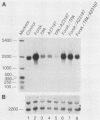
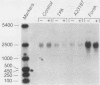
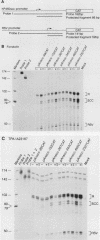
Abstract
Long-term regulation of mammalian steroid hormone synthesis occurs principally by transcriptional regulation of the gene for the rate-limiting cholesterol side-chain cleavage enzyme P450scc. Adrenal steroidogenesis is regulated primarily by two hormones: adrenocorticotropin, which works via cyclic AMP (cAMP) and protein kinase A, and angiotensin II, which works via Ca2+ and protein kinase C. Forskolin and 8-bromo-cAMP stimulated, while prolonged treatment with a phorbol ester (12-O-tetradecanoylphorbol-13-acetate [TPA]) and a calcium ionophore (A23187) additively suppressed accumulation of endogenous P450scc mRNA in transformed murine adrenal Y1 cells. In Y1 cells transfected with 2,327 base pairs of the human P450scc promoter fused to the bacterial gene for chloramphenicol acetyltransferase (CAT), forskolin increased CAT activity 900% while combined TPA plus A23187 reduced CAT activity to 15% of the control level. Forskolin induced the P450scc promoter as rapidly as a promoter containing two cAMP-responsive elements fused to a simian virus 40 promoter, a system known to respond directly to cAMP. Basal expression was increased by sequences between -89 and -152 and was increased further by sequences between -605 and -2327. This upstream region also conferred inducibility by cAMP. TPA plus A23187 transiently increased CAT activity before repressing it, reflecting the complex actions of angiotensin II in vivo. Repression by prolonged treatment with TPA plus A23187 was mediated by multiple elements between -89 and -343. Induction of CAT activity by forskolin was not diminished by treatment with TPA plus A23187, nor were the regions of the promoter responsible for regulation by the two pathways coisolated. Thus, the human gene for P450scc is repressed by TPA plus A23187 by mechanisms and sequences independent of those that mediate induction by cAMP.
