Abstract
Spermatogonial stem cell (SSC) transplantation has been shown to restore fertility in several species and may have application for treating some cases of male infertility (e.g., secondary to gonadotoxic therapy for cancer). To ensure safety of this fertility preservation strategy, methods are needed to isolate and enrich SSCs from human testis cell suspensions and also remove malignant contamination. We used flow cytometry to characterize cell surface antigen expression on human testicular cells and leukemic cells (MOLT-4 and TF-1a). We demonstrated via FACS that EpCAM is expressed by human spermatogonia but not MOLT-4 cells. In contrast, HLA-ABC and CD49e marked >95% of MOLT-4 cells but were not expressed on human spermatogonia. A multiparameter sort of MOLT-4–contaminated human testicular cell suspensions was performed to isolate EpCAM+/HLA-ABC–/CD49e– (putative spermatogonia) and EpCAM–/HLA-ABC+/CD49e+ (putative MOLT-4) cell fractions. The EpCAM+/HLA-ABC–/CD49e– fraction was enriched for spermatogonial colonizing activity and did not form tumors following human-to–nude mouse xenotransplantation. The EpCAM–/HLA-ABC+/CD49e+ fraction produced tumors following xenotransplantation. This approach could be generalized with slight modification to also remove contaminating TF-1a leukemia cells. Thus, FACS provides a method to isolate and enrich human spermatogonia and remove malignant contamination by exploiting differences in cell surface antigen expression.
Introduction
Over 12,000 children are diagnosed with cancer every year in the US, and it has been estimated that a male infant has a 1 in 300 chance of being diagnosed with a malignancy by the age of 20 (1). Fortunately, success rates in treating childhood cancer have increased dramatically over the past few decades, and now approximately 80% of children survive following treatment (2, 3). Given this growing cohort of adult survivors of childhood cancers, emphasis is now being placed on quality of life issues following successful treatment. Many therapies to treat cancer are gonadotoxic and can lead to infertility, and fertility potential has an important impact on quality of life according to cancer survivors (4–7). In fact, the American Society of Clinical Oncology now recommends that the reproductive risks of cancer therapies and fertility preservation options should be routinely discussed with patients before beginning treatment (4, 8).
In men, freezing semen samples is an efficient and well-established technique to preserve fertility for those facing gonadotoxic treatments, such as chemotherapy or radiation. Unfortunately, this is not an option for boys who have not yet entered puberty and do not have sperm. However, these boys do have spermatogonial stem cells (SSCs) in their testes that are poised to produce spermatogenesis at the start of puberty (8–11). SSCs maintain spermatogenesis throughout postpubertal life, and they are defined by their ability to undergo both self-renewing cell divisions and differentiation, leading to the production of haploid sperm. Brinster and colleagues provided the initial demonstration that testicular cells from a fertile mouse could be transplanted into the seminiferous tubules of an infertile recipient, in which they produced complete spermatogenesis and sometimes restored fertility (12–14). Regeneration of spermatogenesis following SSC transplantation has now been established in several animal models, including rodents, goats, sheep, pigs, dogs, and monkeys (13–22).
The potential of using SSCs to preserve and restore fertility in patients receiving gonadotoxic therapies has been extensively discussed (23–32). In theory, testicular cells obtained via biopsy prior to cancer treatment could be cryopreserved and then retransplanted following clinical remission. Several clinics around the world, including our own Fertility Preservation Program in Pittsburgh ( http://www.mwrif.org/220), are now performing testicular biopsies on boys prior to the initiation of cancer therapy in hopes that this tissue can be used in the future to restore fertility (8, 9, 30, 31). However, to make SSC transplantation a realistic clinical option for the prepubertal patient cohort, two major hurdles must be overcome. First, we need to learn the characteristics of human SSCs to facilitate their isolation and enrichment. Second, techniques to remove malignant contamination from the testis cell suspension are needed to eliminate the risk of reintroducing cancer back into survivors.
Unfortunately, there is a real potential for malignant contamination in testicular tissue obtained from patients prior to cancer treatment, especially for those with hematogenous cancers. One study demonstrated that 20% of boys with acute lymphocytic leukemia possessed malignant cells in a testicular biopsy taken prior to the initiation of chemotherapy (33). Furthermore, it has been demonstrated in a rat model of leukemia that transplantation of testicular cells from leukemic donors consistently transmitted leukemia to healthy recipients (34). Fujita and colleagues were the first to demonstrate that FACS could be used to successfully remove malignant cells from a testicular sample prior to SSC transplantation (24). They used antibodies directed against cell surface antigens CD45 and MHC class I (HLA-ABC) on cancer cells to remove the malignant cells from germ cells. Sorted and unsorted cell suspensions were then transplanted into the seminiferous tubules of infertile recipient mice. In this landmark study, recipient mice that received transplants with unsorted cells consistently developed leukemia, whereas those transplanted with sorted cells did not. Additionally, viable offspring were generated from the infertile recipients following transplantation of the sorted germ cells (24). Fujita and colleagues followed up this initial report by demonstrating that 7 out of 8 human leukemic cell lines also expressed the cell surface antigens CD45 and MHC class I, and thus these leukemic markers could theoretically be used to separate leukemic cells from testicular cells in humans as well, but this was not assessed experimentally in that study (25). Hermann and coworkers demonstrated the feasibility of removing contaminating leukemic cells from nonhuman primate testis cell suspensions by FACS sorting with THY-1 (spermatogonial marker) and CD45 (leukemia marker) (35).
However, not all studies using immune-based sorting technologies to separate malignant cells from testicular cell suspensions have been as successful (27, 36). Using a leukemic rat model, Hou and colleagues concluded that a single marker sort is generally not adequate to remove malignant contamination (36). Moreover, studies using human tissue to assess decontamination methods have been very limited to date, likely due to difficulties in obtaining such material for research. However, human studies are needed to demonstrate feasibility and safety before SSC transplantation can be translated to the clinic.
In this study, we characterized the cell surface phenotype of human spermatogonia in testicular tissue obtained from organ donors as well as the MOLT-4 leukemic cell line derived from a patient with acute T cell lymphoblastic leukemia. We used this information to devise sorting strategies to isolate and enrich human SSCs and to remove malignant contamination from human testicular cell suspensions that had been “spiked” with MOLT-4 leukemia cells. A human-to–nude mouse xenotransplantation biological assay was used to assess SSC activity and malignant contamination in fractions obtained from FACS of MOLT-4–contaminated human testis cell suspensions.
Results
Validation of the human-to–nude mouse xenotransplantation as a bioassay of human spermatogonia.
Our laboratory previously established and validated a primate (rhesus)-to–nude mouse xenotransplantation assay for monkey SSCs (37, 38). To enable this assay, we generated a rabbit anti-rhesus testis cell polyclonal antibody that specifically recognizes antigens in rhesus (nonhuman primate) testis cells. This antibody did not exhibit immunoreactivity with untransplanted mouse seminiferous tubules (Figure 1A), but we found that it did recognize colonies of human spermatogonia in mouse seminiferous tubules 2 months after transplantation (Figure 1, C and D). Monkey and human SSCs do not produce complete spermatogenesis in mouse seminiferous tubules (probably due to evolutionary distance between primates and mice). However, the colonization foci are considered to be SSC derived, because (a) they exhibited typical spermatogonial appearance, including arrangement as singles, pairs, and chains on the basement membrane of seminiferous tubules, and expressed the germ cell marker, VASA (Figure 1, E and F). (b) Clusters are not “just survivors of the transplant,” because we transplanted a single cell suspension (confirmed visually on a hemocytometer) that was filtered through a 35-μm strainer. The presence of chains of human germ cells clearly indicates proliferation after engraftment. (c) These colonies are unlikely to arise from differentiating B spermatogonia, because a colonizing human B spermatogonia would produce a spermatocyte at its next division. Therefore, spermatogonial colonies with 4 or more cells must originate from human Adark or Apale spermatogonia, which are considered reserve and active SSCs, respectively (37, 39–41). (d) Finally, human-to–nude mouse colonizing activity in this study always segregated with the distribution of Spalt-like 4–stained (SALL-4–stained) cells (see below). SALL-4 is a zinc-finger transcription factor that is a conserved marker of undifferentiated spermatogonia in rodents, monkeys, and humans (42–45).
Figure 1. Human-to–nude mouse xenotransplantation assay.
