Abstract
Galectin-3 (Gal-3), a member of the β-galactoside-binding gene family, distributes inside and outside the cell and has pleiotropic biological functions such as cell growth, cell adhesion, cell–cell interaction, and mRNA processing in a specific situation. In particular, Gal-3 in the nucleus plays a pivotal role in the regulation of cancer-related gene expression, including cyclin D1, TTF-1 and MUC2, presumably associated with tumor progression. Therefore, to understand the mechanism of nuclear import of Gal-3 is very significant and might be developed to the new approach for the cancer treatment. In this review, we focus on the role of Gal-3 in the nucleus and the molecular mechanism of nuclear import pathways of Gal-3, providing the hints for the inhibition of Gal-3 function.
Keywords: Galectin-3, Transcription factor, Gene expression, Nuclear import
1 Introduction
Galectins are a family of carbohydrate-binding proteins that exhibit diverse biological activities [1, 2]. These proteins contain at least one conserved carbohydrate recognition domain (CRD) of about 130 amino acids and are categorized into three major groups based on the organization of CRD; the prototype, the chimera type and the tandem repeat type [3]. So far, 15 mammalian members of the galectin family have been isolated and identified [2].
Probably, the most studied member of the galectin family concerning with cancer is probably galectin-3 (Gal-3). Gal-3 has the unique structure in the family, i.e. the sole chimera type of galectin, and consists of three distinct structural domains as follows; (1) a short N-terminal domain that contains a serine phosphorylation site, (2) a repeated consensus sequence of glycine, tyrosine and proline, and (3) a C-terminal domain that contain one carbohydrate recognition domain (CRD) [1–3]. It is ubiquitously expressed and has multiple biological functions depending on its subcellular localization. In general, extracellular Gal-3 mediates cell migration, cell adhesion, and cell–cell interactions through its carbohydrate-binding property, whereas cytoplasmic Gal-3 exerts anti-apoptotic activity and modulates several signal transduction pathways [4, 5]. Gal-3 is also found in the nucleus as a nuclear matrix protein involved in pre-messenger RNA splicing or the Hedgehog signal-transduction pathway, mainly interacting with Gemin4 and Sufu (Suppressor of fused), respectively [6–8]. These findings suggest that Gal-3 could be one of the essential factors for the normal cell proliferation and/or development in the nucleus. In addition, nuclear Gal-3 is likely to play a critical role in tumor progression through the regulation of specific gene expression interacting with the transcription factors [9–11].
Because Gal-3 is a key factor in tumor progression and metastasis, many researchers have tried to inhibit its function as a cancer treatment and developed inhibitors targeted to Gal-3 [12]. It is essential to understand the nuclear import mechanism of Gal-3 for the further development of Gal-3 function inhibition, especially in the nucleus. Recently multiple nuclear import pathways of Gal-3 using in vivo and in vitro assay systems have been shown [13, 14]. Therefore, in this review, we provide a brief overview of the role of Gal-3 in the nucleus and the molecular mechanism of its import pathway into the nucleus as it relates to the development of cancer treatment.
2 Involvement of Gal-3 in the regulation of cancer-related gene expression
In 1986, Gal-3, described as CBP-35 at that time, was found in the nucleus of mouse 3T3 fibloblast cells using an immunofluorescence staining [15]. After that, it was revealed that Gal-3 is accumulated in the nucleus of proliferating fibloblast cells and acts as a pre-mRNA splicing factor through the direct interaction with Gemin4 involving in splicesome assembly [6, 16, 17]. Thus, Gal-3 is contained as a nuclear extracts and constitutes a part of an interacting dynamic network of many factors involved in the splicing and transport of mRNA [18].
