar K, Kaijser B. Flies and water as reservoirs for bacterial enteropathogens in urban and rural areas in and around Lahore. Pakistan Epidemiol Infect. 1994;113:435–444. doi: 10.1017/s0950268800068448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oranusi US, Braide W. A study of microbial safety of ready-to-eat foods vended on highways: Onitsha-Owerri, south east Nigeria. Inter Res J Microbiol. 2012;3:66–71. [Google Scholar]
- 24.Mosupye FM, Von Holy A. Microbiological hazards identification and exposure assessment of street food vending in Johannesburg, South Africa. Inter J Food Microbiol. 2000;61:137–145. doi: 10.1016/s0168-1605(00)00264-6. [DOI] [PubMed] [Google Scholar]
- 25.Cheung PY, Kwok KK, Kam KM. Application of BAX system, Tecra Unique TM. Salmonella test, and a conventional culture method for the detection of Salmonella in ready-to-eat and raw foods. J Appl Microbiol. 2007;103:219–227. doi: 10.1111/j.1365-2672.2006.03210.x. [DOI] [PubMed] [Google Scholar]
- 26.Muleta D, Mogessie Ashenafi M. Bacteriological profile and Holding T° at street vended foods from Addis Abeba. Inter J Food Sci Human Nut. 1995;11:95–105. doi: 10.1080/09603120020019683. [DOI] [PubMed] [Google Scholar]
- 27.Bryan F, Jermini M, Schmitt R, Chilufya E, Mwanza M, Matoba A, Mfume E, Chibiya H. Hazards associated with holding and reheating foods at vending sites in a small town in Zambia. J Food Protec. 1997;60:391–398. doi: 10.4315/0362-028X-60.4.391. [DOI] [PubMed] [Google Scholar]
- 28.Alamanos Y, Maipa V, Levidiotou S, Gessouli E. A community waterborne outbreak of gastro-enteritis attributed to Shigella sonnei. Epidemiol Infect. 2000;125:499–503. doi: 10.1017/s0950268800004866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheth M, Gupta A, Ambegaonkar T. Handlers' hygiene practices in small restaurants of Vadodara. Nutr Food Sci. 2011;41:386–392. [Google Scholar]
- 30.Vicas M, Sanchaita S, Singh NP. Multidrug Resistant Acinetobacter. J Glob Infect Dis. 2010;2:291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oluyege AO, Dada AC, Ojo AM, Oluwadare E. Antibiotic resistance profile of bacterial isolates from food sold on a University campus in south western Nigeria. Afr J Biotechnol. 2009;8:5883–5887. [Google Scholar]
- 32.Farzana K, Rouf M, Mahmood S. Prevalence and antibiotics susceptibility patterns of some bacterial isolates from a street-vended fruit product. Afr J Microbiol Res. 2011;5:1277–1284. [Google Scholar]
- 33.Busani L, Graziani C, Battisti A, Franco A, Ricci A, Vio D, Digiannatale E, Paterlini F, D'Incau M, Owczarek S, Caprioli A, Luzzi I. Antibiotic resistance in Salmonella enterica serotypes Typhimurium, Enteritidis and Infantis from human infections, food stuffs and farm animals in Italy. Epidemiol Infec. 2004;132:245–251. doi: 10.1017/s0950268803001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centinkaya F, Cibik G, Soyuteniz E, Ozkin C, Kayali R, Levent B. Shigella and Salmonella contamination in various foodstuffs in Turkey. Food Control. 2008;19:1059–1063. [Google Scholar]