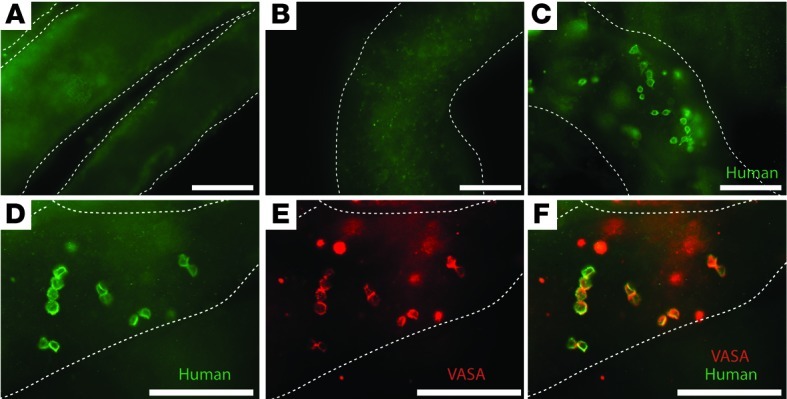
A rabbit anti-primate testis cell polyclonal antibody was previously generated that specifically recognizes antigens on primate (human and nonhuman) testis cells (37). (A) The antibody does not exhibit immunoreactivity with untransplanted mouse seminiferous tubules. (B) An isotype control antibody (rabbit IgG) does not exhibit immunoreactivity with mouse seminiferous tubules transplanted with human testicular cells. (C and D) The primate testis cell antibody cross-reacts with human testis cells and can be used to identify colonies of human spermatogonia in mouse seminiferous tubules 2 months after transplantation. Cells in colonies have a typical spermatogonial appearance, with large nuclear-to-cytoplasmic ratios, and are arranged as singles, pairs, and chains located on the basement membrane of seminiferous tubules. (E and F) The colonizing cells recognized by the primate testis cell antibody also express the germ cell marker VASA. Mouse seminiferous tubules are demarcated by dashed white lines. Scale bar: 100 μm.
Surface antigen expression on human testicular cells and MOLT-4 lymphoblastic leukemia cells.
To characterize cell surface antigens on human testicular cells and MOLT-4 acute lymphoblastic leukemia cells (46), respectively, flow cytometry was performed for a panel of 24 markers, 15 of which exhibited positive immunoreactivity with human testis cells and/or MOLT-4 leukemic cells (Supplemental Figure 1, A and B, and Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI65822DS1). Our aim with this set of experiments was to characterize the cell surface phenotypes of human spermatogonia and human MOLT-4 leukemia cells and also to identify antigens that could be used to distinguish these 2 cell populations. For MOLT-4 markers, we selected antigens that were expressed on greater than 95% of MOLT-4 cells for further study. CD29, CD45, CD49e, CD147, and HLA-ABC met these criteria (Supplemental Figure 1B and Supplemental Table 1), and HLA-ABC, CD49e, and CD147 were selected for use in subsequent experiments. To identify potential spermatogonial markers that could be used to isolate/enrich spermatogonia and were distinct from MOLT-4 cells, our goal was to identify antigens that were expressed by less than 1% of MOLT-4 cells and by 5% or more of human testis cells. CD49f (α6 integrin), CD29 (β1 integrin), CD90 (THY-1), and CD326 (EpCAM) were of particular interest, because these markers have been demonstrated to be expressed on spermatogonia in humans (CD49f, CD90) or in other animal models (25, 36, 47–50). CD49f, CD29, and CD90 were rejected for further consideration, because they had >1% expression on MOLT-4 cells. CD326 (EpCAM) satisfied the criteria (expressed on 0.1% of MOLT-4 cells and 16.4% of human testis cells; see Supplemental Figure 1, A and B, and Supplemental Table 1) and was selected for further study.
Cell sorting for EpCAM to select and enrich undifferentiated spermatogonia from a human testicular cell suspension.
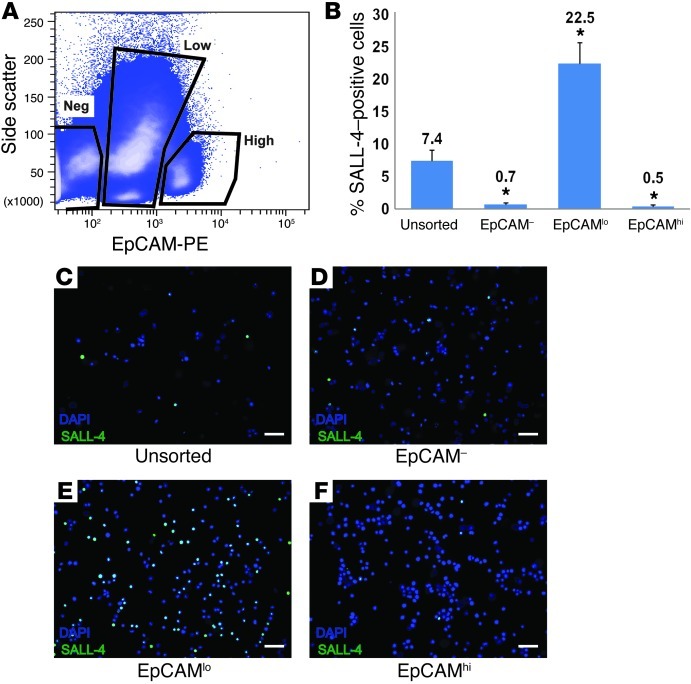
To determine whether EpCAM is expressed on human spermatogonia and could be used as a positive spermatogonial selection marker, human testicular cell suspensions were stained with a PE-conjugated antibody against EpCAM and sorted using FACS. As demonstrated in Figure 2, 3 populations of cells were identified following staining with EpCAM, based on their level of fluorescence and on side scatter of incident light, which provides a measure of intracellular complexity: EpCAM–, EpCAMlo, and EpCAMhi. EpCAM is known to be expressed on SSCs in rats (36, 48). Following sorting, each fraction of cells was fixed and stained with an antibody directed against SALL-4 to quantify undifferentiated human spermatogonia. The majority of SALL-4+ spermatogonia were recovered in the EpCAMlo fraction (Figure 2, B and E). Compared with 7.4% of cells expressing SALL-4 in the unsorted testicular cell population, 22.5% of cells in the EpCAMlo fraction expressed SALL-4 (P < 0.0001). The EpCAM– and EpCAMhi fractions were virtually depleted of SALL-4–expressing cells (P < 0.0001 compared with unsorted).
Figure 2. SALL-4–positive spermatogonia are recovered in the EpCAMlo fraction of human testis cells.
(A) Human testicular cells were stained with EpCAM-PE, and 3 populations were identified based upon EpCAM-PE staining intensity and side scatter of incident light. Negative gates were defined by analysis of human testis cells stained using PE-conjugated isotype control antibodies. (B–F) Following sorting, each fraction of cells was fixed and immunocytochemistry assessing SALL-4 expression was performed. Following SALL-4 staining, cells were counterstained with DAPI. Cells from at least 10 independent images were then counted based on DAPI staining and SALL-4 staining, respectively, to determine the percentage of cells expressing SALL-4. An unsorted fraction of cells was also stained with an isotype antibody to control for nonspecific binding to demonstrate specificity. (B) Relative SALL-4 expression in unsorted and EpCAM-sorted fractions. Bars indicate the mean percentage of SALL-4–positive cells (SALL-4–positive cells/total cells) in each fraction. Error bars represent SEM from 3 replicate sorting experiments. *P < 0.001, compared with unsorted cells. (C–F) Representative images from SALL-4 immunocytochemistry of unsorted and sorted fractions. Scale bar: 50 μm.
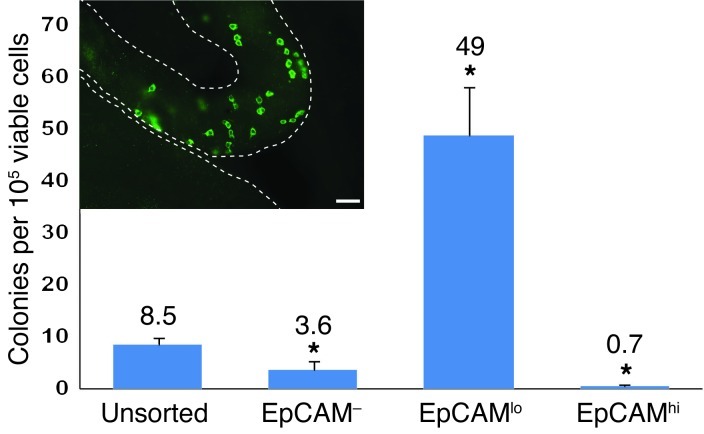
Figure 3. Human-to–nude mouse xenotransplant colonizing activity is enriched in the EpCAMlo fraction of human testis cells.