Many researchers reported the correlation between Gal-3 subcellular distribution and prognosis in various cancers using clinical samples [19–21]. These findings promoted us to investigate the function of nuclear Gal-3 in the cancer cells. Previous studies found persistent overexpression of Gal-3 in the breast cancer cells using transfection promoted the up-regulation of cyclin D1, a key molecule for the cell cycle regulation and a potential oncogene in human cancer [22]. Consequently, the role of Gal-3 on cyclin D1 gene expression was revealed and it showed that Gal-3 induces cyclin D1 promoter activity in human breast epithelial cells independent on cell adhesion. This induction of the cyclin D1 promoter by Gal-3 results from the enhancement and stabilization of nuclear protein-DNA complex formation at the SP1 and cAMP-responsive element (CRE) site of its promoter region. This study provided an evidence of function of nuclear Gal-3 in the regulation of gene transcription for a cancer cell growth promoting activity [9]. Similarly, in the nuclei of papillary thyroid cancer cells, Gal-3 directly interacts with the thyroid-specific TTF-1 transcription factor, whose expression is maintained in papillary cancer, and upregulates the transcriptional activity of TTF-1, contributing to the proliferation of the thyroid cells [10]. This stimulating activity would account for a possible molecular mechanism that Gal-3 controls proliferation in thyroid cells, resulting in cancerous status of thyroid [23]. In addition, Gal-3 can regulate MUC2 mucin expression at the transcriptional level via AP-1 activation in human colon cancer cells [11]. MUC2 mucin, a high molecular weight carbohydrate-rich glycoprotein, is a major secreted mucin in large and small intestines and expressed strongly in the patients with mucinous colorectal carcinomas. Initially, this protein was found to directly bind to Gal-3 [24]. Thereafter, it was revealed alterations in Gal-3 expression levels correlated with both MUC2 protein expression and transcriptional activity. By using MUC2 promoter constructs of different lengths, it was found that Gal-3 could react with a promoter region containing the AP-1 binding site. Detailed analyses suggested an association between Gal-3, c-Jun, and Fra-1 in forming a complex at the AP-1 site on the MUC2 promoter [11]. Thus, Gal-3 functions as an enhancer and modulator of several transcription factors to regulate the gene expression in several cancer cells.
Of note, phosphorylation of Gal-3 seems to be necessary and essential regarding with the exertion of its function in the nucleus. It was reported Gal-3 undergo phosphorylation at the residue of Ser6 by casein kinase 1 and dephosphorylation by protein phosphatase 1 [25, 26], and this phosphorylation regulates the export of Gal-3 from the nucleus [27]. Mutant Gal-3, which cannot be phosphorylated at this Ser6 site, has no effect on the upregulation of the gene expression of cyclin D1, whereas wild type Gal-3 does [28]. This result means the phosphorylation of Ser6 is a critical event for exertion of Gal-3 function as a modulator of gene expression. To support this, a recent study revealed that phosphorylation of Gal-3 contributes to malignant transformation of human epithelial cells via modulation of unique sets of genes [29]. A microarray analysis of 10,000 human genes identified 188 genes that were differentially expressed between wild type Gal-3 and phosphomutant Gal-3 transfectants of BT549 breast carcinoma cell, and, in particular, RT-PCR and immunoblot analysis confirmed that C-type lectin 2, insulin-like growth factor-binding protein 5, protease serine 3, dual specifity phosphatase 6, and cyclin D1 were upregulated in wild type Gal-3 transfectants compared to mutant Gal-3 transfectants. This suggests the promoter region of these genes is also upregulated by direct interaction of Gal-3 and phosphorylation of this protein is necessary for regulation of unique sets of genes that play a role in malignant transformation.
Taken together, Gal-3 play a crucial role in the regulation of specific gene expression dependent on a cell type through the interaction with various transcription factor and phosphorylation is required for exerting its activity (Fig. 1).
Fig. 1.