Full text
PDF

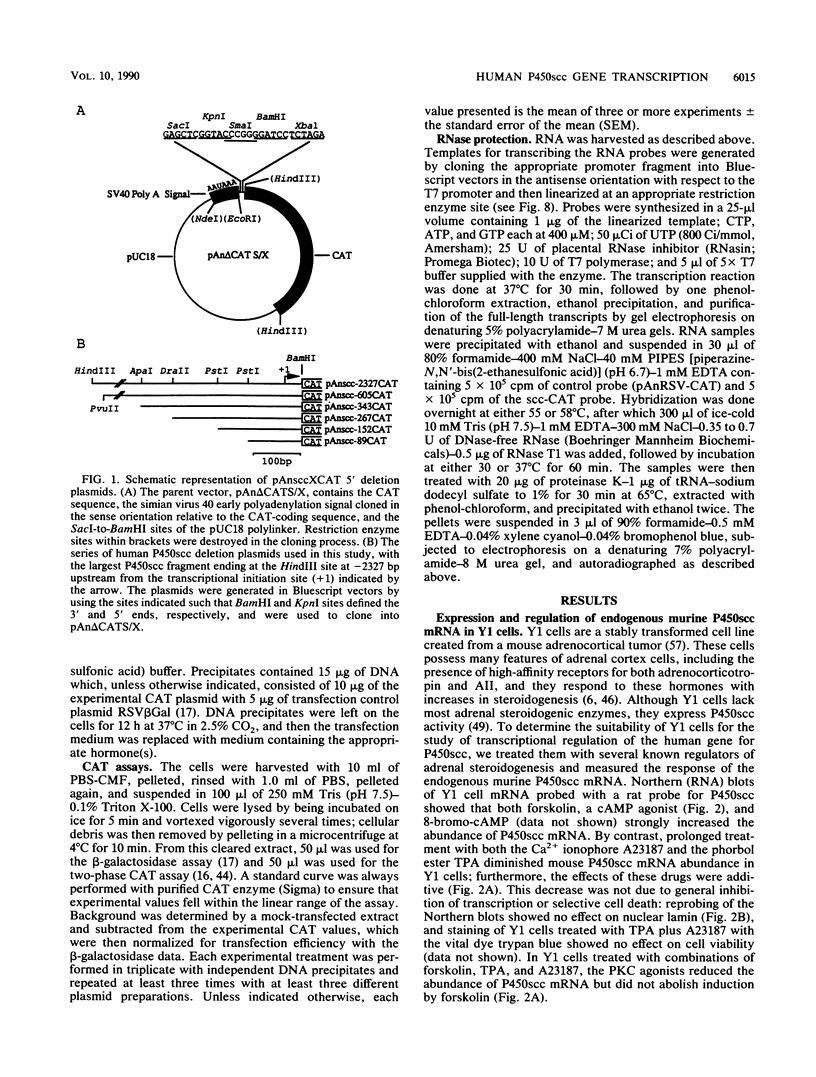
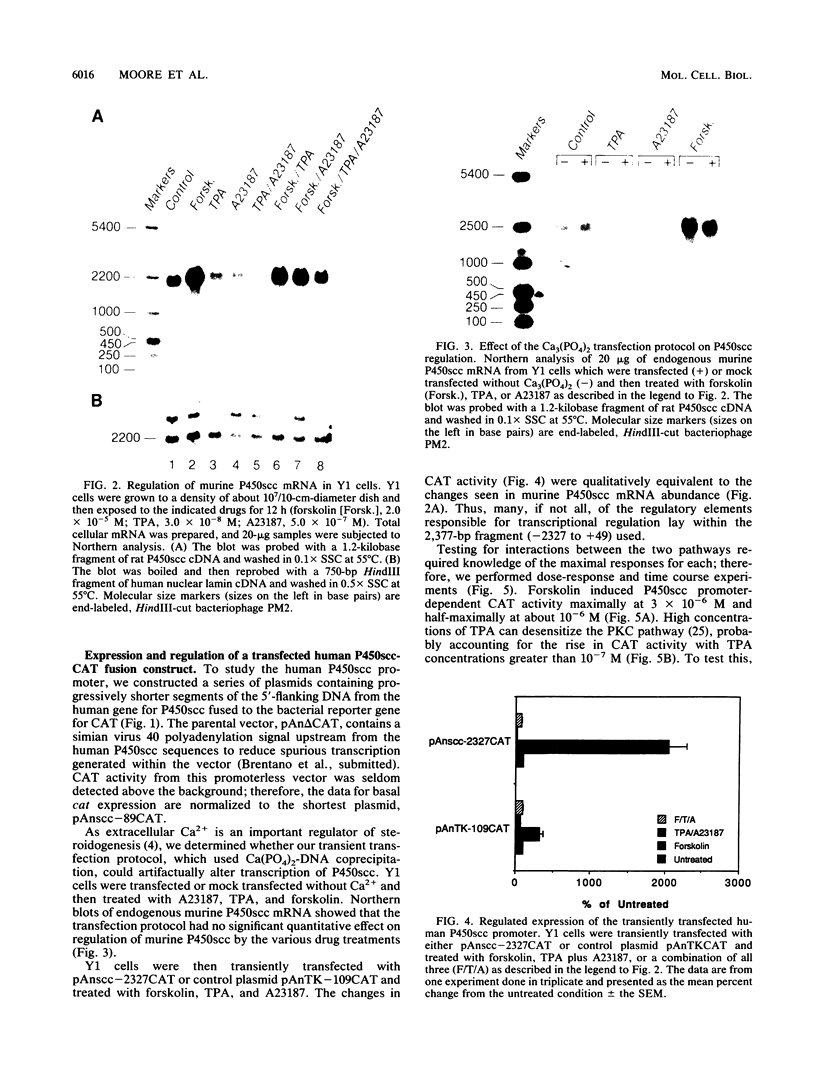
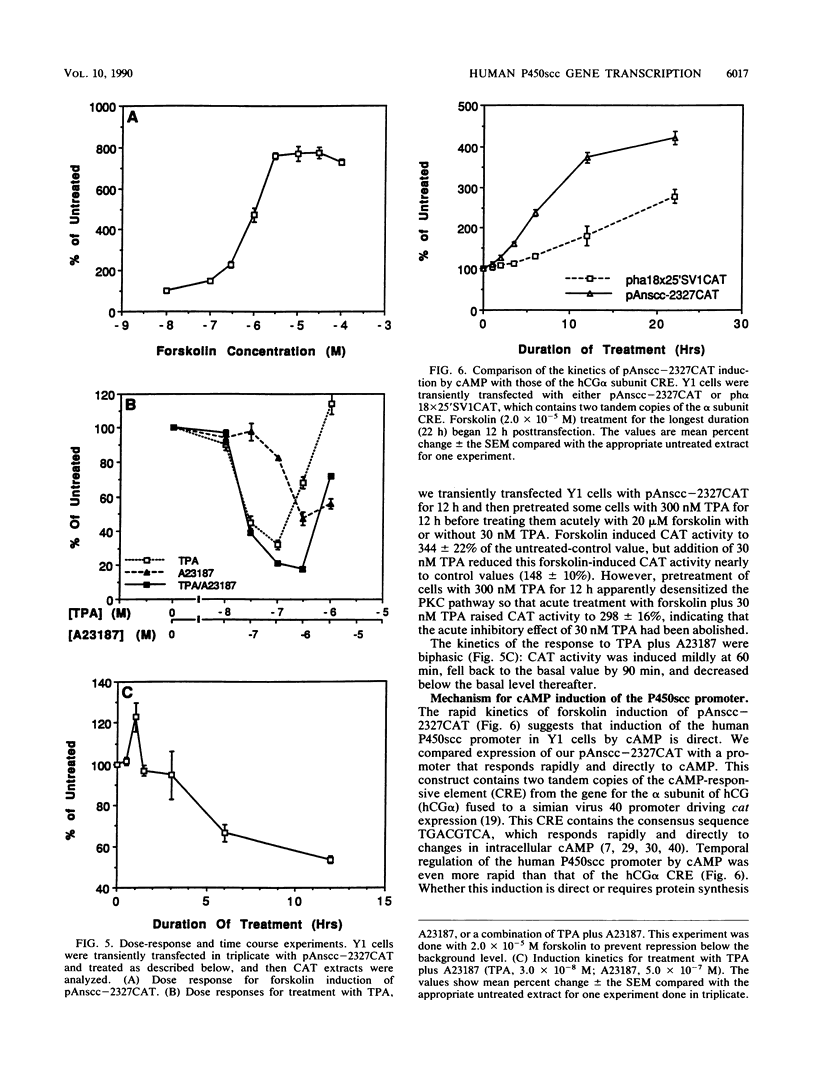
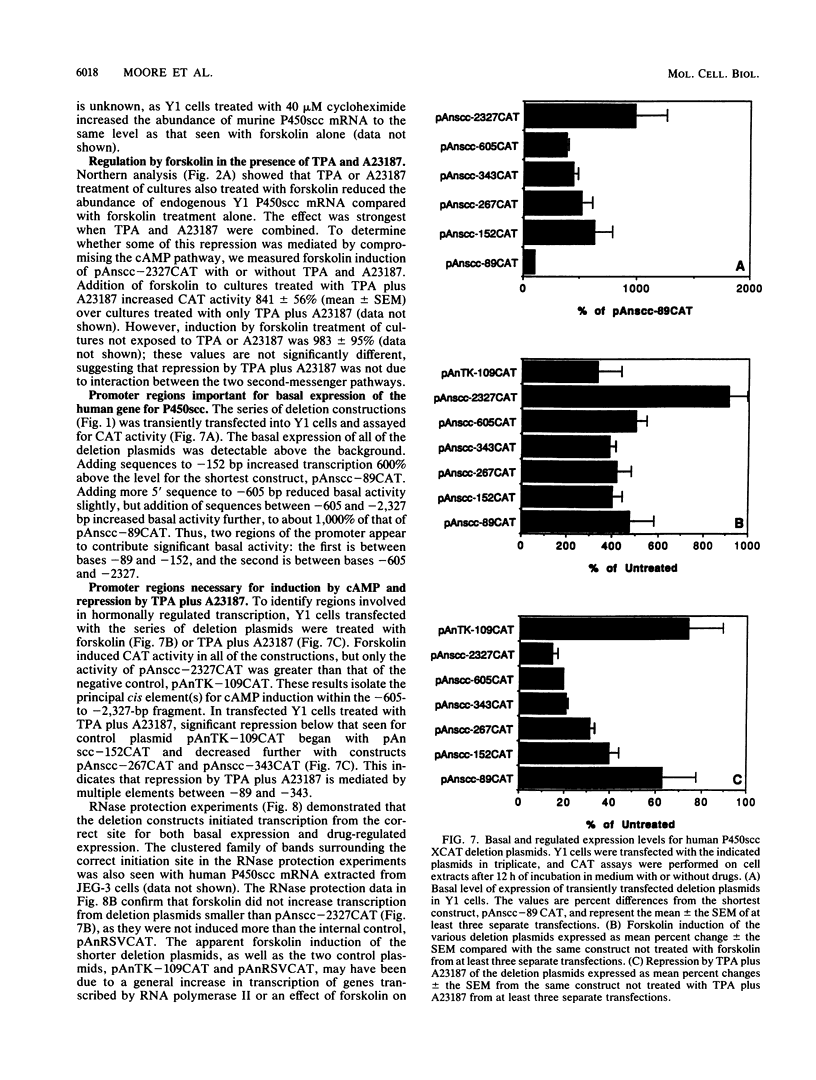
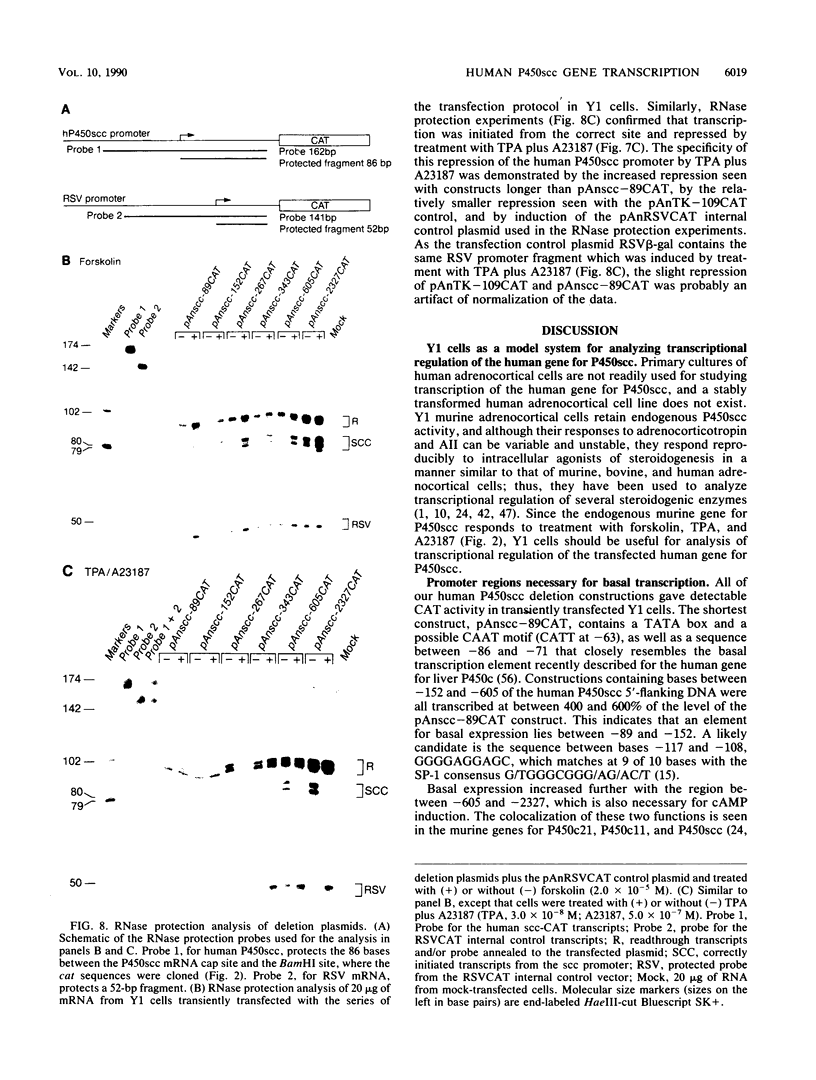




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlgren R., Simpson E. R., Waterman M. R., Lund J. Characterization of the promoter/regulatory region of the bovine CYP11A (P-450scc) gene. Basal and cAMP-dependent expression. J Biol Chem. 1990 Feb 25;265(6):3313–3319. [PubMed] [Google Scholar]
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Barrett P. Q., Bollag W. B., Isales C. M., McCarthy R. T., Rasmussen H. Role of calcium in angiotensin II-mediated aldosterone secretion. Endocr Rev. 1989 Nov;10(4):496–518. doi: 10.1210/edrv-10-4-496. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Begeot M., Langlois D., Vilgrain I., Saez J. M. Angiotensin II (A-II) steroidogenic refractoriness in Y-1 cells in the presence of A-II receptors negatively coupled to adenylate cyclase. Endocr Res. 1987;13(3):301–316. doi: 10.1080/07435808709035460. [DOI] [PubMed] [Google Scholar]
- Bokar J. A., Roesler W. J., Vandenbark G. R., Kaetzel D. M., Hanson R. W., Nilson J. H. Characterization of the cAMP responsive elements from the genes for the alpha-subunit of glycoprotein hormones and phosphoenolpyruvate carboxykinase (GTP). Conserved features of nuclear protein binding between tissues and species. J Biol Chem. 1988 Dec 25;263(36):19740–19747. [PubMed] [Google Scholar]
- Chen C. A., Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988 Jul-Aug;6(7):632–638. [PubMed] [Google Scholar]
- Chiu R., Imagawa M., Imbra R. J., Bockoven J. R., Karin M. Multiple cis- and trans-acting elements mediate the transcriptional response to phorbol esters. Nature. 1987 Oct 15;329(6140):648–651. doi: 10.1038/329648a0. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Hu M. C., Lai C. C., Lin C. H. The 5'-region of the P450XIA1 (P450scc) gene contains a basal promoter and an adrenal-specific activating domain. Biochem Biophys Res Commun. 