Abstract
Geminin is a nuclear protein that performs the related functions of modulating cell cycle progression by binding Cdt1, and controlling differentiation by binding transcription factors. Since embryonic stem cells (ESC) and the epiblast share a similar gene expression profile and an attenuated cell cycle, ESC form an accessible and tractable model system to study lineage choice at gastrulation. We derived several ESC lines in which Geminin can be inducibly expressed, and employed short hairpin RNAs targeting Geminin. As in the embryo, a lack of Geminin protein resulted in DNA damage and cell death. In monolayer culture, in defined medium, Geminin supported neural differentiation; however, in three-dimensional culture, overexpression of Geminin promoted mesendodermal differentiation and epithelial-to-mesenchymal transition. In vitro, ESC overexpressing Geminin rapidly recolonized a wound, downregulated E-cadherin expression, and activated Wnt signaling. We suggest that Geminin may promote differentiation via binding Groucho/TLE proteins and upregulating canonical Wnt signaling.
Introduction
Just before gastrulation, the mouse embryo expands from 20–25 cells to ∼660 cells in roughly 1.5 days [1,2]. During this period of rapid cell division, it is critical that the epiblast remains undifferentiated; cells spend the majority of their cycle in the S phase with a very short G1 [3,4], unlike somatic cells where G1 predominates. This attenuated cell cycle likely underlies the rapid expansion capability of the epiblast and may also play an important role in maintaining its pluripotency and inhibiting differentiation.
At gastrulation, the epiblast is allocated to 3 germ layers: endoderm, mesoderm, and ectoderm. This process involves an epithelial-to-mesenchymal transition (EMT), complex morphogenetic movements, and differential gene expression that culminate in lineage-fate decisions. Despite the importance of understanding cell fate choice in early development, progress has been limited by the early lethality of many gene-null models and the general inaccessibility of the mammalian embryo at this early stage. Since embryonic stem cells (ESC) are derived from the inner cell mass of the blastocyst, they retain the ability to generate all cells of the mouse embryo [5,6]. In addition, ESC and the epiblast have similar gene expression patterns and share an uniquely abbreviated cell cycle. Because of these similarities, differentiation of ESC offers a simple model of cell fate choice at gastrulation and an experimentally tractable system to examine the gene function in development.
One gene that has been shown to modulate both cell cycle progression and differentiation is Geminin, which was identified in 2 independent functional screens in Xenopus embryos: the first screen to identify genes that were degraded during mitosis [7], and the second to identify genes involved in neural induction [8]. Geminin functions as a cell cycle-licensing factor, permitting replication of the genome once and only once during mitosis [9,10]; Geminin-null mouse embryos die by E3.5 due to endoreduplication errors [11,12]. Late in the S phase, Geminin binds and inhibits the interaction of Cdt1 with DNA origins of replication, and must then be degraded during the M phase to allow replication.
Geminin overexpression was reported to expand the Xenopus neural plate by inhibiting BMP signaling, independent of an effect on cell division [8]. Expression of Geminin is restricted to the neural plate in the Xenopus embryos by the Tcf and Vent sites [13], indicating that BMP and Wnt pathway signaling cooperate to control Geminin expression during differentiation. Geminin has also been proposed to control the transition from pluripotency to differentiation in the Xenopus embryo by epigenetically repressing the transcriptional response to the Activin/Nodal, FGF, and BMP pathways in conjunction with polycomb proteins, thus promoting differentiation of the neural ectoderm and inhibiting non-neural lineages [14].
To determine how Geminin functions in lineage specification, we developed several ESC lines in which Geminin can be inducibly overexpressed, and employed short hairpin RNAs (shRNA) to target the native Geminin mRNA. Reduction of Geminin protein via targeted shRNA resulted in cell death due to DNA damage. In monolayer culture, in defined medium, Geminin overexpression supported differentiation of neural precursor cells and neurons. In embryoid bodies (EBs); however, overexpression of Geminin induced mesendodermal differentiation and expression of genes involved in EMT. Initiation of mesendodermal differentiation appears to result from Wnt pathway activation possibly by binding of Geminin to Groucho/Transducin-Like Enhancer of split (TLE) proteins in the nucleus that block Tcf/Lef target gene expression in the absence of activated β-catenin [15].
Materials and Methods
ES cell culture and differentiation
Undifferentiated mouse ESCs were maintained in 0.1% gelatin-coated tissue culture flasks in a complete medium composed of DMEM (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals), 50 mM HEPES (Sigma), and 1 mM β-mercaptoethanol (Sigma) with 5 ng/mL LIF (Chemicon). Neural-permissive culture conditions were achieved by plating cells at low density (∼2.0×104 cells per cm2) in gelatin-coated 6- or 12-well plates in 20% Neural basal medium (Invitrogen), 80% Ham's F12 medium (Invitrogen) with N2 and B27 salts (Invitrogen), 1 mM retinoic acid (Sigma), and 0.05% knockout serum replacement (Invitrogen). To form EBs, cells were plated at 1.25×105 cells per mL in nonadherent Petri dishes in medium consisting of DMEM (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals), 50 mM HEPES (Sigma), and 1 mM β-mercaptoethanol (Sigma) for 4 days, and then transferred to gelatin-coated 12-well dishes for an additional 2 days of adherent culture in the same medium. Cells were maintained at 37°C with 5% CO2.
To test their differentiation potential in the intact animal, control and induced transgenic ESC were injected over the flank of NOD SCID-gamma (NSG) mice (The Jackson Laboratory). Geminin expression was induced for 4 days before implantation, and control cells were maintained in culture for a similar period. Cells were trypsinized, pelleted, and 1×106 cells in a volume of 250 μL injected subcutaneously. After 3–6 weeks, when obvious tumors were present, animals were sacrificed, tumors photographed, and embedded without fixation in OCT. Frozen sections were cut and stained with hematoxylin and eosin or immunohistochemical localization of cell-type-specific antibodies (described in the following section) carried out to confirm the presence of multiple lineages in the teratoma.
Plasmid construction and transfection
Geminin shRNA plasmids were described previously [16]. The H1 promoter was used to drive expression of the shRNA, while a separate eGFP cassette driven by the CMV promoter was used to identify transfected cells and monitor the transfection efficiency (Fig. 7A). ESC were passaged 12 to 24 h before transfection and plated in gelatin-coated 6-well plates (2.5×104 cells per cm2) in complete medium with LIF. ES cells were transfected with Lipofectamine Plus (Invitrogen) as directed by the manufacturer and cultured overnight in serum-free conditions (OptiMem; Invitrogen), followed by EB medium before RNA extraction, western blotting, or immunohistochemistry (IHC), as described below.
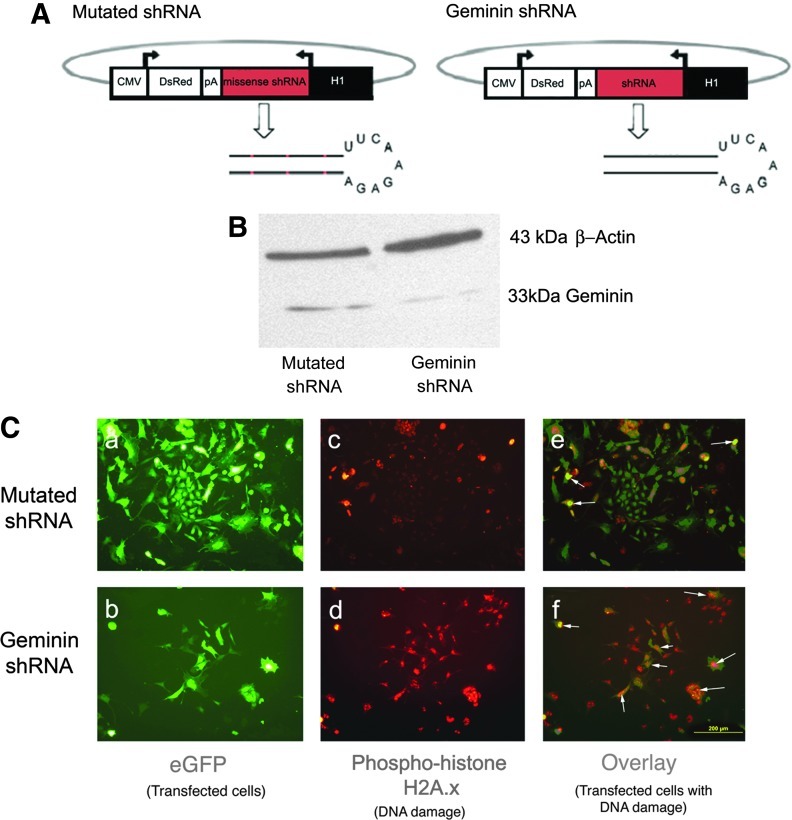
FIG. 7.