Unsorted and EpCAM-sorted human testis cell fractions (see Figure 2) were transplanted into the testes of immune-deficient nude mice. Two months after transplant, colonies of human spermatogonia were identified in mouse recipient testes using the rabbit anti-primate testis antibody and Alexa Fluor 488–conjugated secondary antibody (green) (scale bar: 50 μm) (inset). Mouse seminiferous tubules are demarcated by dashed white lines. Colonies in each recipient testis were counted and normalized to 105 viable cells transplanted per testis. *P < 0.001, compared with unsorted cells. Bars indicate the mean number of colonies per 106 viable cells in each fraction. Error bars represent SEM from 3 replicate sorting experiments.
The human-to–nude mouse xenotransplantation assay was used to quantify SSC activity in unsorted, EpCAM–, EpCAMlo, and EpCAMhi fractions. Each fraction was injected via the efferent ducts into the testes (0.8 × 105 to 3.4 × 105 cells per testis) of busulfan-treated, immune-deficient nude mice. Colonies of human spermatogonia were identified in recipient mouse seminiferous tubules 2 months after transplantation using a rabbit anti-primate testis cell antibody, as described in Figure 1. Colonies contain 4 or more cells (≤100 μm between cells) with typical spermatogonial appearance, often connected by intracytoplasmic bridges, located on the basement membrane of mouse seminiferous tubules. Unsorted human testicular cells produced 8.5 colonies of spermatogonia per 105 viable transplanted cells (Figure 3). The EpCAMlo fraction produced 49 colonies of spermatogonia per 105 viable transplanted cells, representing an approximate 6-fold enrichment compared with the unsorted population (P < 0.0001). Mirroring the SALL-4 data, colony numbers were significantly reduced in the EpCAM– and EpCAMhi fractions (P < 0.01 compared with unsorted controls). Thus, we conclude, based on SALL-4 immunocytochemistry (Figure 2) and the xenotransplantation results (Figure 3), that SSC activity resides in the EpCAMlo fraction of human testis cells.
SALL-4+ spermatogonia do not express HLA-ABC or CD49e.
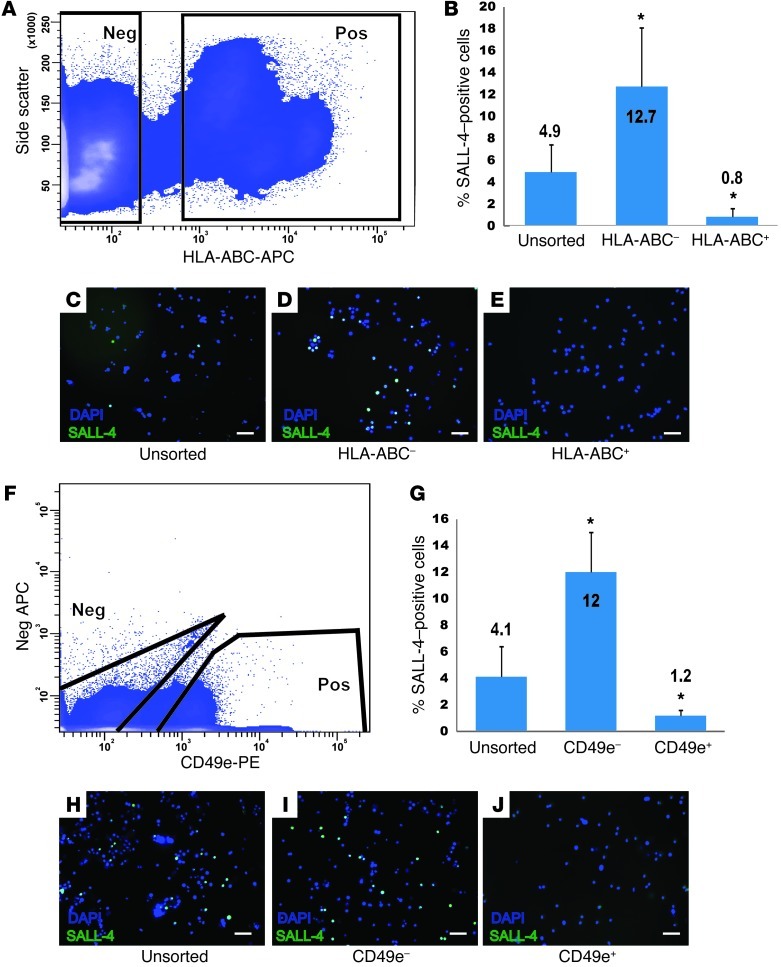
FACS experiments were performed to determine whether putative MOLT-4 markers CD49e, HLA-ABC, and CD147 (expressed by >95% of MOLT-4 cells) were also expressed by SALL-4+ spermatogonia in human testis cell suspensions. The goal of these experiments was to identify markers that can distinguish MOLT-4 leukemia cells from human spermatogonia. FACS analysis of human testis cells for HLA-ABC (Figure 4A), followed by immunocytochemistry of positive and negative fractions for SALL-4 (Figure 4, B–E), revealed that the majority of SALL-4+ human spermatogonia were recovered in the HLA-ABC–negative fraction (P < 0.0001). Similarly, the majority of SALL-4+ spermatogonia were recovered in the CD49e-negative fraction of human testis cells (P < 0.0001; Figure 4, F–J). SALL-4+ spermatogonia were found in both the CD147-positive and -negative fractions (data not shown), and, thus, this marker was not deemed useful for separating MOLT-4 cells from spermatogonia.
Figure 4. SALL-4–positive human spermatogonia do not express HLA-ABC or CD49e.
(A) To determine whether human spermatogonia express HLA-ABC, human testicular cell suspensions were stained with APC-conjugated HLA-ABC antibodies and sorted into positive and negative fractions by FACS. Negative gates were defined by analysis of human testis cells using APC-conjugated isotype control antibodies. (B–E) Following FACS, each fraction of cells was fixed and immunocytochemistry was performed to assess SALL-4 expression; then, fractions were counterstained with DAPI to quantify total cells. (B) The percentage of cells in each unsorted and sorted fraction that displayed SALL-4 staining (SALL-4+ green cells/DAPI-stained total cells). (F–J) A similar experiment was conducted using APC-conjugated CD49e antibodies. Scale bar: 50 μm (C–E and H–J). Bars in B and G indicate the mean percentage of SALL-4–positive cells (SALL-4–positive cells/total cells) in each fraction. Error bars in B and G represent SEM from 3 replicate sorting experiments. *P < 0.001, compared with unsorted cells.
The EpCAMlo/CD49e–/HLA-ABC– cell fraction is enriched for SALL-4+ spermatogonia and colonization activity following xenotransplantation.
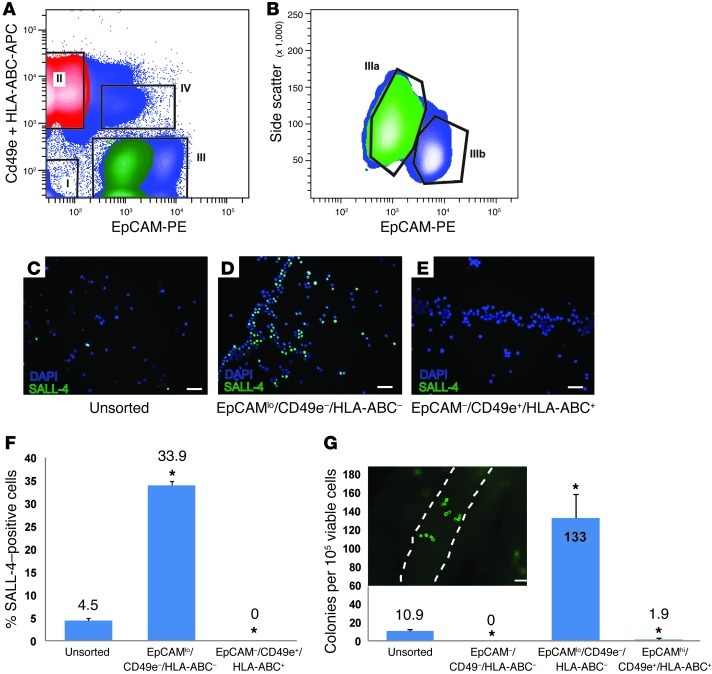
Human testicular cell suspensions were contaminated with 10% MOLT-4 cells in order to simulate a clinical situation in which a patient preserves a testicular biopsy that contains SSCs and might be used in the future to restore fertility (i.e., by SSC transplantation). In order to safely use the preserved tissue for autologous transplantation, the malignant cells must be identified and completely removed. The objectives of this set of experiments were to determine (a) whether spermatogonia could be successfully sorted from a contaminated testicular cell population, (b) whether spermatogonia could be enriched, and (c) whether contaminating malignant cells could successfully be separated from spermatogonia. To achieve these goals, we sorted the contaminated human testis cell suspension into fractions based on relative expression of EpCAM (spermatogonial marker) as well as CD49e and HLA-ABC (MOLT-4 markers). As shown in Figure 5, 4 populations of cells were gated: EpCAM–/CD49e–/HLA-ABC– (fraction I); EpCAM–/CD49e+/HLA-ABC+ (fraction II); EpCAM+/CD49e–/HLA-ABC– (fraction III); and EpCAM+/CD49e+/HLA-ABC+ (fraction IV). The EpCAM+/CD49e–/HLA-ABC– fraction (III) was further fractionated based on level of EpCAM expression and side scatter of incident light (Figure 5B, fractions IIIa and IIIb) into EpCAMlo/SSchi (Figure 5A, fraction III, green) and EpCAMhi/SSclo (Figure 5A, fraction III, blue). Based on data in Figures 2–4 and Supplemental Table 1, we hypothesized that human spermatogonia would be recovered in fraction IIIa (Figure 5, A and B, green) and that MOLT-4 cells would be recovered in fraction II (Figure 5A, red).