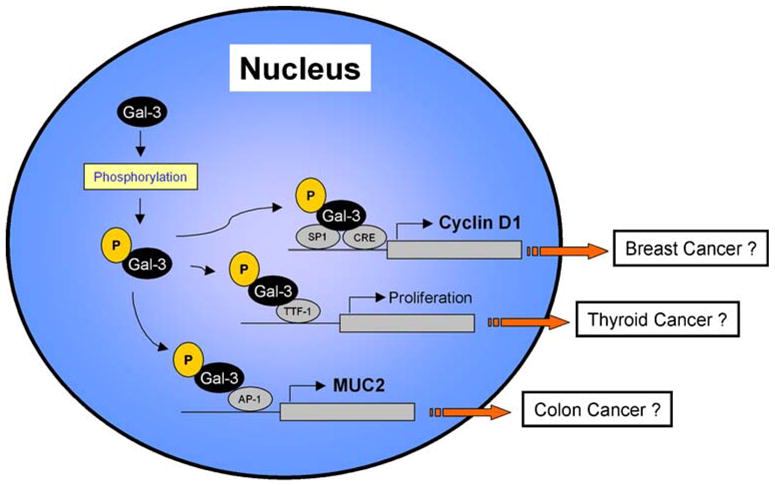
The role of galectin-3 in the nucleus for tumor progression. After galectin-3 is accumulated in the nucleus, it is phosphorylated at the Ser6 site and exerts up-regulation of several cancer-related gene expression
In many ways, this review is largely a review of our recent two papers [13, 14] and less a review of the work of others. We also neglected to indicate a number of references where needed.
3 The molecular mechanism of nuclear import pathway of Gal-3
Despite the better understanding of the function of Gal-3 in the nucleus, the detailed mechanism of how Gal-3 is imported to the nucleus had been unveiled since there is no typical nuclear localization signal (NLS) in its protein sequence [30, 31]. Regarding with nuclear import of Gal-3, three reports have pointed out the essential sequences of Gal-3 for its nuclear localization using transfection assay [32–34]. In these studies, Gong et al. concluded that deletion of the first 11 amino acids of human Gal-3, which contains a Ser6 phosphorylation site, leads to a predominant cytoplasmic distribution, whereas Gaudin et al. [33] showed that an N-terminal deletion mutant of hamster Gal-3 could be localized in the nucleus even when up to 103 amino acids were deleted. In a recent study, Davidson et al. [35] indicated that the C-terminal deletion mutant of mouse Gal-3 showed loss of nuclear localization and suggested that Gal-3 nuclear import was dependent on the IXLT type of nuclear localization sequence in the end of C-terminal region, supporting the conclusion of Gaudin et al. [33]. It seems that these inconsistencies may not result from the use of different cells or the use of different species of Gal-3, but suggest the multiple or complicated nuclear import mechanism of Gal-3 rather than the simple transport system. Moreover, all these reports cannot explain the direct pathway whereby Gal-3 enters the nucleus but just show the essential sequence for the nuclear accumulation of Gal-3 since only transfection assay using various deletion mutants was performed in these studies and Gal-3 is a nucleocytoplasmic protein shuttling between the cytoplasm and the nucleus [35].
Therefore, to examine the detailed molecular mechanism of Gal-3 nuclear import pathways, we introduced an in vitro nuclear import assay using the digitonin-permeabilized cells in addition to an in vivo transfection assay [13]. This in vitro assay was used to show the direct nuclear import mechanism of many nuclear proteins [36, 37]. Firstly, we transfected the constructs of various Gal-3 deletion mutants fused with EGFP and determined the essential domain for its nuclear localization in vivo as shown in other reports. Considering the possible passive diffusion into the nucleus of small molecule under 60 kDa, we fused monomer to trimer of EGFP (approx. 26 kDa) with human Gal-3 (approx. 32 kDa) and examined by transfection assay. We showed that deletion mutant of the last 10 amino acids from the C-terminus (1–240) fused with monomer EGFP can dramatically abrogate the nuclear accumulation, supporting the conclusion of Davison et al. However, the peptide of this last 10 amino acids of Gal-3 fused with trimer of EGFP cannot be accumulated in the nucleus in the transfected cell, suggesting the speculation of Davidson et al. that Gal-3 nuclear import was dependent on the IXLT type of nuclear localization sequence in its C-terminal end is questionable. Interestingly, the deletion mutant of entire CRD (1–115), i.e. the peptide including long N-terminal region of Gal-3, can predominantly translocate into the nucleus in the transfected cells even fused with trimer of EGFP. This result can partially support the conclusion of Gong et al., which was denied by other reports. Collectively, incomplete forms of CRD region of Gal-3 tend to abrogate the nuclear accumulation, whereas N-terminal region of Gal-3 promotes the nuclear import activity in the transfected cells.
