Abstract
Brown adipose tissue (BAT) was thought to disappear after infancy. Recent studies finding BAT in patients undergoing positron emission tomography/computed tomography (PET/CT) have renewed the interest in deciphering the relevance of this tissue in humans. Available data suggests that BAT is more prevalent in children than in adults, and that its activation during adolescence is associated to significantly less gains in weight and adiposity. Data also shows that pediatric patients with metabolically-active BAT on PET/CT examinations have significantly greater muscle volume than patients with no identifiable BAT. Both the activity and the amount of BAT increase during puberty. The magnitude of the increase is higher in boys when compared to girls, and closely related to gains in muscle volume. Hence, concurrent with the great gains in skeletal muscle during infancy and puberty, all infants and adolescents accumulate large amounts of BAT. These observations are consistent with in vitro investigations suggesting close interactions between brown adipocytes, white adipocytes, and myocites. In this review, we discuss the potential role of this tissue in regulating weight and musculoskeletal development in children.
INTRODUCTION
It has been nearly five centuries since Konrad Gessner described a tissue that was “neither fat, nor flesh – but something in between (1),” five decades since Robert E. Smith showed the capacity of brown adipose tissue (BAT) to dissipate energy as heat (2), and five years since the ranks of the obese overtook the number of malnourished in the world (3), but whether BAT has any relevance to human health and disease beyond helping to maintain normal body temperature in newborns remains unknown. The belief that BAT involutes soon after birth and the lack of techniques to adequately measure BAT in humans have limited our understanding of the relevance of this tissue.
New data showing that some adults and most children have BAT, coupled with the development of imaging techniques that provide reliable BAT measurements, has renewed the interest in deciphering the physiology of this tissue (4, 5). In this review, we discuss recent advances in our understanding of the potential role of this tissue in regulating weight and musculoskeletal development in children and adolescents.
ANATOMY, PHYSIOLOGY, AND PATHOLOGY OF BAT
The adipose organ is a complex endocrine system, composed of white and brown fat. White adipose tissue (WAT) serves as the primary site of energy storage, storing triglycerides within individual adipocytes, while BAT stores little fat, burning it instead to produce heat and regulate body temperature (6, 7). Compared to WAT, BAT is highly vascularized and innervated by the sympathetic nervous system. Moreover, white adipocytes are spherical and unilocular, while the brown adipocyte is usually smaller and characterized by multilocular lipid droplets and an abundance of mitochondria expressing uncoupling protein-1 (UCP1) (7). In humans, BAT is recognized primarily in the cervical-supraclavicular area, where it is present across a wide range of ages (4, 5, 8, 9). To date, the sole known function of BAT is to dissipate energy through the uncoupling of oxidative respiration from the production of adenosine triphosphate (ATP), an action regulated by UCP1 (7, 10, 11). This type of heat production is known as nonshivering thermogenesis.
In a thermoneutral state, the activity of UCP1 in the mitochondrial membrane is inhibited by ATP in the cytoplasm. During cold acclimation, sympathetic stimulation triggers the proliferation and differentiation of precursor cells towards the development of BAT (12). Mitochondrial biogenesis and increased synthesis of UCP1 are hallmarks of the thermogenic recruitment process. The release of norepinephrine, associated with a cold environment, interacts with β-adrenergic receptors in the cell membrane of the brown adipocyte leading to the hydrolysis of triglycerides and the production of free fatty acids (13). The increase in free fatty acids in the cytoplasm overcomes ATP inhibition by interacting with UCP1, leading to its activation. UCP1 increases proton leakage across the inner membrane of brown adipocyte mitochondria dissipating energy in the form of heat rather than ATP (12).
Besides UCP1, four additional uncoupling proteins are expressed by the human genome. While UCP1 is exclusive to brown adipose tissue, UCP2 is expressed ubiquitously, UCP3 is mainly expressed in skeletal muscle, and UCP4 and UCP5 are expressed in the brain (14). The physiological functions of these proteins are not well understood, but could have profound significance in our understanding of conditions such as diabetes, obesity, thyroid disease, and aging. Thyroid hormone, an important regulator of energy expenditure, is not only necessary for the full expression of UCP1, but also induces UCP3 expression and UCP3-mediated uncoupling in skeletal muscle mitochondria of rodents (14).
There are two rare pathological disorders closely related to BAT. Hibernomas are benign tumors arising from brown adipocytes that have primarily been described in the neck, axilla, thoracic regions, and retroperitoneum most commonly in young adults (15). Subcutaneous fat necrosis of the newborn is another clinical condition, often associated with asphyxia or hypothermia, that pathologically is characterized by focal areas of fat necrosis that are infiltrated by macrophages and, brown adipocytes at several stages of degeneration (16).
RECENT ADVANCES IN PEDIATRIC BAT RESEARCH
Until recently, the accumulated information on the thermogenic effect of BAT was derived from studies on rodents and hibernating mammals, with little awareness of its potential relevance to humans. Although first described in neonates back in 1902 (17), BAT only began to take on a life of its own a century later with the introduction of positron emission tomography/computed tomography (PET/CT). Today, a large body of evidence indicates that metabolically active BAT is present in a significant number of cancer patients undergoing PET/CT examinations (4, 5, 8, 10). There is, however, a strikingly higher prevalence of BAT depiction in pediatric PET/CT examinations (ranging from 31 to 77%) (4, 18-22) when compared to the prevalence in adults (5) – approximately 1 in 2 children versus 1 in 20 adults.