Figure 5. The EpCAMlo /CD49e–/HLA-ABC– fraction of MOLT-4–spiked human testis cell suspension is enriched for human spermatogonia.
(A) Human testicular cell suspensions were spiked with 10% MOLT-4 cells and then FACS sorted using EpCAM-PE, HLA-ABC-APC and CD49e-APC antibodies. (B) Fraction III in A was further analyzed with side scatter, as described in Figure 2, to identify the SSC fraction, Ep-CAMlo/side scatterhi (green, Fraction IIIa). Thus, only cells that (A) primarily fell within fraction III and (B) secondarily fell within fraction IIIa were collected. (C–F) Immunocytochemistry was performed to assess relative SALL-4 expression in unsorted and sorted fractions. We focused specifically on fractions II and IIIa (green), because this is where we expected to find MOLT-4 leukemia cells and human spermatogonia, respectively, based on data in Figures 2–4. Scale bar: 50 μm (C–E). Bars in F indicate the mean percentage of SALL-4–positive cells (SALL-4–positive cells/total cells) in each fraction. Error bars in F represent SEM from 6 replicate sorting experiments. (G) The human-to–nude mouse xenotransplantation assay was used to assess spermatogonial colonizing activity in unsorted (unspiked) and sorted (spiked) testis cell fractions (I, IIIa, and IV), as described in Figure 3. Bars indicate the mean number of colonies per 106 viable cells in each fraction. Error bars represent SEM from 6 replicate sorting experiments. *P < 0.001, compared with unsorted cells. A typical colony of human spermatogonia in recipient mouse seminiferous tubules is shown in the inset. Scale bar: 50 μm.
As expected, immunocytochemical staining of sorted fractions revealed significant enrichment of SALL-4+ cells in the EpCAMlo/SSchi/CD49e–/HLA-ABC– fraction (IIIa) compared with unsorted testicular cells (33.9% vs. 4.5% SALL-4+ cells in the unsorted population, P = 0.0005; Figure 5, C–F). This fraction will be described as EpCAMlo/CD49e–/HLA-ABC– from this point forward and in Figures 5 and 6. No SALL-4+ cells were found in the EpCAM–/CD49e+/HLA-ABC+ fraction (II). Furthermore, the xenotransplantation analysis of spermatogonial colonies in the seminiferous tubules of nude mice confirmed that colonization activity was enriched in the EpCAMlo/CD49e–/HLA-ABC– fraction compared with unsorted (unspiked) testicular cells (133 colonies per 105 viable transplanted cells vs. 10.9 colonies per 105 viable transplanted cells in the unsorted control, P < 0.0001; Figure 5G). This represents approximately 12-fold enrichment of spermatogonial colonizing activity in the human-to–nude mouse xenotransplant assay.
Figure 6. EpCAM–/CD49e+/HLA-ABC+ cells form testicular tumors following transplantation into nude mice, but EpCAMlo /CD49e–/HLA-ABC– cells do not form tumors.
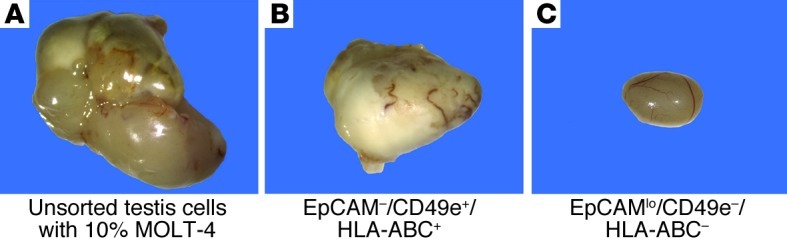
(A and B) Unsorted spiked testicular cells and cells from fraction II (see Figure 5A) produced tumors in recipient mouse testes. (C) Cells from fraction IIIa (see Figure 5, A and B) that contained human spermatogonia colonizing the seminiferous tubule of nude mice (see Figure 5G) did not produce tumors.
EpCAM–/CD49e+/HLA-ABC+ cells form testicular tumors following transplantation into the testes of nude mice, but EpCAMlo /CD49e–/HLA-ABC– do not.
To determine whether MOLT-4 cells had been successfully removed from the sorted population of spermatogonia, tumor formation was assessed following transplantation of the sorted fractions into the testes of nude mice. In a prior sensitivity analysis, it was demonstrated that transplantation of as few as 10 MOLT-4 cells into the testes of nude mice could induce tumor formation (35).
Following sorting of the spiked testicular cell population, the EpCAMlo/CD49e–/HLA-ABC–– (putative SSCs, fraction IIIa) and EpCAM–/CD49e+/HLA-ABC+–sorted (putative MOLT-4 cells, fraction II) fractions (Figure 5, A and B) were transplanted into the seminiferous tubules of nude mice. Uncontaminated testicular cells, a pure population of MOLT-4 cells, and unsorted spiked cells were transplanted in the same manner to serve as negative and positive controls, respectively. When a pure population of MOLT-4 cells was transplanted into the seminiferous tubules, tumor formation was observed 18% of the time (Table 1). The unsorted spiked population of cells produced tumors in 41% of testes transplanted (Figure 6A and Table 1). The EpCAM–/CD49e+/HLA-ABC+ fraction produced tumors in 23% of transplanted testes (Figure 6B and Table 1), whereas tumors were never observed in the EpCAMlo/CD49e–/HLA-ABC– fraction (Figure 6C and Table 1).
Table 1.
Quantitative assessment of tumor formation in recipient mouse testes
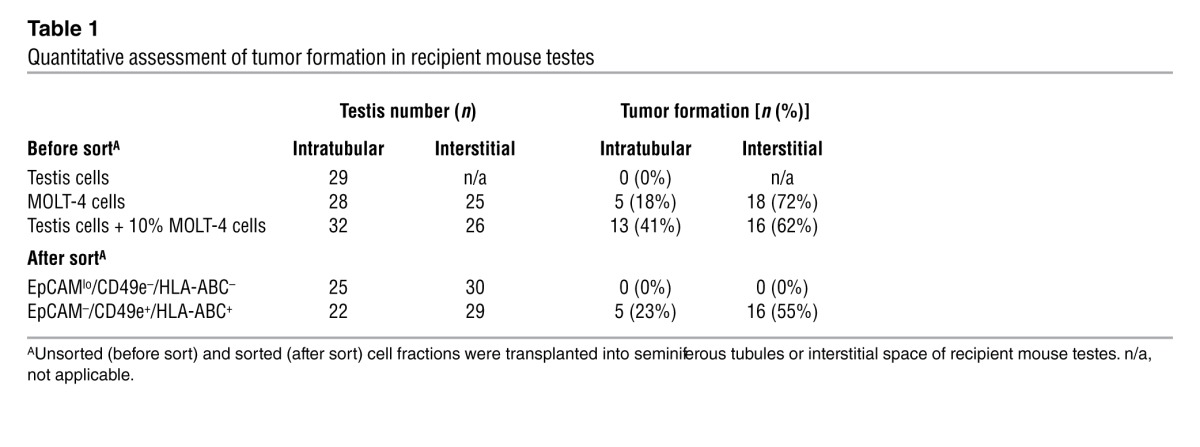
For additional confirmation that MOLT-4 contamination had been successfully removed from the EpCAMlo/CD49e–/HLA-ABC– fraction, interstitial testicular transplants were performed. Earlier work with MOLT-4 testicular transplantation suggested that tumor formation may be more efficient when cells are introduced into the interstitial space, thus increasing the sensitivity of the tumor bioassay. The same cell fractions were transplanted into the interstitial space of the testes in nude mice. Approximately 5,000 MOLT-4 cells were transplanted per testis in the control arms of this cancer cell–spiking experiment (i.e., 5,000 MOLT-4 cells or 50,000 unsorted testis cells spiked with 10% MOLT-4 cells). Unlike in the intratubular transplantation experiments above, tumor analysis was not performed until 16 weeks following transplantation, or sooner if palpable tumors were present, to maximize the sensitivity of the tumor bioassay. With the interstitial transplants, 72% of testes transplanted with pure MOLT-4 cells developed tumors, as did 62% of testes transplanted with an unsorted spiked suspension of cells (Table 1). Following sorting, tumor formation was observed in 55% of testes transplanted with the EpCAM–/CD49e+/HLA-ABC+ (putative MOLT-4) fraction, whereas there was no tumor formation in any of the testes transplanted with the EpCAMlo/CD49e–/HLA-ABC– (putative SSC) fraction. Pathological analyses of the samples (by C.A. Castro) indicated that they are consistent with lymphocytic tumoral growth, with characteristic malignant invasion through the tunica albuginea and into abdominal organs. Furthermore, immunohistochemical analyses of testicular tumors with a human-specific antibody directed against the nuclear mitosis apparatus protein (NuMA) demonstrated that the tumors are of human origin (see Supplemental Figure 2). Thus, a multiparameter sort strategy effectively segregated undifferentiated spermatogonia from MOLT-4 leukemia cells. FACS reanalysis of the EpCAMlo/CD49e–/HLA-ABC– fraction demonstrated a purity range of 98.8%–99.8%.