1989 Apr 14;160(1):276–281. doi: 10.1016/0006-291x(89)91652-5. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Voutilainen R., Mohandas T. K., Miller W. L. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8962–8966. doi: 10.1073/pnas.83.23.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozza E. N., Vila M. C., Acevedo-Duncan M., Farese R. V., Gómez-Sánchez C. E. Treatment of primary cultures of calf adrenal glomerulosa cells with adrenocorticotropin (ACTH) and phorbol esters: a comparative study of the effects on aldosterone production and ACTH signaling system. Endocrinology. 1990 Apr;126(4):2169–2176. doi: 10.1210/endo-126-4-2169. [DOI] [PubMed] [Google Scholar]
- Dean D. C., McQuillan J. J., Weintraub S. Serum stimulation of fibronectin gene expression appears to result from rapid serum-induced binding of nuclear proteins to a cAMP response element. J Biol Chem. 1990 Feb 25;265(6):3522–3527. [PubMed] [Google Scholar]
- Di Blasio A. M., Voutilainen R., Jaffe R. B., Miller W. L. Hormonal regulation of messenger ribonucleic acids for P450scc (cholesterol side-chain cleavage enzyme) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human fetal adrenal cells. J Clin Endocrinol Metab. 1987 Jul;65(1):170–175. doi: 10.1210/jcem-65-1-170. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Sazer S., Tjian R., Schimke R. T. Transcription factor Sp1 recognizes a DNA sequence in the mouse dihydrofolate reductase promoter. Nature. 1986 Jan 16;319(6050):246–248. doi: 10.1038/319246a0. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Enyedi P., Szabó B., Spät A. Reduced responsiveness of glomerulosa cells after prolonged stimulation with angiotensin II. Am J Physiol. 1985 Feb;248(2 Pt 1):E209–E214. doi: 10.1152/ajpendo.1985.248.2.E209. [DOI] [PubMed] [Google Scholar]