Transfection of ESC with a Geminin short hairpin RNA (shRNA) plasmid causes DNA damage. (A) Diagrams of a control plasmid containing 3 nucleotide substitutions producing a mutated shRNA and the shRNA targeting Geminin. (B) Western blot of protein from ESC transfected for 24h with mutated shRNA or Geminin shRNA, demonstrating a 6-fold reduction in Geminin protein. (C) ESC were transfected with mutated (a, c, e) or Geminin shRNA (b, d, f). 11.4%±0.6% of cells exposed to the control (mutated) shRNA express phosphohistone H2A.X (an indicator of DNA damage, arrows), whereas the loss of Geminin protein induced widespread DNA damage (84.3%±0.1% of transfected cells, P≤0.001). Scale bar=200 μM. Color images available online at www.liebertpub.com/scd
Inducible expression of Geminin in ESC
We obtained the MGZRTcH2 ESC cell line and the corresponding exchange vector from Dr. Shinji Masui [17]. This cell line contains a tetracycline-regulated transactivator, a tetracycline-response element, followed by the minimal promoter of the human cytomegalovirus (hCMV*-1) immediate early gene, a hygromycin resistance cassette, directional loxP sites, and an IRES-Venus (yellow fluorescent protein) cassette knocked-in to the endogenous ROSA26 promoter. The corresponding exchange vector replaces the hygromycin resistance cassette with the desired cDNA in addition to adding a puromycin resistance cassette. When the exchange vector is correctly enzymatically recombined into the ROSA26 locus, the resulting clones are no longer hygromycin resistant, but are puromycin resistant—allowing both positive and negative selection, thereby ensuring the elimination of cells with random integration of the exchange vector (Fig. 1A).
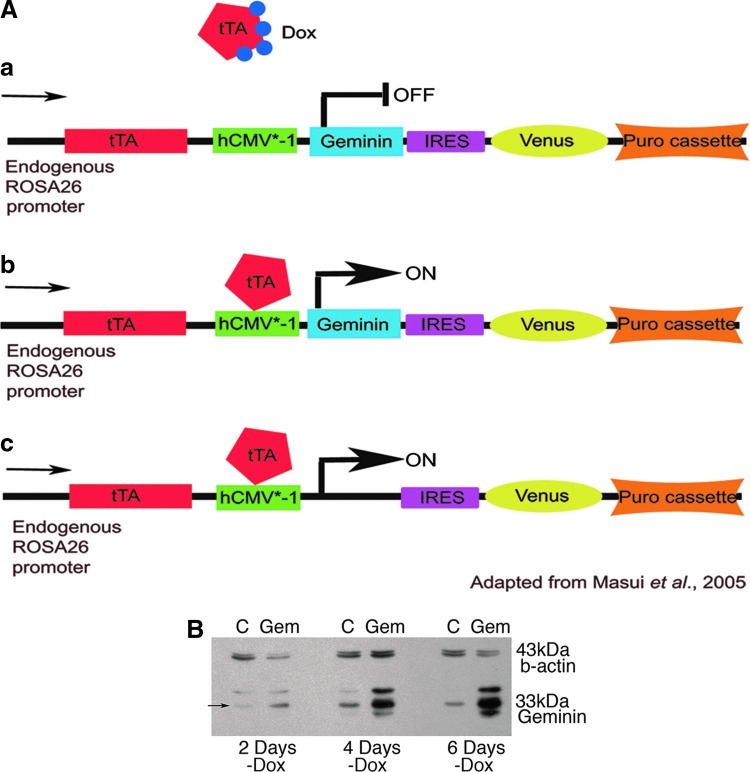
FIG. 1.
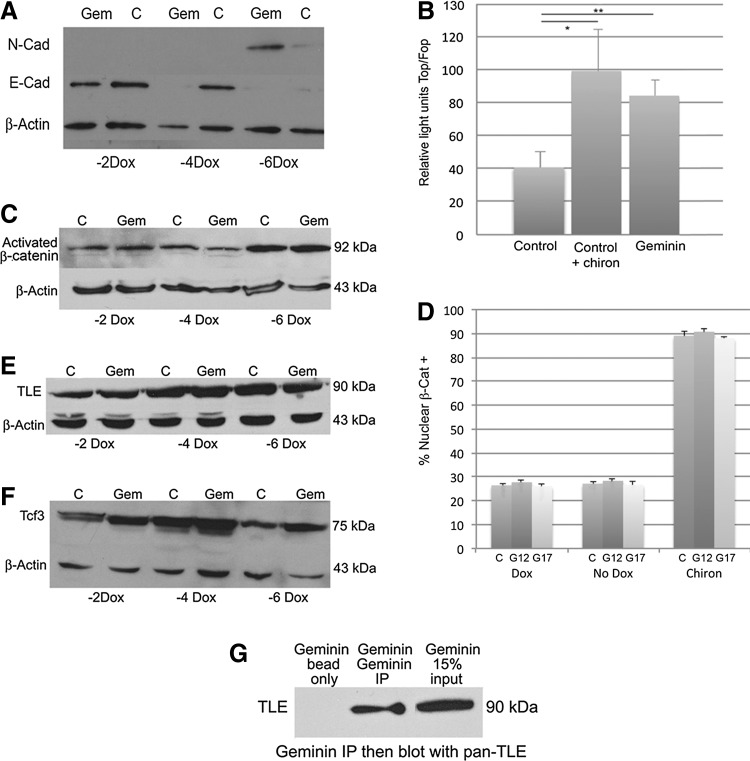
Geminin can be inducibly expressed in embryonic stem cells (ESC). (A) Expression of Geminin from the ROSA26 locus can be controlled by doxycycline (Dox). (a) In the presence of the tetracycline analog Dox, the tetracycline-regulated transactivator (tTA) is unable to bind to the hCMV*-1 promoter (tetracycline-response element, followed by the minimal promoter of the human cytomegalovirus immediate early gene) to initiate downstream gene expression. (b) Once Dox is removed from the tissue culture medium, the tTA binds the hCMV*-1 promoter, and Geminin and Venus proteins are transcribed. (c) Control cells inducibly express Venus, but lack the Geminin cDNA. (B) Geminin can be inducibly expressed in ESC. Western blot of protein from control (C) and Geminin (Gem) ESC lines grown for 2, 4, and 6 days without Dox to induce transgene expression. Full-length Geminin is the middle band (arrow) at 33 kDa. At 2 days of induction Geminin expression was increased in Geminin ESC, and by 4 days and continuing through 6 days, Geminin was strongly overexpressed compared to control cells. Color images available online at www.liebertpub.com/scd
A polymerase chain reaction (PCR)-cloned and sequence-verified Geminin cDNA was inserted into one exchange vector. A control exchange vector with no cDNA was also generated to develop control cell lines that are puromycin resistant and express the Venus protein upon doxycycline (Dox) withdrawal. Each exchange vector was transfected into the MGZRTcH2 cell line with a Cre recombinase vector, followed by selection with 2 mg/mL puromycin (Sigma). Clones were expanded in complete medium with 1 mg/mL Dox (Sigma) to inhibit transgene expression and 2 mg/mL puromycin to maintain selection, and several selected to determine their differentiation capability. Clones were checked for random integration with 100 mg/mL hygromycin (Sigma). Overexpression of Geminin was verified by western blot and quantitative PCR. During all differentiation experiments, selection was maintained with 4 mg/mL puromycin, and Geminin expression was induced by Dox withdrawal (tet-off).
Cell cycle analysis
Cells were grown in EB medium for 6 days. On day 4, cells were plated at 2×105 cells/well in 6-well plates in triplicate in EB medium. After 48 and 96 h, cells were trypsinized and counted using a Coulter Counter (BD). For cell cycle analysis, samples were fixed by adding cold absolute ethanol and kept at −20°C until analysis. Fixed cells were resuspended in PBS, and DNAse-free RNase A (Sigma) was added to a 20 mg/mL final concentration. Samples were incubated at 37°C for 30 min, and DNA was labeled with 0.1 mg/mL propidium iodide (Sigma). Cells were sorted in a FACSCalibur using Cell Quest Pro MAC 9.0 and Mod Fit LT Mac 3.1 SP3 for cell cycle analysis.