To demonstrate that the multiparameter sorting strategy could be generalized to other cancer cell lines, we contaminated human testis cell suspensions with TF-1a human leukemia cells (51). TF-1a cells did not efficiently make solid tumors following xenotransplantation to nude mouse testes, so we labeled them with ubiquitin-C-GFP to enable tracking and assess contamination of sorted fractions (Supplemental Methods and Supplemental Figure 3). TF-1a cells did not express HLA-ABC to the same degree as the MOLT-4 cell line, so an alternate epitope, CD45, was used instead. A multiparameter FACS procedure was performed using EpCAM-APC (spermatogonial marker), CD49e-PE (TF-1a marker), and CD45-PE (TF-1a marker) on a human testicular cell suspension spiked with TF-1a cells, as described for the MOLT-4 line above (see Supplemental Methods and Supplemental Figure 3). Purity check indicated that the putative spermatogonial fraction (IIIa) was 99.4% pure (Supplemental Figure 3, C and E). This fraction contained SALL-4+ spermatogonia (Supplemental Figure 3H) but was devoid of GFP+ TF-1a cells (Supplemental Figure 3, F and H).
Discussion
SSCs may have application for preserving and restoring spermatogenesis in men who are rendered infertile due to chemotherapy or radiation treatment for cancer or other conditions. A boy or man could theoretically cryopreserve testicular tissue or cells (containing SSCs) before the gonadotoxic therapy and have these cells reintroduced into his testis after he is cured of his primary disease. For a cancer survivor, this approach has the inherent and unacceptable risk of reintroducing malignant cells. We created this scenario in this study by contaminating human testis cell suspensions with MOLT-4 leukemia cells. We then used a multiparameter sorting approach to prove that it is feasible to isolate and enrich SSCs from a heterogeneous human testis cell suspension and also remove malignant contamination. To enable these studies, we validated two experimental approaches for studying human spermatogonia. First, we optimized SALL-4 immunocytochemistry to provide a rapid quantification of human spermatogonia in different cell fractions. Second, we validated human-to–nude mouse xenotransplantation as a routine bioassay for human spermatogonia.
Brinster and colleagues introduced the SSC transplantation technique in 1994 (12, 13), and it has become the “gold standard” for assessing stem cell activity in rodent testes. In the absence of any specific SSC markers, the transplant technique identified stem cells by demonstrating their biologic potential to produce and maintain spermatogenesis in infertile recipient animals. SSC transplantation is a quantitative bioassay because each colony of spermatogenesis arises from the clonogenic self-renewal and differentiation from a single transplanted SSC (52–54). This technique revolutionized the field of SSC biology and has stimulated nearly two decades of discovery on the basic biology and regenerative potential of this adult tissue stem cell.
Although human-to-human SSC transplant is not possible as a routine bioassay, Nagano and colleagues demonstrated that human and nonhuman primate spermatogonia could colonize and survive for extended periods of time after transplantation into the seminiferous tubules of immune-deficient nude mice (55, 56). The transplanted cells from higher primates did not produce complete spermatogenesis in recipient mice (probably due to evolutionary distance between mice and humans), but the investigators concluded that the colonizing spermatogonia were derived from SSCs because they survived long term on the basement membrane of seminiferous tubules and retained spermatogonial morphology and phenotype (staining for RBMY in primates) (55, 56). In addition, clusters of undifferentiated spermatogonia were observed 1 month following transplant, suggesting that the transplanted SSCs underwent replication. We obtained similar results in this study using a rabbit anti-primate testis cell antibody (37) to detect human spermatogonia in mouse testes. Transplanted human testis cells produced colonies with typical spermatogonial appearance that expressed the germ cell marker, VASA (Figure 1). Furthermore, colonizing activity in every experiment coincided with the distribution of SALL-4+ spermatogonia (Figures 2, 3, and 5). Although xenotransplantation of primate SSCs to nude mice has the limitation that it does not regenerate complete spermatogenesis, it is gradually becoming the gold standard for primate SSCs (9, 29, 37, 38, 50, 55–58).
EpCAM, our selected spermatogonial marker, is a calcium-independent adhesion molecule that is expressed by murine embryonic stem cells, primordial germ cells of both sexes, and spermatogonia in adult mice (59). Furthermore, Ryu and colleagues demonstrated that EpCAM could be used as a cell surface marker to isolate and enrich transplantable SSCs in the rat (48). Here we provide the first demonstration to our knowledge that EpCAM is a conserved spermatogonial marker in humans. The majority of SALL-4+ spermatogonia were recovered in the EpCAMlo fraction of human testis cells, and xenotransplant colonizing activity in this fraction was enriched nearly 6 fold compared with that in unsorted controls. It is important to confirm experimentally that rodent spermatogonial markers are conserved in humans. CD29 (β1 integrin), for example, is a marker of rodent SSCs that does not appear to be conserved in humans (60) (S.L. Dovey and K.E. Orwig, data not shown). Others have reported that SSEA4 (50) and GPR125 (61) are cell surface markers of human spermatogonia. We did not observe immunoreactivity for either marker with human testis cell suspensions in this study. These disparate results might be attributed to differences in cell processing (i.e., trypsin concentration) that affect cell surface antigens or the use of different antibodies.
We further refined our sorting strategy by adding 2 MOLT-4 leukemia cell markers (CD49e and HLA-ABC) to the staining cocktail that was then used to analyze and fractionate MOLT-4–contaminated human testis cell suspensions. The putative spermatogonial fraction (EpCAMlo/CD49e–/HLA-ABC–) was enriched 12 fold for colonizing activity in the human-to–nude mouse xenotransplant assay. This fraction never produced a tumor following transplantation into seminiferous tubules or into the testicular interstitial space. In contrast, the putative MOLT-4 leukemia cell fraction was depleted of SALL-4+ spermatogonia and produced tumors in seminiferous tubules as well as in the testicular interstitial space. Similar results were obtained by Hou and colleagues, who used EpCAM in combination with leukemia markers to remove malignant contamination in a rat model of Roser’s T cell leukemia (36) and concluded that a multiparameter sorting strategy that included both spermatogonial and leukemia markers was required to eliminate malignant contamination and leukemia transmission.
We then replicated this finding using a different human leukemic cell line, TF-1a, to demonstrate that the multiparameter FACS strategy to remove malignant cells from therapeutic spermatogonia can be applied across different malignancies (Supplemental Figure 3). It is important to note, however, that we needed to use different cell surface antigens when sorting the TF-1a cells from spermatogonia, as their cell surface phenotype was somewhat different than that of MOLT-4 cells. Through a series of similar experiments, it may be possible to identify a broad panel of markers that can be used in a generalized approach to remove a variety of malignant cell types from human testis cell suspensions.
Two prior studies have attempted to separate spermatogonia from cancer cells in a human model. In 2006, Fujita and colleagues demonstrated via flow cytometry that several human leukemic cell lines uniformly expressed cell surface antigens MHC class I and CD45 (25). They then performed FACS on human testicular cells and demonstrated that the MHC class I–/CD45– fraction contained germ cells (assessed qualitatively by RT-PCR for germ cell markers), suggesting that these cell surface antigens could be used to sort leukemic cells away from germ cells. However, the authors of that study did not report sorting and transplantation of contaminated human testis cell suspensions, as they had previously reported for mice (24). Geens and coworkers did contaminate human testis cell suspensions with B cell acute lymphoblastic leukemic cells but were not able to remove the malignant contamination using FACS-based selection for HLA-ABC (27).