- Fenstermaker R. A., Milsted A., Virgin J. B., Miller W. L., Nilson J. H. The transcriptional response of the human chorionic gonadotropin beta-subunit gene to cAMP is cycloheximide sensitive and is mediated by cis-acting sequences different from that found in the alpha-subunit gene. Mol Endocrinol. 1989 Jul;3(7):1070–1076. doi: 10.1210/mend-3-7-1070. [DOI] [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring N. B., Durica J. M., Lifka J., Hedin L., Ratoosh S. L., Miller W. L., Orly J., Richards J. S. Cholesterol side-chain cleavage P450 messenger ribonucleic acid: evidence for hormonal regulation in rat ovarian follicles and constitutive expression in corpora lutea. Endocrinology. 1987 May;120(5):1942–1950. doi: 10.1210/endo-120-5-1942. [DOI] [PubMed] [Google Scholar]
- Golos T. G., Miller W. L., Strauss J. F., 3rd Human chorionic gonadotropin and 8-bromo cyclic adenosine monophosphate promote an acute increase in cytochrome P450scc and adrenodoxin messenger RNAs in cultured human granulosa cells by a cycloheximide-insensitive mechanism. J Clin Invest. 1987 Sep;80(3):896–899. doi: 10.1172/JCI113149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Handler J. D., Schimmer B. P., Flynn T. R., Szyf M., Seidman J. G., Parker K. L. An enhancer element and a functional cyclic AMP-dependent protein kinase are required for expression of adrenocortical 21-hydroxylase. J Biol Chem. 1988 Sep 15;263(26):13068–13073. [PubMed] [Google Scholar]
- Hoeffler J. P., Deutsch P. J., Lin J., Habener J. F. Distinct adenosine 3',5'-monophosphate and phorbol ester-responsive signal transduction pathways converge at the level of transcriptional activation by the interactions of DNA-binding proteins. Mol Endocrinol. 1989 May;3(5):868–880. doi: 10.1210/mend-3-5-868. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Inoue H., Higashi Y., Morohashi K., Fujii-Kuriyama Y. The 5'-flanking region of the human P-450(SCC) gene shows responsiveness to cAMP-dependent regulation in a transient gene-expression system of Y-1 adrenal tumor cells. Eur J Biochem. 