Immunohistochemistry
ES cultures were fixed with 2% paraformaldehyde (PFA) for 15 min at room temperature and washed twice with PBS. Fixed cells were blocked in 10% donkey serum for 30 min, followed by an overnight incubation at 4°C with primary antibody (rabbit anti-Sox3: Mike Klymkowski, University of Colorado, 1:1,000; goat anti-Oct3/4: Santa Cruz, 1:500; rabbit anti-Foxa2: Upstate Biotechnology, 1:500; mouse anti-E-cadherin: Cell Signaling, 1:500; goat anti-Brachyury: Santa Cruz, 1:500; anti-activated β-catenin: Millipore; 1:250; or rabbit anti-phospho-histone H2A.X antibody: Cell Signaling, 1:500). Cells were then washed with PBS and incubated with secondary antibodies (1:400) conjugated to Cy3 or FITC (Jackson Immunoresearch) for 2 h at room temperature. Nuclei were visualized with Hoechst 33258 (Sigma), and images were obtained using an Leica DM inverted fluorescence microscope and an Olympus DP70 camera with associated software.
Quantitative RT-PCR
RNA was harvested with Trizol (Invitrogen) following the manufacturer's protocol, and genomic DNA was removed by DNAse digestion (Sigma). Complete DNA digestion was confirmed by semiquantitative PCR using primers for β-actin before reverse transcription. About 0.5 to 1.0 mg RNA sample was used for reverse transcription with Verso RT (Thermo Scientific) using random nonamers (Invitrogen) following the manufacturer's protocols. For quantitative PCR, cDNAs were diluted 1:2, and 1 μL was used per reaction with the ABgene SYBR green master mix. All primer pairs were rigorously screened to eliminate the primer dimer, and reaction conditions were optimized, producing reaction efficiencies between 90% and 110%. Quantitative PCR results were calculated, and statistical analysis performed with REST2008 software [18]. Primer sequences and detailed reaction conditions are available upon request.
Western blotting
ESC were lysed in an RIPA buffer (50 mM Tris–HCL, pH7.4, 1% NP-40, 0.25% Na-deoxycholate, and 150 mM NaCl2) with Complete protease inhibitor (Roche), cell debris was pelleted by centrifugation, and the protein-containing supernatant was removed and analyzed using the Pierce Protein Assay to determine total protein concentration. Ten to 30 mg of protein was loaded onto 10% or 12% polyacrylamide SDS PAGE gels and then transferred to PVDF membranes. Membranes were blocked with 5% milk powder/TBST (Tris-buffered saline and 0.2% Tween 20) and incubated with rabbit anti-Geminin (1:2,000; Santa Cruz), anti-activated β-catenin (1:2,000; Millipore), goat anti-Tcf3 (1:500; Santa Cruz), goat anti-panTLE (1:500; Santa Cruz), mouse anti-E-Cadherin (1:20,000; Cell Signaling), mouse anti-N-Cadherin (1:5,000; Life Technologies), and/or mouse anti-β-actin (1:10,000; Sigma) primary antibodies in 5% milk powder/TBST overnight at 4°C. Membranes were washed in TBST and incubated for 1 h at room temperature in a horseradish peroxidase-conjugated secondary antibody (1:1,000–1:10,000; Jackson Immunoresearch). Membranes were developed using a Pierce Supersignal West Pico chemiluminescent substrate. Western blotting was done on 3 Geminin transgenic cell lines and was repeated at least 3 times each.
Cell migration assay
To determine if cells overexpressing Geminin would more rapidly recolonize a wound, 3 lines of Geminin transgenic ESC were plated in triplicate at 5×105 cells per well in gelatin coated 6-well plates in EB medium±Dox. After 5 days of transgene induction, a cell scraper was used to remove a strip of cells from the middle of each dish, and the medium changed immediately. On 5 sequential days of culture, 5 photomicrographs (at different levels of the scrape) were taken from each of 3 replicate cultures±Dox, with 3 biological replicates (n=45 photomicrographs per timepoint) for each of the 3 lines. The distance between the cut edges was measured on each day and averaged, and significant differences were determined by matched-pair t-test.
TOP-/FOPFlash assay
Control and Geminin ESC were grown in adherent culture in EB medium for 48 h to induce transgene expression, in the presence and absence of the GSK3β inhibitor Chiron99021 (10 μM) as a positive control. Cells were transfected with TOPFlash or FOPFlash plasmids (Millipore) using Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. A Renilla plasmid (pRL-CMV; Promega) was cotransfected as an internal vector control. After 24 h, cells were lysed with passive lysis buffer and subjected to the Dual-luciferase assay (Promega) according to the manufacturer's directions. Samples were read with a Veritas microplate luminometer (Turner Biosystems). Data are expressed as the ratio of TOPFlash relative to Renilla over FOPFlash relative to Renilla, and significance was assessed using the Wilcoxon signed-rank test.
Nuclear β-catenin assay
To further interrogate the status of Wnt-signaling pathway members in Geminin-overexpressing ES cells, we determined the percentage of cells in which β-catenin was present in the nuclei. Control and 2 Geminin transgenic cell lines (G12 and G17) were grown without Dox to induce transgene expression, with Dox (negative control), or cells gown with Dox and the GSK3-β inhibitor Chiron99021 (10 μM) to stimulate the Wnt pathway (positive control). After 72h, cells were fixed in 2% PFA and stained with anti-β-catenin antibodies and with the Hoechst dye to label nuclear DNA. Quantification was carried out by counting the number of β-catenin+ nuclei divided by the total number of (Hoechst+) nuclei in 10 fields from 4 replicate cultures with 3 biological replicates (120 fields per group). Data are expressed as means of the 3 biological replicates±SD, and statistically significant differences between the treatment groups were determined using the Wilcoxon signed-rank test, followed by matched-pair tests.
Coimmunoprecipitation
Control and Geminin ESC were grown in EB medium for 4 days to induce transgene expression. Cells were scraped from the tissue culture dishes and lysed in Blenis lysis buffer (10 mM KPO4, 1 mM EDTA, pH7.0, 5 mM EGTA, pH7.2, 10 mM MgCl2, 50 mM β-glycerol phosphate, and 0.3% Chaps) with Complete protease inhibitor (Roche). Protein samples were divided in half, and 2 mL of goat anti-TLE (Santa Cruz) or rabbit anti-Geminin (Santa Cruz) antibodies were added to an aliquot of each sample set and incubated overnight at 4°C with end-over-end agitation. Preblocked Protein A or Protein G Sepharose beads (GE) were added to samples with antibody as well as to samples with input protein alone as a negative control and incubated for 1 h at 4°C with end-over-end agitation. Samples were centrifuged, and the supernatant was saved for a loading control. Sepharose beads were washed 3 times with a lysis buffer at 4°C. The loading buffer was added to the Sepharose beads, and the samples were boiled for 5 min to release the antibody/antigen complexes from the beads before analysis by western blot as described above.
Results
Geminin can be inducibly expressed in ESC
To explore the effects of increasing levels of Geminin protein in ESC, we created cell lines that express Geminin when Dox is removed from the culture medium (Tet-off; Fig. 1A). Twenty-four Geminin and 24 control (puromycin-resistant cells that inducibly express only Venus yellow fluorescent protein) ESC lines were cloned and expanded for further study. Accurate targeting was verified by testing for both hygromycin sensitivity and puromycin resistance, as well as verification that Geminin was overexpressed only in the absence of Dox. Several lines were selected for additional study based on Dox-regulated Geminin protein overexpression and/or Venus protein expression. Geminin protein levels were increased 2 days after Dox withdrawal, and by 4 days, Geminin expression was significantly elevated compared to control ESC and remained overexpressed at 6 days (Fig. 1B). These data indicate that the Geminin protein can be reliably overexpressed in response to Dox withdrawal.
Geminin promotes neural precursor and neuronal differentiation in monolayer culture
To examine their neural differentiation potential, cells were grown in defined, neural-permissive medium at low density for 6 days of transgene induction. In all 3 lines tested, compared with the same uninduced cell lines, Geminin overexpression promoted differentiation of both Sox3+ neural precursors and Tuj1-positive primitive neurons (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). ImageJ analysis of cell numbers indicated that there are modest increases in the number of Sox3-positive neural precursors compared with uninduced control cells (P≤0.12) and a small, but not significant (P≤0.08), increase in Tuj1-positive neurons in Geminin-expressing, compared with uninduced, cells.
Geminin promotes EMT and mesendodermal differentiation in EBs
To examine the role of the Geminin protein in ESC differentiation, we also employed a three-dimensional (3D) differentiation assay. Cells were plated in nonadherent Petri dishes in the absence of LIF and Dox, but with 10% serum, promoting the formation of EBs and inducing differentiation. Because some tetracycline derivatives, including Dox, inhibit matrix metalloproteinases [19], potentially reducing EMT in control cells grown in the presence of Dox, we compared 3 Geminin-inducible lines and a control line that lacked the inserted Geminin cDNA both cultured without Dox.