Further examination of the nuclear import system of Gal-3, involved an in vitro nuclear import assay using recombinant Gal-3 fused with fluorescent material or EGFP in a digitonin-permeabilized cell. Recombinant wild type Gal-3 protein was prepared that is conjugated with fluorescent material (F/Gal-3) or with monomer EGFP (Gal-3/GFP), whose molecular mass is approx. 32 and 60 kDa, respectively, to discriminate the potential activity of accommodation within the nuclear pore complex by a passive diffusion system that allows free entry of small proteins up to 60 kDa. In the typical transporting system, both Gal-3 recombinant proteins were not able to migrate into the nucleus, presumably due to the stronger binding to the cell surface glycoproteins. To block this interaction, lactose, which is the inhibitor of Gal-3 binding capacity, was added to the transport buffer, and assayed in this modified condition. Interestingly, F/Gal-3 can be imported into the nucleus without any soluble factors in this condition, suggesting that wild type Gal-3 can be imported to the nucleus by a passive diffusion. One may consider that lactose addition with in vitro assay has an artificial effect to assess the nuclear import mechanisms in vivo. In the case of highly expressed Gal-3 in cancer cells or inhibition of its sugar binding capacity with a small cytoplasmic binding partner, there is the possibility of Gal-3 nuclear import by a passive diffusion. On the other hand, it was shown that Gal-3/GFP, whose molecular mass is too large to pass through the nuclear pore complex, can be imported to the nucleus by active transporting system in the condition of lactose addition. This transport seems to be mainly dependent on the N-terminal region of Gal-3, supporting the result from the transfection assay. In addition, this is independent of any soluble factor or ATP, similarly to the importin-β and β-catenin nuclear import pathway [38, 39].
Unfortunately, with this in vitro nuclear import assay system, we cannot prove another possible Gal-3 nuclear import pathway through its carbohydrate-binding capacity or dependent on the CRD since Gal-3 predominantly binds to the cell surface with the lack of lactose addition, therefore different approach is necessary to show the CRD dependent nuclear import pathway of Gal-3 if possible. We have searched the protein sequence of Gal-3 and identified an NLS-like sequence, 223HRVKKL228, in the C-terminal region of the human Gal-3, and showed the novel pathway of Gal-3 into the nucleus by importin-mediated nuclear import system [14]. Importin-α is the receptor subunit that recognizes the NLS, a cluster of basic amino acid residues that complex with importin-β for passage through the nuclear pore, meaning NLS-containing proteins, like p53, are transported into the nucleus by this importin-mediated transporting system [40, 41]. This nuclear import is rapidly and strongly performed and the localization of Gal-3 fused with SV40 T-antigen NLS is predominantly detected in the nucleus, so the interaction between Gal-3 and importin-α proteins is relatively weak through its NLS-like sequence. In this sequence, substitution of Arg to Ala at position 224 (R224A), but not the substitution of Lys to Ala at position 226 or 227 (K226A, K227A) of the human Gal-3 efficiently abolished nuclear localization in the transfected cells, indicating the pivotal role of Arg224 for Gal-3 nuclear accumulation. We also showed the direct interaction between Gal-3 and importin-α proteins dependent on this Arg224 residue using GST pull down assay and BiFC analysis. Thus, Gal-3 can also migrate into the nucleus by means of importin-α/β complex and the residue of Arg224 is a key factor for this novel pathway.
Taking this into consideration, there is also the possibility that several cytoplasmic anchor proteins lead Gal-3 nuclear translocation by a passive diffusion or an active transporting system whereas no more evidence has been shown about the concrete binding molecule of Gal-3 regarding with the nuclear translocation. Moreover, Gal-3 can form a dimer or even a pentamer in a specific situation, so it is also possible for Gal-3 to be imported by active transport as a dimer [42] or a pentamer [43] formation dependent on the condition or proliferation status of cell.