1988 Feb 1;171(3):435–440. doi: 10.1111/j.1432-1033.1988.tb13808.x. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Lindell C. M. Isolation and characterization of the human chorionic gonadotropin beta subunit (CG beta) gene cluster: regulation of transcriptionally active CG beta gene by cyclic AMP. Mol Cell Biol. 1988 Dec;8(12):5100–5107. doi: 10.1128/mcb.8.12.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J. L., Powers A. C., Gallagher G. D., Habener J. F. Enhancer and promoter element interactions dictate cyclic adenosine monophosphate mediated and cell-specific expression of the glycoprotein hormone alpha-gene. Mol Endocrinol. 1989 May;3(5):763–772. doi: 10.1210/mend-3-5-763. [DOI] [PubMed] [Google Scholar]
- John M. E., John M. C., Boggaram V., Simpson E. R., Waterman M. R. Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4715–4719. doi: 10.1073/pnas.83.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Complexities of gene regulation by cAMP. Trends Genet. 1989 Mar;5(3):65–67. doi: 10.1016/0168-9525(89)90027-9. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lund J., Ahlgren R., Wu D. H., Kagimoto M., Simpson E. R., Waterman M. R. Transcriptional regulation of the bovine CYP17 (P-450(17)alpha) gene. Identification of two cAMP regulatory regions lacking the consensus cAMP-responsive element (CRE). J Biol Chem. 1990 Feb 25;265(6):3304–3312. [PubMed] [Google Scholar]
- Mason J. I., Carr B. R., Rainey W. E. The action of phorbol ester on steroidogenesis in cultured human fetal adrenal cells. Endocr Res. 1986;12(4):447–467. doi: 10.3109/07435808609035450. [DOI] [PubMed] [Google Scholar]
- McAllister J. M., Hornsby P. J. Dual regulation of 3 beta-hydroxysteroid dehydrogenase, 17 alpha-hydroxylase, and dehydroepiandrosterone sulfotransferase by adenosine 3',5'-monophosphate and activators of protein kinase C in cultured human adrenocortical cells. Endocrinology. 1988 May;122(5):2012–2018. doi: 10.1210/endo-122-5-2012. [DOI] [PubMed] [Google Scholar]