Most Geminin-overexpressing EBs attached to the nonadherent Petri dish 3 to 4 days after Dox withdrawal, and the cells rapidly migrated from the EB (Fig. 2). However, the vast majority of control EBs remained intact and floating for the entire 6-day assay. Therefore, to force the attachment of control EBs and promote differentiation, cells were grown as floating EBs for 4 days and then replated onto adherent tissue culture dishes for an additional 2 days before fixation for IHC or harvesting of RNA. Geminin EBs expressed strikingly less E-cadherin protein compared with control EBs (Fig. 2A) and downregulated E-cadherin at the level of transcription in quantitative PCR (Fig. 3B). We also examined E-cadherin and N-cadherin expression by western blot in 3 cell lines cultured as EBs with and without Dox for 2, 4, and 6 days (Fig. 5A), which identified a reduction in E-cadherin protein levels after 4 and 6 days of transgene induction and a concomitant upregulation of N-cadherin protein and mRNA 6 days after transgene induction.
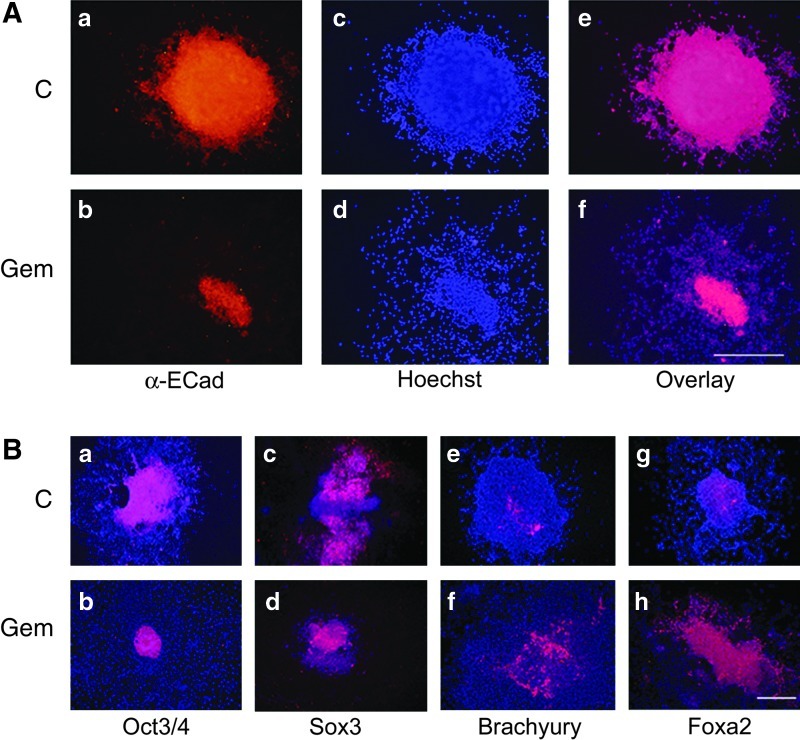
FIG. 2.
In three-dimensional culture, Geminin promotes epithelial-to-mesenchymal transition (EMT) and mesendodermal differentiation. (A) Immunohistochemical localization of E-Cadherin in a control (C) cell line and in Geminin (Gem) ESC grown without Dox as embryoid bodies (EBs) for 4 days, followed by 2 days in adherent culture. Compared to controls that express high levels of E-Cadherin (a), Geminin overexpression dramatically downregulated the expression of E-Cadherin protein (b), a hallmark of cells that have undergone EMT. Control EB have attached, but cells have not yet migrated from them as illustrated by Hoechst staining (c), unlike Geminin-overexpressing EBs that attach, and cells migrate rapidly from the EB (d). (e) and (f) are overlays of ac and bd. Scale bar=200 μM. (B) Localization of cell-type-restricted proteins in control (C) and Geminin (Gem) ESC grown as in (A) above. Geminin overexpression decreased the expression of the pluripotency marker (Oct3/4; a, b) and of a neural precursor marker (Sox3; c, d), but increased expression of a mesodermal marker (Brachyury; e, f) and a marker of the endoderm lineage (Foxa2; g, h). The nuclei were stained with Hoechst 33258 (blue). Scale bar=200 μM. Color images available online at www.liebertpub.com/scd
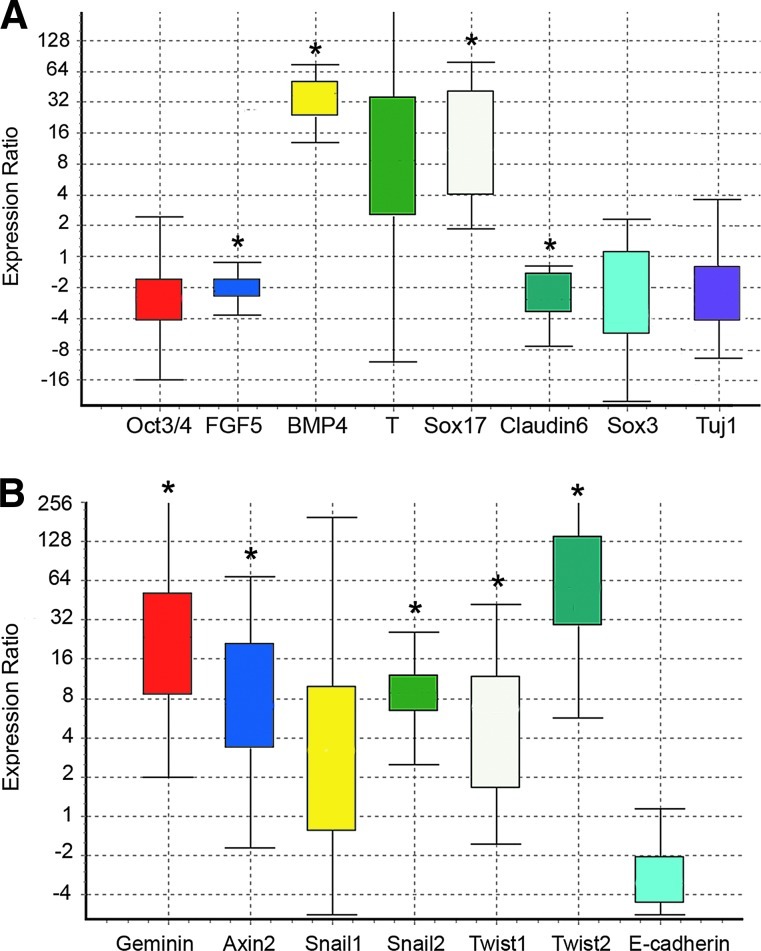
FIG. 3.
Analysis of gene expression in Geminin versus control EBs. Quantitative PCR of mRNAs from EBs grown for 6 days as described in Fig. 2A. Box-and-whisker plots were produced with REST software [18]. The top and bottom whiskers indicate the range, while the box indicates the upper- and lower-quartile values. Gene expression was calculated relative to β-actin and set to 1 in the control ESC line. Asterisks indicate statistically significant differences (Geminin vs. control, P≤0.05) calculated by the REST software (n=3 replicates). (A) Geminin overexpression decreased expression of the pluripotency gene Oct3/4 (−2.5-fold) and of the epiblast gene FGF5 (−2.0-fold). There was increased expression of the mesendodermal markers BMP4 (35.2-fold) and Sox17 (12.8-fold), and there was a trend toward increasing Brachyury expression (10.3-fold) in Geminin EBs. Finally, there was reduced expression of the epidermal ectoderm marker Claudin6 (−2.5-fold) and of the neural precursor marker Sox3 (−2.5), and of Tuj1 (−2.3-fold). (B) Geminin overexpression (21.9-fold) increased the expression of the Wnt pathway target Axin2 (7.7-fold). There was a trend toward increased Snail1 expression (3.1-fold), as well as significant alterations in several other genes involved in EMT after Geminin overexpression, including Snail2 (8.8-fold), Twist1 (4.8-fold), and Twist2 (73.4-fold). E-cadherin expression was decreased 3.3-fold in Geminin cells compared to control cells. Color images available online at www.liebertpub.com/scd
FIG. 5.