We have described a scenario for the outlines of the overall process of Gal-3 nuclear translocation including the possible mechanism and pathways (Fig. 2). After Gal-3 is synthesized in the cytoplasm, it exists mainly as a monomer form and partially as a dimer or pentamer. It also could localize in the cytoplasm by binding to the cytoplasmic anchor proteins through its β-galactoside binding activity or interact with importin-α/β complex through its NLS-like sequence. Then, Gal-3 enters the nucleus by either passive diffusion or active transport system. These diverse and complicated manners might indicate that the nuclear import after synthesis of Gal-3 protein in the cytoplasm is necessary and essential to be phosphorylated and maintain its molecular stability.
Fig. 2.
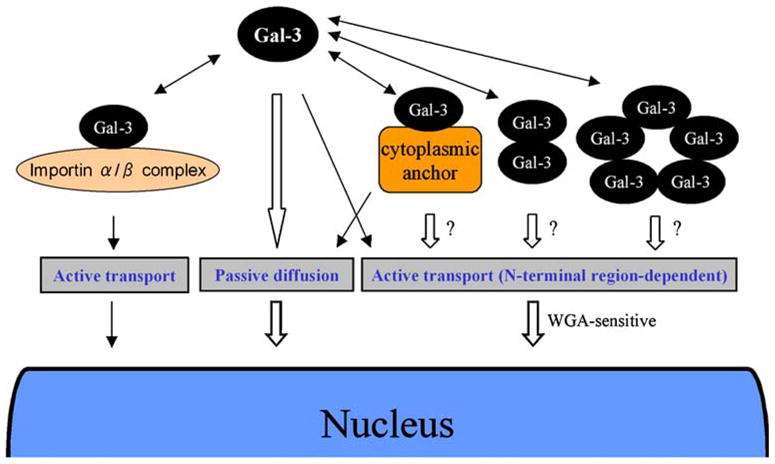
Possible mechanism of the nuclear import of galectin-3. The nuclear import pathways of galectin-3 are governed by a passive diffusion and the complicated active transporting system
4 Conclusion
Gal-3 is considered as a therapeutic target for several cancers, and many researchers have invented and developed the inhibitor for it. For example, modified citrus pectin (MCP), which is modified from a natural plant polysaccharide, i.e. citrus pectin, has been reported to inhibit Gal-3 mediated cellular functions, such as the formation of tumor cell emboli in blood circulation [44]. Several reports have also indicated the establishment of various Gal-3 inhibitors, but all inhibitors have been established to target the CRD region of Gal-3 for the inhibition of its sugar binding capacity. Details concerning with the inhibitors of Gal-3 were described in another review [12].
Meanwhile, it seems difficult to perfectly inhibit the nuclear entry of Gal-3 considering the existence of multiple and complicated nuclear import pathways as described above. However, to inhibit its nuclear import and function in the nucleus efficiently as possible, we suggest it is reasonable to be targeted the N-terminal region of Gal-3. The N-terminal region of Gal-3 contains a glycine, tyrosine, and proline repeat motif as well as the serine phosphorylation site, and this unique structure is not observed in any other galectins. Moreover, this region can mainly regulate the active nuclear import of Gal-3 and phosphorylation should be essential for the exertion of nuclear Gal-3 function as a regulator of gene expression, so the targeting to the N-terminal region of Gal-3 is significant and useful in this point. Of course, the permeability of inhibitors to the cell membrane is also important and should be considered in their development.
Acknowledgments
This work was supported by the NIH/NCI grant R37 CA-46120.
Contributor Information
Susumu Nakahara, Email: suita3387@yahoo.co.jp, Tumor Progression and Metastasis Program, Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA. Suita Municipal Hospital, Suita, Osaka, Japan.
Avraham Raz, Email: raza@karmanos.org, Tumor Progression and Metastasis Program, Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA.