- Mellon S. H., Vaisse C. cAMP regulates P450scc gene expression by a cycloheximide-insensitive mechanism in cultured mouse Leydig MA-10 cells. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7775–7779. doi: 10.1073/pnas.86.20.7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. L. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988 Aug;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Milsted A., Cox R. P., Nilson J. H. Cyclic AMP regulates transcription of the genes encoding human chorionic gonadotropin with different kinetics. DNA. 1987 Jun;6(3):213–219. doi: 10.1089/dna.1987.6.213. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Yoshida M., Arai K. T-cell activation signals and human T-cell leukemia virus type I-encoded p40x protein activate the mouse granulocyte-macrophage colony-stimulating factor gene through a common DNA element. Mol Cell Biol. 1988 Dec;8(12):5581–5587. doi: 10.1128/mcb.8.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Sogawa K., Omura T., Fujii-Kuriyama Y. Gene structure of human cytochrome P-450(SCC), cholesterol desmolase. J Biochem. 1987 Apr;101(4):879–887. doi: 10.1093/oxfordjournals.jbchem.a121955. [DOI] [PubMed] [Google Scholar]
- Mouw A. R., Rice D. A., Meade J. C., Chua S. C., White P. C., Schimmer B. P., Parker K. L. Structural and functional analysis of the promoter region of the gene encoding mouse steroid 11 beta-hydroxylase. J Biol Chem. 1989 Jan 15;264(2):1305–1309. [PubMed] [Google Scholar]
- Picado-Leonard J., Voutilainen R., Kao L. C., Chung B. C., Strauss J. F., 3rd, Miller W. L. Human adrenodoxin: cloning of three cDNAs and cycloheximide enhancement in JEG-3 cells. J Biol Chem. 1988 Mar 5;263(7):3240–3244. [PubMed] [Google Scholar]
- Pierson R. W., Jr Metabolism of steroid hormones in adrenal cortex tumor cultures. Endocrinology. 1967 Oct;81(4):693–707. doi: 10.1210/endo-81-4-693. [DOI] [PubMed] [Google Scholar]