Geminin overexpression stimulates Wnt signaling. (A) Geminin overexpression promotes expression of N-cadherin and decreases expression of E-cadherin. (B) Geminin strikingly upregulated Wnt signaling, to nearly the same level as in control cells treated with the GSK3β inhibitor Chir99021 (chiron—a Wnt pathway agonist), as assessed in luciferase assays. The mean change (Relative Light Units TOP Flash/FOP Flash) in the Geminin line was significantly higher than the control line grown without Dox (**P≤0.01) as well as in control cells+chiron compared with control cells (*P≤0.02) Student's t-test (n=5). Error bars represent standard error of the mean. (D–F) Geminin does not influence expression of Wnt pathway members, but binds nuclear Transducin-Like Enhancer of split (TLE) proteins. Control (C) and Geminin (Gem) mESC were grown 2 (−2 Dox), 4 (−4 Dox), and 6 (−6 Dox) days without Dox to induce transgene expression, and then protein was harvested and used in western blotting. There was a little change in the expression or localization of activated β-catenin (C, D) of TLE proteins that function to inhibit Tcf/Lef function (E) or of Tcf3, an inhibitory Tcf/Lef factor (F), with Geminin overexpression. However, there was a consistent interaction of Geminin with TLE proteins in samples grown 4 days without Dox as assayed by coimmunoprecipitation using an anti-Geminin antibody to pull-down and anti-TLE to blot (G). (D) Control (C), and 2 Geminin transgenic cell lines (G12 and G17) were grown + Dox (negative controls), were grown in the absence of Dox (−Dox) to induce Geminin transgene expression, or were treated with the GSK3β inhibitor chiron (+Chir) to stimulate the Wnt pathway. Cells that expressed nuclear β-catenin and Hoechest 33258 were counted, and data expressed as a percentage of the β-catenin+/Hoechst + cells (% nuclear β-cat+).
When the expression of lineage-restricted markers was examined by IHC (Fig. 2B), compared with controls, Geminin overexpression decreased the expression of the pluripotent stem cell marker Oct3/4 and of the neural precursor gene Sox3, while the mesodermal marker Brachyury and the endoderm-restricted protein Foxa2 increased (Fig. 2B). We also examined the expression of lineage-restricted markers (Fig. 3A) and genes involved in EMT (Fig. 3B) in quantitative PCR. Geminin overexpression decreased Oct3/4 and the epiblast marker FGF5, indicating that the Geminin-overexpressing cells differentiated more rapidly than control ESC. Markers of the mesendodermal lineage, BMP4, Brachyury, and Sox17, were significantly increased, whereas the epidermal ectoderm marker Claudin6, Sox3, and the neuronal differentiation marker Tuj1 were decreased. Expression of genes involved in EMT, Snail1, Snail2, Twist1, and Twist2, was also increased in Geminin-overexpressing cells, and E-cadherin downregulated. Taken together, these data indicate that Geminin controls the expression of genes involved in EMT.
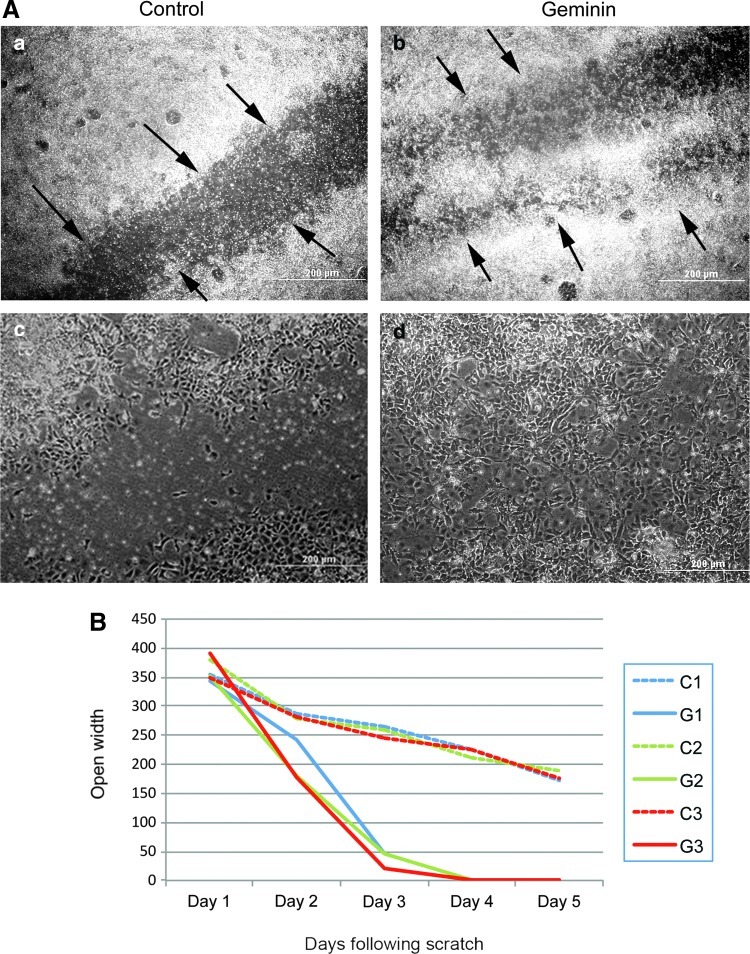
A wounding assay was used (Fig. 4) to characterize the highly migratory phenotype resulting from Geminin overexpression. Geminin-overexpressing cells (n=3 transgenic lines±Dox) migrated rapidly from the edge of a scraped monolayer; after 4 days, the denuded region had been completely recolonized by Geminin-overexpressing ESC, while in uninduced control cultures after 5 days, a mean gap of 178.3±7.1 μm remained (Fig. 5). This is consistent with our observations that Geminin promotes rapid EMT both in EBs and in the early embryo [20], and with the induction of genes involved in cell migration in Geminin-overexpressing ESC (Fig. 3B).
FIG. 4.
Geminin promotes rapid recolonization of a scrape wound. (A) Low- (a, b) and high- (c, d) magnification phase-contrast micrographs of Control (a, c) and Geminin-overexpressing (b, d) ES cells, demonstrating that Geminin overexpression produces highly migratory cells that rapidly colonize a wound in the cell monolayer after 4 days. Arrows=edge of scraped cells. Scale bars=200 μM. (B) Three lines of Geminin ESC (lines 12, 14, and 17) grown±Dox were tested for their ability to close a scrape wound. When Geminin was overexpressed, cells (n=3 lines, G1, G2, and G3) completely closed a 350-μM wound in the cell monolayer a full 2 days earlier than uninduced ESC (dashed lines, C1, C2, and C3). On day 1, the mean gap Control versus Geminin was 362±13.1 micrometers versus 361±20.8; on day 2: 282.3±4.2 versus 199.3±29.4, P≤0.01; on day 3: 250.7±6.6 versus 37±12, P≤1.4×10−6; by day 4 of culture controls: 220.7±6.8; and on day 5: 178.3±7.1 μM. Color images available online at www.liebertpub.com/scd
Geminin does not affect trilineage differentiation in teratomas
Geminin overexpression increased the rate at which teratomas formed in NSG mice, but all the 3 germ lineages were present in the tumors. Of the 6 subcutaneous injections, all formed teratomas >1 cm in diameter after 3 weeks, while injection of uninduced ESC in 4 flanks produced similar-sized tumors after 4–6 weeks in vivo. Histological analysis of the teratomas indicated that the Geminin-overexpressing teratomas contained derivatives of all 3 germ layers, but also contained undifferentiated Oct3/4-positive cells, and did not exhibit the complex tissue differentiation characteristic of control teratomas, possibly because of the short interval before the animals were sacrificed (data not shown). Teratomas developed from control ESC contained a complex mixture of endoderm, mesoderm (muscle and cartilage), and neuronal cells (data not shown). The tet-off system is particularly useful in transplantation paradigms, as it insures continued expression of the transgene in vivo, unlike constructs that require the continued presence of an inducing agent.
Geminin overexpression stimulates Wnt signaling in ESC
Because Wnt signaling plays a critical role in formation of the primitive streak and mesendodermal differentiation in the early mouse embryo, we examined the status of Wnt pathway signaling in Geminin-overexpressing ESC. Induction of Geminin protein expression significantly increased canonical Wnt signaling in the TopFlash luciferase assay compared to controls (Fig. 5B, Control vs. Geminin, **P≤0.01), which was approximately equal to the increase in Wnt signaling produced by exposure of control cells to the Gsk3β inhibitor Chir99021 (Control+chiron vs. Geminin, P≤0.70, and Control vs. Control+chiron *P≤0.02), previously shown to promote Wnt signaling [21]. In addition, a well-characterized canonical Wnt target gene, Axin2 [22–25], was upregulated over 7-fold after Dox withdrawal in an EB culture (Fig. 3B). Geminin ES cells failed to activate the TopFlash reporter, and Axin2 gene expression was unchanged in the presence of Dox (data not shown). These data indicate that in ESC, Geminin overexpression functions to stimulate Wnt signaling as was observed in Wnt indicator mice [20]. Since BMP signaling is also required for mesendodermal differentiation in the mouse embryo, we examined BMP4 and Axin2 expression after 3 days of EB differentiation by quantitative PCR. Axin2 was increased 57-fold, while BMP4 was only increased 3-fold, suggesting that Geminin promotes Wnt signaling that may in turn upregulate BMP4 expression.