Our study adds significantly to the current literature by demonstrating that a multiparameter sorting strategy can enrich spermatogonia and eliminate cancer contamination from a human testis cell suspension. These conclusions are supported by quantitative in vitro and in vivo assessments, including transplant of selected fractions into the seminiferous tubules of recipient mice. This human-to–nude mouse xenotransplant assay is most relevant to the cancer survivor paradigm in which the ultimate objective will be to transplant a patient’s cells back into the seminiferous tubules of his testes to reinitiate spermatogenesis. However, these bioassays require a large number of cells and time. Ultimately, it will be necessary to identify specific, sensitive markers of SSCs and cancers cells so that assessment of stem cell activity and malignant contamination can be conducted quickly and with a relatively smaller portion of the patient’s tissue. Molecular readouts, such as PCR, are rapid and likely have the best sensitivity to detect occult tumor cells, and, indeed, evaluation of minimal residual disease (MRD) with PCR is now being investigated as a more precise means to screen tissue for transplantation (62). Alarmingly, assessment of MRD in ovarian tissue destined for autotransplantation in patients with leukemia identified malignant contamination in the majority of samples, even after a negative histology and immunohistochemistry examination (62, 63).
One current limitation to performing MRD screening routinely prior to transplantation is the need to identify a PCR target unique to the cancer of interest. However, identifying a distinctive PCR target for MRD screening is just half of the equation. What is the clinical significance of very-low-level contamination detected only by PCR for a given malignancy? How likely is this to result in clinical relapse if the tissue is transplanted? Courbiere and colleagues discussed this issue eloquently in an editorial describing a patient with chronic myeloid leukemia who underwent ovarian tissue harvesting in which autotransplantation of the tissue was debated after histology evaluation was negative but PCR demonstrated a small number of BCR-ABL transcripts in the cortical tissue (0.001%) (64). Considering that the survival and engraftment of tumor cells will depend on the type of cancer and number infused, it was felt clinically that the likelihood of inducing relapse was low if transplantation was performed, but the absolute risk is virtually impossible to quantify.
The findings in our study parallel this clinical dilemma, in that the FACS reanalysis purity check demonstrated that the EpCAMlo/CD49e–/HLA-ABC– fraction was 98.8%–99.8% pure. Furthermore, this fraction did not produce tumors in 55 transplanted testes (intratubular and interstitial). Do these results indicate that approximately 99% purity should be considered safe for autologous transplantation? In the bone marrow transplant field, “purging” malignant cells from HSC samples prior to autologous transplant has been studied extensively for over 2 decades, as autologous bone marrow transplant is considered standard treatment for patients with various malignancies (65). Overall, there is limited convincing evidence that transfusing a small number of cancer cells in HSC grafts causes relapse or that purging HSC grafts decreases rates of relapse, and results from phase II and III clinical trials have been mixed (65, 66). Clearly, HSC transplantation and spermatogonial and/or ovarian transplantation are not clinically equivalent, considering that HSC transplantation is required to treat or cure life-threatening conditions, whereas fertility preservation procedures are elective. Nonetheless, HSC graft purging studies do highlight the point that in vitro measures of decontamination efficiency, such as PCR, may not always be appropriate surrogates of clinical outcome. Short of performing a clinical trial, biological readouts, such as xenotransplantation, may be the most relevant end points to assess the adequacy of decontamination. Indeed, as our ability to detect MRD through molecular methods improves, it is likely that clinicians will face this challenging scenario on a more frequent basis. Thus, it will be important to not only improve MRD screening techniques, but also to correlate MRD screening results with xenotransplantation studies, so that the clinical risk of inducing a relapse following transplantation of tissue with trace MRD can be estimated.
Progress in culturing human SSCs has been reported by several laboratories in the past few years (9, 29, 57, 61, 67) and may provide an alternative approach for removing malignant contamination. In theory, it may be possible to amplify human SSCs clonally from individual cells or from small enriched fractions of testis cells and thereby alleviate malignant contamination. This will require continued progress to establish robust culture conditions in which human SSCs survive and can be expanded over several passages to produce a sufficient number of stem cells for therapeutic application.
We have demonstrated that it is feasible to enrich SSCs and remove malignant contamination from a heterogeneous human testis cell suspension. In these studies, we validated new tools to study spermatogonia in human testes and demonstrated that SALL-4 and EpCAM are conserved markers of human spermatogonia. As the panels of spermatogonial and cancer markers expand, it will be important to test sorting strategies on primary human cancers, which are likely to be more heterogeneous than the MOLT-4 and TF-1a leukemia lines used in this study. In addition, it will be important to develop methods to rapidly screen cell populations for malignant contamination and establish criteria for assessing safety prior to transplant. Continued work in this field is important, because clinics are already cryopreserving testicular tissue and ovarian tissue for patients with cancer in anticipation that this tissue can be used in the future to restore fertility. Autologous transplantation of tissue or cells is among the techniques being considered for both sexes, so risk of reintroducing cancer is of paramount concern.
Methods
Animals.
All experiments using animals were approved by the Institutional Animal Care and Use Committees of the Magee-Womens Research Institute and the University of Pittsburgh and performed in accordance with the NIH guidelines for the care and use of animals (assurance no. A3654-01).
Procurement and processing of human testicular tissue.
Deidentified human testicular tissue was obtained through the Center for Organ Recovery and Education and the University of Pittsburgh Health Sciences Tissue Bank under University of Pittsburgh IRB no. 0506140. Tissue was obtained from postpubertal male organ donors (Supplemental Table 2) and transported on ice in Lactated Ringer’s solution following procurement. Tissue processing ranged from 7 to 21 hours following organ procurement. A single cell suspension of human testicular parenchyma was prepared by sequential enzymatic digestion with 2 mg/ml collagenase mixture (1:0.4:0.15 [w/w/w] collagenase I/collagenase II/thermolysin; Liberase HI or Liberase MTF/C; Roche Applied Science), followed by 0.25% trypsin (Invitrogen) plus 1.4 mg/ml DNase I (Sigma-Aldrich), essentially as described by Hermann et al. (37, 38). Cells were cryopreserved at a final concentration of 20 × 106 cells/ml in cryoprotectant medium containing 15% FBS, 10% DMSO, 0.15 M trehalose using a controlled-rate freezer (CryoMed, Thermo Scientific) and then transferred to liquid nitrogen. For experiments, frozen testis cells were thawed rapidly, washed, and suspended in MEMα medium containing 10% FBS.
MOLT-4 cell line culture.
The MOLT-4 cell line, derived from a 19-year-old man with acute T cell lymphoblastic leukemia in relapse (46), was obtained from ATCC. Cultures were established in RPMI-1640 media (GIBCO, Invitrogen) with 10% FBS and supplemented with antibiotic-antimycotic solution containing penicillin, streptomycin, and amphotericin (Antibiotic-Antimycotic, GIBCO Cell Culture, Invitrogen). Fresh media was added every 2 to 3 days, and cells were subcultured at or before they reached a density of 2 × 106 cells/ml, as per manufacturer recommendations.
Flow cytometry.
Flow cytometry was used to characterize the expression of a panel of cell surface antigens on MOLT-4 and human testicular cells. To assess antigen expression, 0.5 × 106 cells were stained with fluorophore-conjugated primary antibodies (Supplemental Table 3) for 20 minutes on ice. Cells were also stained with isotype control antibodies to correct for nonspecific antibody binding. Preliminary titration experiments were carried out with each antibody to determine the optimal antibody concentration for both MOLT-4 cells and human testicular cells. Following staining, cells were washed 3 times with cold Dulbecco’s PBS (D-PBS; GIBCO, Invitrogen) containing 10% FBS. A FACSDiva (Becton Dickinson) machine was used to perform flow cytometry, and the percentage of cells expressing the antigen of interest was determined by quantifying the percentage of cells with a higher fluorescence intensity than the isotype control. Each experiment was replicated 3–5 times.
FACS.
Based on flow cytometry results, markers that were expressed on >95% of MOLT-4 cells were considered markers of MOLT-4 leukemia cells. In contrast, markers expressed by <1% of MOLT-4 cells and 5% or more of human testis cells were considered potential SSC markers. CD49e (α5 integrin), HLA-ABC, and CD326 (EpCAM) met these criteria and were selected for further analysis by FACS and immunocytochemical analysis of human testis cell fractions.
Human testis cell suspensions were stained with fluorescent-conjugated antibodies (anti-human CD49e clone NKI-SAM-1, BioLegend; anti-human HLA-ABC clone G46-2.6, BD Biosciences; anti-human CD326 clone 9C4, BioLegend) as described above and sorted into 2 to 3 fractions based on expression level of the candidate antigen using a FACSvantage SE fluorescence-activated cell sorter (Becton Dickinson). Prior to sorting, the cell suspensions were filtered through a 35-μm nylon membrane. Propidium iodide (0.5 μg/ml) was used to distinguish nonviable cells. Negative gates were drawn based on analysis of unstained control cells or cells stained with the isotype control antibodies.