- Rice D. A., Aitken L. D., Vandenbark G. R., Mouw A. R., Franklin A., Schimmer B. P., Parker K. L. A cAMP-responsive element regulates expression of the mouse steroid 11 beta-hydroxylase gene. J Biol Chem. 1989 Aug 25;264(24):14011–14015. [PubMed] [Google Scholar]
- STONE D., HECHTER O. Studies on ACTH action in perfused bovine adrenals: aspects of progesterone as an intermediary in corticosteroidogenesis. Arch Biochem Biophys. 1955 Jan;54(1):121–128. doi: 10.1016/0003-9861(55)90014-x. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Schimmer B. P. Adrenocortical Y1 cells. Methods Enzymol. 1979;58:570–574. doi: 10.1016/s0076-6879(79)58173-7. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Miller W. L. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc [corrected], in human steroidogenic tissues. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1590–1594. doi: 10.1073/pnas.84.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen R., Tapanainen J., Chung B. C., Matteson K. J., Miller W. L. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986 Jul;63(1):202–207. doi: 10.1210/jcem-63-1-202. [DOI] [PubMed] [Google Scholar]
- Wong M., Rice D. A., Parker K. L., Schimmer B. P. The roles of cAMP and cAMP-dependent protein kinase in the expression of cholesterol side chain cleavage and steroid 11 beta-hydroxylase genes in mouse adrenocortical tumor cells. J Biol Chem. 1989 Aug 5;264(22):12867–12871. [PubMed] [Google Scholar]
- Woodcock E. A., Johnston C. I. Inhibition of adenylate cyclase in rat adrenal glomerulosa cells by angiotensin II. Endocrinology. 1984 Jul;115(1):337–341. doi: 10.1210/endo-115-1-337. [DOI] [PubMed] [Google Scholar]
- Yanagida A., Sogawa K., Yasumoto K. I., Fujii-Kuriyama Y. A novel cis-acting DNA element required for a high level of inducible expression of the rat P-450c gene. Mol Cell Biol. 1990 Apr;10(4):1470–1475. doi: 10.1128/mcb.10.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumura Y., Buonassisi V., Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966 Mar;26(3):529–535. [PubMed] [Google Scholar]