To examine the mechanisms underlying Geminin's ability to stimulate Wnt signaling, we examined the percentage of cells in which β-catenin was present in the nucleus, indicating pathway activation. In the presence of Dox, 26.4%±0.7% of Control, 27.7%±1.4% of Geminin line12, and 26.1%±0.7% Geminin line17 cells exhibited nuclear β-catenin. The pathway agonist chiron strongly stimulated nuclear localization of β-catenin: 89.0%±3.0% of Control, 90.7%±4.0% of G12, and 88±0.6% of the G17 cells exhibited nuclear β-catenin. When grown in the absence of Dox to activate transgene expression, there were baseline levels of nuclear β-catenin in both Control cells (27.1%±0.9%) and in Geminin-overexpressing cells (28.3%±1.1%; Line G12, and 26.7%±1.9%; Line G17) (Fig. 5D). Differences between the means were not statistically significant. These data suggest that Geminin stimulates Wnt signaling via a mechanism that is unlikely to involve β-catenin.
Geminin may induce Wnt signaling by binding TLE proteins
To begin to identify the mechanisms by which Geminin promotes Wnt signaling, we first examined the expression of the Wnt pathway members. Geminin did not dramatically increase the level of activated β-catenin protein (Fig. 5C) or affect its localization (Fig. 5D), suggesting that Geminin does not regulate expression of Wnt ligands or inhibit GSK3β. Expression of TLE proteins, which are required for Wnt pathway inhibition, was largely unchanged by Geminin overexpression (Fig. 5E). There was a small, but consistent, increase in expression of Tcf3 (Fig. 5F), which typically inhibits the Wnt pathway, in response to increased Geminin. While this may seem inconsistent with the observed increase in the Wnt pathway activity, it is likely a negative feedback mechanism.
Since Geminin has been reported to bind proteins in the nucleus and inhibit their function, we performed coimmunoprecipitation experiments with Tcf3 and Geminin antibodies, but were unable to detect an interaction (data not shown). However, there was a weak, but consistent, interaction of Geminin with TLE proteins. Based on this interaction, we hypothesize that Geminin may bind and inhibit TLE proteins from repressing Wnt signaling in the nucleus, thereby activating Wnt target gene expression.
Geminin reduces proliferation and alters cell cycle of ESC
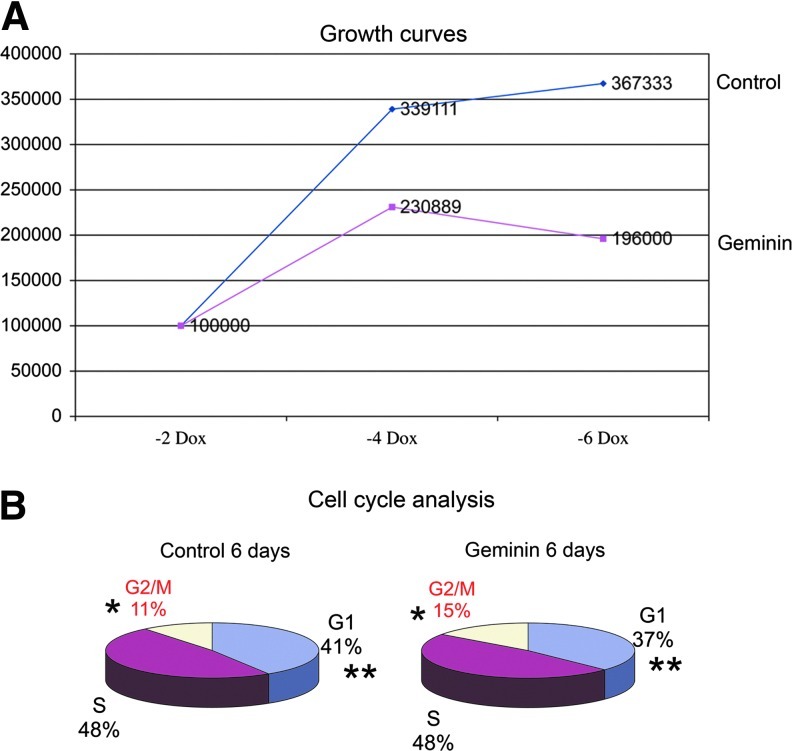
Since Geminin inhibits DNA replication to prevent endoreduplication, we assayed proliferation and cell cycle characteristics of Geminin and control ESC in monolayer culture. In the presence of Dox, Geminin cell lines grow similarly to the control cell line (data not shown). However, overexpression of Geminin significantly reduced proliferation (Fig. 6A), increased the length of time the cells spent in the G2/M phase of the cell cycle, and reduced the time spent in the G1 phase (Fig. 6B). Decreased proliferation may itself promote differentiation, since exit from the cell cycle is required for many cell fate decisions. Additionally, cells may be unable to progress from G2/M, because they are unable to initiate new rounds of DNA replication, since Geminin is normally destroyed by the proteasome during the M phase. Clearly, Geminin plays an important role in controlling cell cycle and proliferation in ESC similar to its role in the inner cell mass.
FIG. 6.
Geminin overexpression decreases proliferation and increases the length of the G2/M phase. (A) Control and Geminin ESC were grown for 2 days in the absence of LIF and Dox and then plated to assay proliferation. Geminin overexpression reduced proliferation at 4 (*P≤0.005) and 6 days (**P≤0.0001) after Dox withdrawal; Student's t-test, n=3. (B) Cell cycle analysis after 6 days of Dox withdrawal. Geminin overexpression increased the length of G2/M (*P≤0.05) and decreased the length of G1 (**P≤0.03 Student's t-test, n=3). Color images available online at www.liebertpub.com/scd
Geminin shRNA results in DNA damage and cell death
To determine if Geminin protein is necessary for Wnt signaling, lineage differentiation, and/or EMT, we employed shRNAs (Fig. 7A) to reduce Geminin protein levels. We were able to achieve a 6-fold knockdown of Geminin protein 24 h post-transfection (Fig. 7B); however, cells transfected with the Geminin shRNA exhibited increased DNA damage as illustrated by phosphohistone H2A.X antibody staining (Fig. 7C), precluding the analysis of differentiation and Wnt signaling. Cells transfected with the control shRNA showed some DNA damage (11.4%±0.6%) that increased dramatically when Geminin was knocked down (84.3%±0.1%), P≤0.001.
Discussion
Geminin has previously been characterized by its ability to promote neural differentiation at the expense of alternative lineages in Xenopus [8,14] and Drosophila embryos [26]. Our results demonstrate that the outcome of Geminin overexpression in ESC is highly context dependent: in a sparse monolayer culture, in defined medium, it can promote neural differentiation, while in 3D culture, as EBs with serum, it promotes EMT and mesendodermal differentiation.
Given its context-dependent effects, it is not surprising that 2 recent reports of Geminin gain and loss of function in ESC come to remarkably different conclusions. The first proposed that Geminin escapes degradation in ESC and inhibits the action of Brg1 to sustain Oct4, Sox2, and Nanog expression, thereby maintaining pluripotency [27]. The second study reported that Geminin controls neuronal differentiation via regulation of chromatin, although without corresponding alterations in protein expression or in promoting differentiation [28]. Low levels of overexpression and downregulation obtained in that study may have created a state of chromatin readiness to respond to lineage differentiation factors, rather than neuronal differentiation per se. Recently, targeted deletion of Geminin in neural stem cells suggested that in vivo Geminin may have a primary role in cycle regulation rather than in lineage choice [29], although another analysis demonstrated that deletion expanded the neural precursor population, and overexpression promoted migration and premature neuronal differentiation [30].
Our overexpression studies consistently demonstrate that Geminin promotes, rather than inhibits, differentiation in EBs; however, it remains possible that the outcome is dependent on the level of expression or on the differentiation state of the cell—producing different outcomes in ESC versus lineage-committed neural precursors. Additionally, we were unable to assess ESC differentiation in the absence of Geminin protein, as we observed DNA damage in shRNA transfected cells, likely not observed using siRNAs, since Geminin is degraded and resynthesized during each cycle.