References
- 1.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 2.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nature Reviews Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 3.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochimica et Biophysica Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 4.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconjugate Journal. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 5.Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267–275. doi: 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- 6.Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Research. 2001;29:3595–3602. doi: 10.1093/nar/29.17.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JL, Gray RM, Haudek KC, Patterson RJ. Nucleocytoplasmic lectins. Biochimica et Biophysica Acta. 2004;1673:75–93. doi: 10.1016/j.bbagen.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Paces-Fessy M, Boucher D, Petit E, Paute-Briand S, Blanchet-Tournier MF. The negative regulator of Gli, Suppressor of fused (Sufu), interacts with SAP18, Galectin3 and other nuclear proteins. Biochemical Journal. 2004;378:353–362. doi: 10.1042/BJ20030786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin HM, Pestell RG, Raz A, Kim HR. Galectin-3 enhances cyclin D(1) promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene. 2002;21:8001–8010. doi: 10.1038/sj.onc.1205820. [DOI] [PubMed] [Google Scholar]
- 10.Paron I, Scaloni A, Pines A, Bachi A, Liu FT, Puppin C, et al. Nuclear localization of Galectin-3 in transformed thyroid cells: A role in transcriptional regulation. Biochemical and Biophysical Research Communications. 2003;302:545–553. doi: 10.1016/s0006-291x(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 11.Song S, Byrd JC, Mazurek N, Liu K, Koo JS, Bresalier RS. Galectin-3 modulates MUC2 mucin expression in human colon cancer cells at the level of transcription via AP-1 activation. Gastroenterology. 2005;129:1581–1591. doi: 10.1053/j.gastro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Pieters RJ. Inhibition and detection of galectins. Chembiochem. 2006;7:721–728. doi: 10.1002/cbic.200600011. [DOI] [PubMed] [Google Scholar]
- 13.Nakahara S, Oka N, Wang Y, Hogan V, Inohara H, Raz A. Characterization of the nuclear import pathways of galectin-3. Cancer Research. 2006;66:9995–10006. doi: 10.1158/0008-5472.CAN-06-1772. [DOI] [PubMed] [Google Scholar]
- 14.Nakahara S, Hogan V, Inohara H, Raz A. Importin-mediated nuclear translocation of galectin-3. Journal of Biological Chemistry. 2006;281:39649–39659. doi: 10.1074/jbc.M608069200. [DOI] [PubMed] [Google Scholar]
- 15.Moutsatsos IK, Davis JM, Wang JL. Endogenous lectins from cultured cells: subcellular localization of carbohydrate-binding protein 35 in 3T3 fibroblasts. Journal of Cell Biology. 1986;102:477–483. doi: 10.1083/jcb.102.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moutsatsos IK, Wade M, Schindler M, Wang JL. Endogenous lectins from cultured cells: Nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:6452–6456. doi: 10.1073/pnas.84.18.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1213–1217. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson RJ, Wang W, Wang JL. Understanding the biochemical activities of galectin-1 and galectin-3 in the nucleus. Glycoconjugate Journal. 2004;19:499–506. doi: 10.1023/B:GLYC.0000014079.87862.c7. [DOI] [PubMed] [Google Scholar]
- 19.Lotz MM, Andrews CW, Jr, Korzelius CA, Lee EC, Steele GD, Jr, Clarke A, et al. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3466–3470. doi: 10.1073/pnas.90.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honjo Y, Inohara H, Akahani S, Yoshii T, Takenaka Y, Yoshida J, et al. Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clinical Cancer Research. 2000;6:4635–4640. [PubMed] [Google Scholar]
- 21.Mathieu A, Saal I, Vuckovic A, Ransy V, Vereerstraten P, Kaltner H, et al. Nuclear galectin-3 expression is an independent predictive factor of recurrence for adenocarcinoma and squamous cell carcinoma of the lung. Modern Pathology. 2005;18:1264–1271. doi: 10.1038/modpathol.3800416. [DOI] [PubMed] [Google Scholar]
- 22.Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Research. 1999;59:4148–4154. [PubMed] [Google Scholar]