Immunocytochemistry.
Cell fractions were collected in Opti-MEM (GIBCO, Invitrogen) supplemented with 10% FBS, spotted onto slides (Superfrost Plus; Fisher Scientific), and fixed with methanol. Once dry, immunocytochemistry was performed by rehydrating the cells with D-PBS, incubating them with a blocking buffer containing goat serum (D-PBS plus 0.1% Triton X-100, 5% goat serum, 3% BSA), and staining them with rabbit anti–SALL-4 (spermatogonial marker; Abcam) for 90 minutes in the dark. Slides were then washed 3 times, and SALL-4 staining was detected by indirect immunofluorescence by incubating with goat anti-rabbit IgG Alexa Fluor 488 (Invitrogen) for 45 minutes. After staining, cells were washed and mounted with VectaShield mounting media containing DAPI (Vector Laboratories) and imaged via fluorescent microscopy. To quantify the percentage of cells expressing SALL-4 in each sorted fraction, at least 10 random images of each fraction were recorded and the number of SALL-4+ cells as well as the total number of cells was quantified. An unsorted sample of testicular cells was also stained to determine the percentage of unsorted testicular cells that express SALL-4. These experiments were replicated 3 times for each representative antibody (HLA-ABC, CD49e, and EpCAM) using testicular tissue from different male donors.
Xenotransplantation and whole mount immunofluorescent quantification of colonization activity of undifferentiated spermatogonia.
Following FACS, unsorted and sorted testicular cell fractions were transplanted into the testes of busulfan-treated, immune-deficient nude mice (NCr nu/nu; Taconic) as previously described (37). Briefly, immunodeficient nude mice were treated with a single dose of busulfan (40 mg/kg, Sigma-Aldrich) at 6 weeks of age to eliminate endogenous spermatogenesis. Xenotransplantation was then performed 5 weeks after busulfan treatment by injecting cell suspensions containing 10% trypan blue (Invitrogen) into the seminiferous tubules of recipient testes via cannulation of the efferent ducts. Approximately 7 μl of cell suspension was injected per testis. For experiments involving FACS of contaminated testicular cell suspensions, MOLT-4 cells were mixed with human testicular cells such that MOLT-4 cells made up approximately 10% of the final cell suspension prior to sorting. The concentration of cells transplanted into seminiferous tubules from each fraction varied based on the total number of cells collected following sorting. An average of 996,845 cells were transplanted per recipient mouse testis from the unsorted spiked cell suspension, 63,780 cells were transplanted from the EpCAMlo/CD49e–/HLA-ABC– (spermatogonial) fraction, and 5,000 cells were transplanted from the the EpCAM–/CD49e+/HLA-ABC+ (MOLT-4) fraction. As discussed above, a prior sensitivity analysis demonstrated that as few as 10 MOLT-4 cells were capable of inducing tumor formation when transplanted into the testes of immunodeficient mice treated with busulfan, and injection of 1,000 MOLT-4 cells reliably induced tumor formation in the majority of mice (83%) (35). This experiment was designed primarily to assess SSC activity in each fraction. Malignant contamination of each fraction was also evaluated by injection into the testicular interstitial space, which is an excellent environment for tumor formation (see Human-to–nude mouse tumor bioassay below).
In order to quantify human testis cell colonization of the mouse recipient seminiferous tubules, the testes were recovered 8 weeks following transplantation and removed from the tunica, and the seminiferous tubules were dispersed gently with collagenase IV (1 mg/ml) and DNase I (1 mg/ml) in D-PBS. Samples were then fixed for 4 hours in 4% paraformaldehyde. Whole mount immunofluorescence was carried out by dehydrating samples in a graded series of methanol dilutions before incubating in MeOH/DMSO/H2O2 (4:1:1) solution for 3 hours. Samples were then rehydrated, blocked with a blotto milk solution in D-PBS (D-PBS plus 0.02 gm/ml blotto dry milk powder plus 5% Triton-X), and stained with a rabbit anti-primate testis cell primary antibody (37) at a 1:200 dilution overnight at 4°C. Immunoreactivity with primate cells was detected indirectly using goat anti-rabbit IgG Alexa Fluor 488 (1:200, Invitrogen). All dehydration, rehydration, and staining steps were carried out in 12-mm Transwell baskets (Corning Life Sciences) in order to prevent loss of seminiferous tubules. Finally, the seminiferous tubules were mounted on slides with VectaShield mounting media containing DAPI (Vector Laboratories) with raised cover slips and imaged with fluorescent microscopy. Spermatogonial colonies were counted if they met the following criteria: at least 4 cells exhibiting spermatogonial morphology (ovoid shape with high nuclear-to-cytoplasmic ratio), located on the basement membrane in a continuous area of recipient seminiferous tubule (≤100 μm between cells; see Figure 1).
Human-to–nude mouse tumor bioassay.
In addition to the intratubular transplant bioassay for human spermatogonia, xenotransplants into the interstitial space (between seminiferous tubules) of nude mouse testes were performed to assess tumorigenic potential of unsorted and sorted cell fractions. We found that the interstitial space was more susceptible to tumor formation than the intratubular space and was therefore a more sensitive bioassay for malignant contamination. Approximately 10 μl of cell suspension was transplanted into the interstitial space at cell concentrations of 0.5 × 106 cells/ml to 5 × 106 cells/ml (50,000 cells per recipient mouse testis in the unsorted spiked arm, 5,000 cells per testis in all other experimental arms) by initially cannulating the efferent duct and then visibly pushing the needle through the efferent duct into the interstitial space. As indicated above, as few as 10 MOLT-4 cells are sufficient to produce tumors following transplantation into the testes of nude mice (35). Therefore, the human-to–nude mouse tumor assay has the sensitivity to detect a 0.2% contamination with cancer cells (10 cells in a transplanted fraction of 5,000 cells). Following interstitial transplantation, the mice were monitored and palpated regularly to assess for tumor formation and sacrificed for analysis when palpable tumors were present or by 4 months following transplantation. The testes were removed and examined grossly for tumor formation.
Statistics.
Analysis of variance on nested linear mixed-effect models was used to compare differences among the percentage of SALL-4+ cells in unsorted versus sorted cell fractions in the immunohistochemistry experiments and colonizing activity in the human-to–nude mouse xenotransplant bioassay. P values of less than 0.05 were considered significant.
Study approval.
Animal studies were approved by the Institutional Animal Care and Use Committees of Magee-Womens Research Institute and the University of Pittsburgh and performed in accordance with the NIH guidelines for the care and use of animals (assurance no. A3654-01). Deidentified human testicular tissues were obtained through the Center for Organ Recovery and Education and the University of Pittsburgh Health Sciences Tissue Bank with approval from the University of Pittsburgh Institutional Review Board (IRB approval no. 0506140).
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 HD055475 and R21 HD061289, Magee-Womens Research Institute and Foundation, United States – Israel Binational Science Foundation, and the Richard King Mellon Foundation. Additional thanks to McGowan Institute for Regenerative Medicine FACS facility and Lynda Guzik for help with cell sorting and to the Center for Organ Recovery and Education and the Tissue and Research Pathology Services at the University of Pittsburgh Cancer Institute for human tissues.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(4):1833–1843. doi:10.1172/JCI65822.
Brian P. Hermann’s present address is: Department of Biology, University of Texas at San Antonio, San Antonio, Texas, USA.