Geminin has been shown to bind and inhibit the function of several nuclear factors, including AP4 [31], the chromatin-remodeling protein Brg-1 [32], Hox proteins [33], and Six3 [34]. Geminin also has the potential to act as a molecular switch between proliferation and differentiation by binding additional transcription factors [35], including TLE proteins. Tcf/Lef transcription factors inhibit Wnt signaling in the absence of nuclear β-catenin [36,37], and our results suggest that Geminin may inhibit interactions between Tcf/Lefs and TLEs to de-repress Wnt signaling.
Wnt signaling is critically involved in establishing the primitive streak and promoting the EMT required for mesendodermal differentiation in the embryo [38–44]. The transcriptional repressors Snail1 and Snail2 both downregulate the expression of E-cadherin, and in the absence of Snail1, mesendoderm is still specified; however, gastrulation fails because cells are unable to leave the primitive streak [44,45]. It has been hypothesized that downregulation of E-cadherin releases β-catenin from cytoskeletal junctional complexes, increasing Wnt signaling [46,47] that in turn feeds back to increase Snail1 [48–52]. However, it appears that in our EB model, Geminin de-represses Wnt signaling to promote Snail1 and Snail2 expression, suggesting that Geminin should now be included in the list of genes that initiate EMT and mesendodermal differentiation.
Wnt signaling is required for the proliferation of neural precursors [53–56], for neuronal differentiation [57,58], and patterning of the developing nervous system [59,60]. The key bHLH transcription factors that drive neuronal specification, including Neurog1 and NeuroD1, are transcriptional targets of the canonical Wnt pathway [54,57,61]. Therefore, while Wnt signal inhibition may initially be required to promote neural precursor differentiation by inhibiting mesendodermal lineage differentiation, active signaling appears to be required for precursor proliferation and neuronal differentiation [62].
Wnt signaling has previously been shown to be required for the differentiation of ESC to mesendoderm [63–65] and is also implicated in the switch from the pluripotent to the differentiated state via Tcf3 binding to a protein complex with Oct4, Sox2, and Nanog on gene promoters [66–68]. In many cell types, cell fate decisions are tightly coupled to the cell cycle exit [36,69,70], and the most crucial decision in the cell cycle, that is, to proliferate, differentiate, quiesce, senesce, or apoptose, occurs at the G1 checkpoint [71]. The attenuated G1 phase in adult stem cells, ESC, and the epiblast may prevent differentiation by insulating cells from growth factor exposure [72–74]. Overexpression of Geminin decreased proliferation and increased the length of G2/M in both ESC (above) and in early postimplantation embryos [20]. Persistent Geminin protein expression without cyclic degradation is likely to maintain the sequestration of Cdt1 and block progression from the M phase. In turn, lengthening G2/M would increase the time Geminin is available to interact with other proteins in the nucleus and promote differentiation. Our results suggest that Geminin, via its ability to de-repress Wnt signaling, may be a key regulatory factor controlling cell cycle, EMT, and differentiation at gastrulation.
Geminin expression has been negatively correlated with patient prognosis in several cancers [10,75,76], where it may promote EMT and therefore metastasis. In fact, when Geminin was overexpressed in the MCF-10 nontumorigenic mammary epithelial cell line, there was increased proliferation and anchorage-independent growth [77,78]. Knockdown of Geminin protein might therefore be a powerful approach to both inhibit metastasis and initiate endoreduplication and apoptotic cell death in dividing tumor cells.
Supplementary Material
Acknowledgments
The authors are great to Brian Magnuson and Diane Fingar for help with western blotting and immunoprecipitation, Maria Morell for cell cycle analysis and DNA damage assessment, Lisa S.D. Emmett for developing ESC lines, Yao-Chang Tsan for thoughtful discussions, Mike Klymkowsky for Sox3 antibody, and Yuji Masui for cell lines and plasmids, the Flow Core at the University of Michigan; supported by the NIH grants RR-023187 and NS-39438.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ciemerych MA. Sicinski P. Cell cycle in mouse development. Oncogene. 2005;24:2877–2898. doi: 10.1038/sj.onc.1208608. [DOI] [PubMed] [Google Scholar]
- 2.Stead E. White J. Faast R. Conn S. Goldstone S. Rathjen J. Dhingra U. Rathjen P. Walker D. Dalton S. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 2002;21:8320–8333. doi: 10.1038/sj.onc.1206015. [DOI] [PubMed] [Google Scholar]
- 3.Burdon T. Smith A. Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 4.White J. Stead E. Faast R. Conn S. Cartwright P. Dalton S. Developmental activation of the Rb-E2F pathway and establishment of cell cycle-regulated cyclin-dependent kinase activity during embryonic stem cell differentiation. Mol Biol Cell. 2005;16:2018–2027. doi: 10.1091/mbc.E04-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans MJ. Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 6.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGarry TJ. Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 8.Kroll KL. Salic AN. Evans LM. Kirschner MW. Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development. 1998;125:3247–3258. doi: 10.1242/dev.125.16.3247. [DOI] [PubMed] [Google Scholar]
- 9.Luo L. Kessel M. Geminin coordinates cell cycle and developmental control. Cell Cycle. 2004;3:711–714. [PubMed] [Google Scholar]
- 10.Wohlschlegel JA. Kutok JL. Weng AP. Dutta A. Expression of geminin as a marker of cell proliferation in normal tissues and malignancies. Am J Pathol. 2002;161:267–273. doi: 10.1016/S0002-9440(10)64178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez MA. Tachibana KE. Adams DJ. van der Weyden L. Hemberger M. Coleman N. Bradley A. Laskey RA. Geminin is essential to prevent endoreduplication and to form pluripotent cells during mammalian development. Genes Dev. 2006;20:1880–1884. doi: 10.1101/gad.379706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara K. Nakayama KI. Nakayama K. Geminin is essential for the development of preimplantation mouse embryos. Genes Cells. 2006;11:1281–1293. doi: 10.1111/j.1365-2443.2006.01019.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor JJ. Wang T. Kroll KL. Tcf- and Vent-binding sites regulate neural-specific geminin expression in the gastrula embryo. Dev Biol. 2006;289:494–506. doi: 10.1016/j.ydbio.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 14.Lim JW. Hummert P. Mills JC. Kroll KL. Geminin cooperates with Polycomb to restrain multi-lineage commitment in the early embryo. Development. 2011;138:33–44. doi: 10.1242/dev.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brantjes H. Roose J. van De Wetering M. Clevers H. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 2001;29:1410–1419. doi: 10.1093/nar/29.7.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Shea KS. De Boer LS. Slawny NA. Gratsch TE. Transplacental RNAi: deciphering gene function in the postimplantation-staged embryo. J Biomed Biotechnol. 2006;2006:18657. doi: 10.1155/JBB/2006/18657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masui S. Shimosato D. Toyooka Y. Yagi R. Takahashi K. Niwa H. An efficient system to establish multiple embryonic stem cell lines carrying an inducible expression unit. Nucleic Acids Res. 2005;33:e43. doi: 10.1093/nar/gni043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaffl MW. Horgan GW. Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin MO. Ceballos G. Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63:102–107. doi: 10.1016/j.phrs.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmett LS. O'Shea KS. Geminin is required for epithelial to mesenchymal transition at gastrulation. Stem Cells Dev. 2012;21:2395–2409. doi: 10.1089/scd.2011.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finlay D. Patel S. Dickson LM. Shpiro N. Marquez R. Rhodes CJ. Sutherland C. Glycogen synthase kinase-3 regulates IGFBP-1 gene transcription through the thymine-rich insulin response element. BMC Mol Biol. 2004;5:15. doi: 10.1186/1471-2199-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes TA. Brady HJ. Regulation of axin2 expression at the levels of transcription, translation and protein stability in lung and colon cancer. Cancer Lett. 2006;233:338–347. doi: 10.1016/j.canlet.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Jho EH. Zhang T. Domon C. Joo CK. Freund JN. Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung JY. Kolligs FT. Wu R. Zhai Y. Kuick R. Hanash S. Cho KR. Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 25.Yan D. Wiesmann M. Rohan M. Chan V. Jefferson AB. Guo L. Sakamoto D. Caothien RH. Fuller JH, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn LM. Herr A. McGarry TJ. Richardson H. The Drosophila Geminin homolog: roles for Geminin in limiting DNA replication, in anaphase and in neurogenesis. Genes Dev. 2001;15:2741–2754. doi: 10.1101/gad.916201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang VS. Carter SA. Hyland SJ. Tachibana-Konwalski K. Laskey RA. Gonzalez MA. Geminin escapes degradation in G1 of mouse pluripotent cells and mediates the expression of Oct4, Sox2, and Nanog. Curr Biol. 2011;21:692–699. doi: 10.1016/j.cub.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yellajoshyula D. Patterson ES. Elitt MS. Kroll KL. Geminin promotes neural fate acquisition of embryonic stem cells by maintaining chromatin in an accessible and hyperacetylated state. Proc Natl Acad Sci U S A. 2011;108:3294–3299. doi: 10.1073/pnas.1012053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz KM. Banisadr G. Lastra RO. McGuire T. Kessler JA. Miller RJ. McGarry TJ. Geminin-deficient neural stem cells exhibit normal cell division and normal neurogenesis. PLoS One. 2011;6:e17736. doi: 10.1371/journal.pone.0017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spella M. Kyrousi C. Kritikou E. Stathopoulou A. Guillemot F. Koussis D. Pachnis VP. Lygerou Z. Taraviras S. Geminin regulates cortical progenitor proliferation and differentiation. Stem Cells. 2011;29:1269–1282. doi: 10.1002/stem.678. [DOI] [PubMed] [Google Scholar]