- 23.Takenaka Y, Inohara H, Yoshii T, Oshima K, Nakahara S, Akahani S, et al. Malignant transformation of thyroid follicular cells by galectin-3. Cancer Letter. 2003;195:111–119. doi: 10.1016/s0304-3835(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 24.Bresalier RS, Byrd JC, Wang L, Raz A. Colon cancer mucin: A new ligand for the beta-galactoside-binding protein galectin-3. Cancer Research. 1996;56:4354–4357. [PubMed] [Google Scholar]
- 25.Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. Journal of Biological Chemistry. 1993;268:26712–26718. [PubMed] [Google Scholar]
- 26.Mazurek N, Conklin J, Byrd JC, Raz A, Bresalier RS. Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. Journal of Biological Chemistry. 2000;275:36311–36315. doi: 10.1074/jbc.M003831200. [DOI] [PubMed] [Google Scholar]
- 27.Tsay YG, Lin NY, Voss PG, Patterson RJ, Wang JL. Export of galectin-3 from nuclei of digitonin-permeabilized mouse 3T3 fibroblasts. Experimental Cell Research. 1999;252(2):250–261. doi: 10.1006/excr.1999.4643. [DOI] [PubMed] [Google Scholar]
- 28.Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. Journal of Biological Chemistry. 2002;277:6852–6857. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- 29.Mazurek N, Sun YJ, Price JE, Ramdas L, Schober W, Nangia-Makker P, et al. Phosphorylation of galectin-3 contributes to malignant transformation of human epithelial cells via modulation of unique sets of genes. Cancer Research. 2005;65(23):10767–10775. doi: 10.1158/0008-5472.CAN-04-3333. [DOI] [PubMed] [Google Scholar]
- 30.Gorlich D, Mattaj IW. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 31.Pante N, Aebi U. Sequential binding of import ligands to distinct nucleopore regions during their nuclear import. Science. 1996;273:1729–1732. doi: 10.1126/science.273.5282.1729. [DOI] [PubMed] [Google Scholar]
- 32.Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, et al. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Research. 1999;59:6239–6245. [PubMed] [Google Scholar]
- 33.Gaudin JC, Mehul B, Hughes RC. Nuclear localisation of wild type and mutant galectin-3 in transfected cells. Biology of the Cell. 2000;92:49–58. doi: 10.1016/S0248-4900(00)88763-8. [DOI] [PubMed] [Google Scholar]
- 34.Davidson PJ, Li SY, Lohse AG, Vandergaast R, Verde E, Pearson A, et al. Transport of galectin-3 between the nucleus and cytoplasm. I. Conditions and signals for nuclear import. Glycobiology. 2006;16(7):602–611. doi: 10.1093/glycob/cwj088. [DOI] [PubMed] [Google Scholar]
- 35.Davidson PJ, Davis MJ, Patterson RJ, Ripoche MA, Poirier F, Wang JL. Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology. 2002;12:329–337. doi: 10.1093/glycob/12.5.329. [DOI] [PubMed] [Google Scholar]
- 36.Adam SA, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- 37.Imamoto N, Shimamoto T, Takao T, Tachibana T, Kose S, Matsubae M, et al. In vivo evidence for involvement of a 58 kDa component of nuclear pore-targeting complex in nuclear protein import. EMBO Journal. 1995;14:3617–3626. doi: 10.1002/j.1460-2075.1995.tb00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kose S, Imamoto N, Tachibana T, Shimamoto T, Yoneda Y. Ran-unassisted nuclear migration of a 97-kD component of nuclear pore-targeting complex. Journal of Cell Biology. 1997;139:841–849. doi: 10.1083/jcb.139.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokoya F, Imamoto N, Tachibana T, Yoneda Y. beta-catenin can be transported into the nucleus in a Ran-unassisted manner. Molecular Biology of the Cell. 1999;10:1119–1131. doi: 10.1091/mbc.10.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annual Review of Cell and Developmental Biology. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 41.Allen TD, Cronshaw JM, Bagley S, Kiseleva E, Goldberg MW. The nuclear pore complex: Mediator of translocation between nucleus and cytoplasm. Journal of Cell Science. 2000;113(Pt 10):1651–1659. doi: 10.1242/jcs.113.10.1651. [DOI] [PubMed] [Google Scholar]
- 42.Yang RY, Hill PN, Hsu DK, Liu FT. Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry. 1998;37:4086–4092. doi: 10.1021/bi971409c. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. Journal of Biological Chemistry. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 44.Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. Journal of the National Cancer Institute. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]