References
- 1.Ries LAG, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, Maryland, USA: National Cancer Institute; 2007. [Google Scholar]
- 2.Steliarova-Foucher E, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet. 2004;364(9451):2097–2105. doi: 10.1016/S0140-6736(04)17550-8. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, et al. SEER Cancer Statistic Review 1975–2008. Bethesda, Maryland, USA: National Cancer Institute; 2010. [Google Scholar]
- 4.Lee SJ, et al. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 5.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer. 1999;86:697–709. doi: 10.1002/(SICI)1097-0142(19990815)86:4<697::AID-CNCR20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Carter J, et al. Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecol Oncol. 2005;97(1):90–95. doi: 10.1016/j.ygyno.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Partridge AH, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 8.Keros V, Hultenby K, Borgström B, Fridström M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22(5):1384–1395. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- 9.Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AMM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305(23):2416–2418. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- 10.Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today. 2009;87(1):1–26. doi: 10.1002/bdrc.20142. [DOI] [PubMed] [Google Scholar]
- 11.Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update. 2006;12(3):275–282. doi: 10.1093/humupd/dmk001. [DOI] [PubMed] [Google Scholar]
- 12.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6(1):29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci U S A. 2001;98(11):6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2001;98(23):13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003;69(2):412–420. doi: 10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- 18.Honaramooz A, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69(4):1260–1264. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- 19.Mikkola M, et al. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim. 2006;41(2):124–128. doi: 10.1111/j.1439-0531.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136(6):823–831. doi: 10.1530/REP-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrid M, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81(5):898–905. doi: 10.1095/biolreprod.109.078279. [DOI] [PubMed] [Google Scholar]
- 22.Hermann BP, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11(5):715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005;34(34):51–56. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- 24.Fujita K, et al. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest. 2005;115(7):1855–1861. doi: 10.1172/JCI24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita K, et al. Isolation of germ cells from leukemia and lymphoma cells in a human in vitro model: potential clinical application for restoring human fertility after anticancer therapy. Cancer Res. 2006;66(23):11166–11171. doi: 10.1158/0008-5472.CAN-06-2326. [DOI] [PubMed] [Google Scholar]
- 26.Hou M, Andersson M, Eksborg S, Soder O, Jahnukainen K. Xenotransplantation of testicular tissue into nude mice can be used for detecting leukemic cell contamination. Hum Reprod. 2007;22(7):1899–1906. doi: 10.1093/humrep/dem085. [DOI] [PubMed] [Google Scholar]
- 27.Geens M, Van de Velde H, De Block G, Goossens E, Van Steirteghem A, Tournaye H. The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Hum Reprod. 2007;22(3):733–742. doi: 10.1093/humrep/del418. [DOI] [PubMed] [Google Scholar]
- 28.Schlatt S, Ehmcke J, Jahnukainen K. Testicular stem cells for fertility preservation: preclinical studies on male germ cell transplantation and testicular grafting. Pediatr Blood Cancer. 2009;53(2):274–280. doi: 10.1002/pbc.22002. [DOI] [PubMed] [Google Scholar]
- 29.Sadri-Ardekani H, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302(19):2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg JP, et al. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod. 2010;25(1):37–41. doi: 10.1093/humrep/dep371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyns C, et al. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod. 2011;26(4):737–747. doi: 10.1093/humrep/deq387. [DOI] [PubMed] [Google Scholar]
- 32.Wyns C, Curaba M, Vanabelle B, Van Langendonckt A, Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update. 2010;16(3):312–328. doi: 10.1093/humupd/dmp054. [DOI] [PubMed] [Google Scholar]
- 33.Kim TH, et al. Pretreatment testicular biopsy in childhood acute lymphocytic leukaemia. Lancet. 1981;2(8248):657–658. doi: 10.1016/s0140-6736(81)90996-x. [DOI] [PubMed] [Google Scholar]
- 34.Jahnukainen K, Hou M, Petersen C, Setchell B, Soder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61(2):706–710. [PubMed] [Google Scholar]
- 35.Hermann BP, Sukhwani M, Salati J, Sheng Y, Chu T, Orwig KE. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Hum Reprod. 2011;26(12):3222–3231. doi: 10.1093/humrep/der343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou M, Andersson M, Zheng C, Sundblad A, Soder O, Jahnukainen K. Decontamination of leukemic cells and enrichment of germ cells from testicular samples from rats with Roser’s T-cell leukemia by flow cytometric sorting. Reproduction. 2007;134(6):767–779. doi: 10.1530/REP-07-0240. [DOI] [PubMed] [Google Scholar]
- 37.Hermann BP, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25(9):2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod. 2009;24(7):1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Alphen MM, van de Kant HJ, de Rooij DG. Repopulation of the seminiferous epithelium of the rhesus monkey after X irradiation. Radiat Res. 1988;113(3):487–500. doi: 10.2307/3577245. [DOI] [PubMed] [Google Scholar]
- 40.Ehmcke J, Simorangkir DR, Schlatt S. Identification of the starting point for spermatogenesis and characterization of the testicular stem cell in adult male rhesus monkeys. Hum Reprod. 2005;20(5):1185–1193. doi: 10.1093/humrep/deh766. [DOI] [PubMed] [Google Scholar]
- 41.Ehmcke J, Schlatt S. Identification and characterization of spermatogonial subtypes and their expansion in whole mounts and tissue sections from primate testes. Methods Mol Biol. 2008;450:109–118. doi: 10.1007/978-1-60327-214-8_7. [DOI] [PubMed] [Google Scholar]
- 42.Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27(4):422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- 43.Hobbs RM, et al. Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell. 2012;10(3):284–298. doi: 10.1016/j.stem.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eildermann K, et al. Developmental expression of the pluripotency factor sal-like protein 4 in the monkey, human and mouse testis: restriction to premeiotic germ cells. Cells Tissues Organs. 2012;196(3):206–220. doi: 10.1159/000335031. [DOI] [PubMed] [Google Scholar]
- 45.Gassei K, Orwig KE. SALL4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PLoS One. 2013;8(1):e53976. doi: 10.1371/journal.pone.0053976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minowada J, Onuma T, Moore GE. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972;49(3):891–895. [PubMed] [Google Scholar]
- 47.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96(10):5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryu BY, Orwig KE, Kubota H, Avarbock MR, Brinster RL. Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev Biol. 2004;274(1):158–170. doi: 10.1016/j.ydbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Conrad S, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456(7220):344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 50.Izadyar F, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26(6):1296–1306. doi: 10.1093/humrep/der026. [DOI] [PubMed] [Google Scholar]
- 51.Hu X, et al. Characterization of a unique factor-independent variant derived from human factor-dependent TF-1 cells: a transformed event. Leuk Res. 1998;22(9):817–826. doi: 10.1016/S0145-2126(98)00073-3. [DOI] [PubMed] [Google Scholar]
- 52.Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol Reprod Dev. 1999;53(2):142–148. doi: 10.1002/(SICI)1098-2795(199906)53:2<142::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Ebata KT, Nagano MC. Genetic analysis of the clonal origin of regenerating mouse spermatogenesis following transplantation. Biol Reprod. 2003;69(6):1872–1878. doi: 10.1095/biolreprod.103.019273. [DOI] [PubMed] [Google Scholar]
- 54.Kanatsu-Shinohara M, et al. Clonal origin of germ cell colonies after spermatogonial transplantation in mice. Biol Reprod. 2006;75(1):68–74. doi: 10.1095/biolreprod.106.051193. [DOI] [PubMed] [Google Scholar]
- 55.Nagano M, McCarrey JR, Brinster RL. Primate spermatogonial stem cells colonize mouse testes. Biol Reprod. 2001;64(5):1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 56.Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril. 2002;78(6):1225–1233. doi: 10.1016/S0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- 57.Wu X, et al. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A. 2009;106(51):21672–21677. doi: 10.1073/pnas.0912432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zohni K, Zhang X, Tan SL, Chan P, Nagano M. CD9 is expressed on human male germ cells that have a long-term repopulation potential after transplantation into mouse testes. Biol Reprod. 2012;87(2):27. doi: 10.1095/biolreprod.112.098913. [DOI] [PubMed] [Google Scholar]
- 59.Anderson R, Schaible K, Heasman J, Wylie C. Expression of the homophilic adhesion molecule, Ep-CAM, in the mammalian germ line. J Reprod Fertil. 1999;116(2):379–384. doi: 10.1530/jrf.0.1160379. [DOI] [PubMed] [Google Scholar]
- 60.Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today. 2009;87(1):27–34. doi: 10.1002/bdrc.20141. [DOI] [PubMed] [Google Scholar]
- 61.He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82(2):363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116(16):2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 63.Rosendahl M, Andersen MT, Ralfkiaer E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril. 2010;94(6):2186–2190. doi: 10.1016/j.fertnstert.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 64.Courbiere B, Prebet T, Mozziconacci MJ, Metzler-Guillemain C, Saias-Magnan J, Gamerre M. Tumor cell contamination in ovarian tissue cryopreserved before gonadotoxic treatment: should we systematically exclude ovarian autograft in a cancer survivor? Bone Marrow Transplant. 2010;45(7):1247–1248. doi: 10.1038/bmt.2009.313. [DOI] [PubMed] [Google Scholar]
- 65.Alvarnas JC, Forman SJ. Graft purging in autologous bone marrow transplantation: a promise not quite fulfilled. Oncology (Williston Park). 2004;18(7):867–876. [PubMed] [Google Scholar]
- 66.Bensinger WI. Should we purge? Bone Marrow Transplant. 1998;21(2):113–115. doi: 10.1038/sj.bmt.1701051. [DOI] [PubMed] [Google Scholar]
- 67.Mirzapour T, et al. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012;44(suppl 1):41–55. doi: 10.1111/j.1439-0272.2010.01135.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.