- 31.Kim MY. Jeong BC. Lee JH. Kee HJ. Kook H. Kim NS. Kim YH. Kim JK. Ahn KY. Kim KK. A repressor complex, AP4 transcription factor and geminin, negatively regulates expression of target genes in nonneuronal cells. Proc Natl Acad Sci U S A. 2006;103:13074–13079. doi: 10.1073/pnas.0601915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo S. Herr A. Lim JW. Richardson GA. Richardson H. Kroll KL. Geminin regulates neuronal differentiation by antagonizing Brg1 activity. Genes Dev. 2005;19:1723–1734. doi: 10.1101/gad.1319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo L. Yang X. Takihara Y. Knoetgen H. Kessel M. The cell-cycle regulator geminin inhibits Hox function through direct and polycomb-mediated interactions. Nature. 2004;427:749–753. doi: 10.1038/nature02305. [DOI] [PubMed] [Google Scholar]
- 34.Del Bene F. Tessmar-Raible K. Wittbrodt J. Direct interaction of geminin and Six3 in eye development. Nature. 2004;427:745–749. doi: 10.1038/nature02292. [DOI] [PubMed] [Google Scholar]
- 35.Pitulescu M. Kessel M. Luo L. The regulation of embryonic patterning and DNA replication by geminin. Cell Mol Life Sci. 2005;62:1425–1433. doi: 10.1007/s00018-005-4553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniels DL. Weiss WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 37.Blauwkamp TA. Chang MV. Cadigan KM. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 2008;27:1436–1446. doi: 10.1038/emboj.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly OG. Pinson KI. Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 39.Kemler R. Hierholzer A. Kanzler B. Kuppig S. Hansen K. Taketo MM. de Vries WN. Knowles BB. Solter D. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- 40.Liu X. Huang S. Ma J. Li C. Zhang Y. Luo L. NF-kappaB and Snail1a coordinate the cell cycle with gastrulation. J Cell Biol. 2009;184:805–815. doi: 10.1083/jcb.200806074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maretto S. Cordenonsi M. Dupont S. Braghetta P. Broccoli V. Hassan AB. Volpin D. Bressan GM. Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohamed OA. Clarke HJ. Dufort D. Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn. 2004;231:416–424. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- 43.Rivera-Perez JA. Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Carver EA. Jiang R. Lan Y. Oram KF. Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieto MA. Sargent MG. Wilkinson DG. Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- 46.Medici D. Hay ED. Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stemmer V. de Craene B. Berx G. Behrens J. Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene. 2008;27:5075–5080. doi: 10.1038/onc.2008.140. [DOI] [PubMed] [Google Scholar]
- 48.Bachelder RE. Yoon SO. Franci C. de Herreros AG. Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray SA. Gridley T. Snail1 gene function during early embryo patterning in mice. Cell Cycle. 2006;5:2566–2570. doi: 10.4161/cc.5.22.3502. [DOI] [PubMed] [Google Scholar]
- 50.Sakai D. Tanaka Y. Endo Y. Osumi N. Okamoto H. Wakamatsu Y. Regulation of Slug transcription in embryonic ectoderm by beta-catenin-Lef/Tcf and BMP-Smad signaling. Dev Growth Differ. 2005;47:471–482. doi: 10.1111/j.1440-169X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 51.Yook JI. Li XY. Ota I. Hu C. Kim HS. Kim NH. Cha SY. Ryu JK. Choi YJ, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 52.Zhou BP. Deng J. Xia W. Xu J. Li YM. Gunduz M. Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 53.Chesnutt C. Burrus LW. Brown AM. Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 54.Israsena N. Hu M. Fu W. Kan L. Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–231. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 55.Kalani MY. Cheshier SH. Cord BJ. Bababeygy SR. Vogel H. Weissman IL. Palmer TD. Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zechner D. Fujita Y. Hulsken J. Muller T. Walther I. Taketo MM. Crenshaw EB., 3rd Birchmeier W. Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 57.Hirabayashi Y. Itoh Y. Tabata H. Nakajima K. Akiyama T. Masuyama N. Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 58.Muroyama Y. Fujihara M. Ikeya M. Kondoh H. Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ciani L. Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 60.Nordstrom U. Jessell TM. Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- 61.Kuwabara T. Hsieh J. Muotri A. Yeo G. Warashina M. Lie DC. Moore L. Nakashima K. Asashima M. Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slawny NA. O'Shea KS. Dynamic changes in Wnt signaling are required for neuronal differentiation of mouse embryonic stem cells. Mol Cell Neurosci. 2011;48:205–216. doi: 10.1016/j.mcn.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gadue P. Huber TL. Paddison PJ. Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindsley RC. Gill JG. Kyba M. Murphy TL. Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 65.Nakanishi M. Kurisaki A. Hayashi Y. Warashina M. Ishiura S. Kusuda-Furue M. Asashima M. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 2009;23:114–122. doi: 10.1096/fj.08-111203. [DOI] [PubMed] [Google Scholar]
- 66.Cole MF. Johnstone SE. Newman JJ. Kagey MH. Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pereira L. Yi F. Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–7491. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tam WL. Lim CY. Han J. Zhang J. Ang YS. Ng HH. Yang H. Lim B. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells. 2008;26:2019–2031. doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grosshans J. Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell. 2000;101:523–531. doi: 10.1016/s0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- 70.Seher TC. Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol. 2000;10:623–629. doi: 10.1016/s0960-9822(00)00502-9. [DOI] [PubMed] [Google Scholar]
- 71.Blomen VA. Boonstra J. Cell fate determination during G1 phase progression. Cell Mol Life Sci. 2007;64:3084–3104. doi: 10.1007/s00018-007-7271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becker KA. Ghule PN. Therrien JA. Lian JB. Stein JL. Van Wijnen A. Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–893. doi: 10.1002/jcp.20776. [DOI] [PubMed] [Google Scholar]
- 73.Burdon T. Stracey C. Chambers I. Nichols J. Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol. 1999;210:30–43. doi: 10.1006/dbio.1999.9265. [DOI] [PubMed] [Google Scholar]
- 74.Orford KW. Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 75.Nishihara K. Shomori K. Tamura T. Fujioka S. Ogawa T. Ito H. Immunohistochemical expression of geminin in colorectal cancer: implication of prognostic significance. Oncol Rep. 2009;21:1189–1195. doi: 10.3892/or_00000340. [DOI] [PubMed] [Google Scholar]
- 76.Xouri G. Lygerou Z. Nishitani H. Pachnis V. Nurse P. Taraviras S. Cdt1 and geminin are down-regulated upon cell cycle exit and are over-expressed in cancer-derived cell lines. Eur J Biochem. 2004;271:3368–3378. doi: 10.1111/j.1432-1033.2004.04271.x. [DOI] [PubMed] [Google Scholar]
- 77.Montanari M. Boninsegna A. Faraglia B. Coco C. Giordano A. Cittadini A. Sgambato A. Increased expression of geminin stimulates the growth of mammary epithelial cells and is a frequent event in human tumors. J Cell Physiol. 2005;202:215–222. doi: 10.1002/jcp.20120. [DOI] [PubMed] [Google Scholar]
- 78.Aokage K. Ishii G. Ohtaki Y. Yamaguchi Y. Hishida T. Yoshida J. Nishimura M. Nagai K. Ochiai A. Dynamic molecular changes associated with epithelial-mesenchymal transition and subsequent mesenchymal-epithelial transition in the early phase of metastatic tumor formation. Int J Cancer. 2011;128:1585–1595. doi: 10.1002/ijc.25500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.