The depiction of BAT by PET/CT in children, like in adults, is related to environmental temperature and season. BAT activity is observed in most studies during cold exposure (4, 23), consistent with histological evidence indicating that BAT is universally present in children and adults (24, 25). The percentage of PET/CT studies with metabolically active BAT is also higher in the winter months. This is true even in children living in warmer climates and when studies are obtained under thermoneutral conditions (4, 18). Indeed, it is becoming increasingly apparent that photoperiod and day length are strong determinants of BAT activity, independent of environmental temperature (6, 26).
Visualization of BAT in children is also dependent on disease status, weight, body composition, and the degree of sexual development (4, 18, 27, 28). Although excessive calorie intake has also been suggested to stimulate BAT activity in mice, whether diet induces BAT activation and thermogenesis in humans is the subject of considerable discussion (29). However, UCP1 polymorphisms have been reported to influence postprandial thermogenesis after a high fat meal in healthy boys (30).
A recent study in children with lymphoma found that while only about 10% of the PET/CT exams at diagnosis exhibited BAT, close to 80% of the follow-up exams displayed BAT when the patients were in remission (27). Although, the mechanism responsible for the suppression of BAT activity is unknown, patients with lymphomas have high circulating levels of tumor necrosis factor alpha (TNF-α) (31), a pluripotent cytokine reported to elicit apoptotic degeneration of brown adipocytes (32). This cytotoxic effect is known to be mediated by the p55 TNF-α receptor subtype, and its deletion has been shown to increase thermogenesis with an associated increase of UCP1 expression in BAT (33).
In contrast, the visualization of metabolically active BAT in adult patients with cancer is thought to be independent of disease status (34). Unfortunately, the low BAT prevalence, long treatment courses, and relatively poor survival rates greatly hinder longitudinal assessments of BAT in adult populations.
Association of BAT to Weight and Measures of White Adiposity in Children
Data from animal studies indicate that a reduced amount or function of BAT leads to obesity, insulin resistance, and dyslipidemia, while an increased amount or function protects against weight gain and its co-morbidities (35-38). Studies in humans also suggest that WAT and BAT are inversely related (5, 6, 8, 11, 34). Lean patients exhibit greater BAT activity than obese subjects, and most studies in adult patients report a negative relation between body mass and/or body fat and the degree of metabolically active BAT (5, 6, 9). Other studies, however, find no relation between BAT and body mass (39, 40) or measures of adiposity (40, 41). Similar discrepancies are reported in pediatric cross-sectional studies; while one observed an inverse relationship between body mass index (BMI) and BAT activity (20), others found no significant differences in the weight, BMI, or measures of subcutaneous adiposity between children with and without functioning BAT (18, 23).
The activation of BAT in children has been shown to be related to changes in weight and adiposity (28). Pediatric cancer patients with no visualization of BAT at diagnoses, but with evidence of BAT activity at follow-up PET/CT studies gained significantly less weight and subcutaneous and visceral adiposity than those who remained without BAT activity when disease-free. On average, increases in weight and subcutaneous fat were three times greater, and those in visceral fat six times greater, in children who did not demonstrate any BAT activity when compared to children who had metabolically active BAT (28).
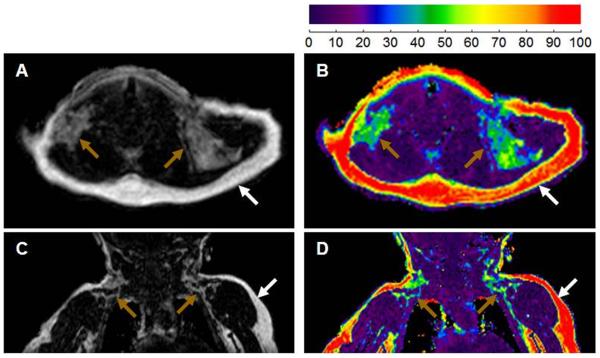
The results of a recent study based on magnetic resonance imaging (MRI), provide additional evidence that overweight/obese children have significantly less total (functional and non-functional) BAT in the supraclavicular area than lean, healthy children (81) (Figure 1). Moreover, there appears to be a strong inverse relation between magnetic resonance imaging measures of BAT and weight or BMI% in overweight/obese children. Interestingly, the strength of this negative relation is similar to the strength of the positive correlation between values of WAT and these anthropometric measures.
Figure 1.
Presence of BAT and BMI percentile. Coronal MR views of a (A) healthy 11-year old boy with a BMI in the 79th percentile depicting both white and brown fat (represented by a yellow/green fat fraction) and of an (B) obese 11-year old boy with a BMI in the 98th percentile showing only white fat (represented by a red fat fraction). The fat fraction (%) scale is represented on the right.
Although BAT activation could decrease WAT as a result of increased energy consumption (8), it is also possible that WAT suppresses BAT function. The white adipocyte is known to produce cytokines and chemokines, such as TNF-α, IL-6, and monocyte chemoattractant protein 1, that induce inflammation and could potentially have cytotoxic effects on BAT (42, 43). Additionally, an increase in BAT could directly lead to an increase in muscle function and energy expenditure, which in turn leads to a decrease in adiposity. Studies are needed to establish the direction of causality between BAT activity and WAT accumulation and the degree to which this relationship is mediated by muscle.
Association of BAT to Muscle Development in Children
Recent data shows that pediatric patients with metabolically active BAT on PET/CT examinations have significantly greater muscle volume than patients with no identifiable BAT (18). On average, boys and girls who exhibit BAT have approximately 33 to 50% greater muscle volume than patients who do not exhibit BAT (18). This clinical observation is consistent with information that brown adipocytes and myocytes share many features, including an abundance of mitochondria, energy expenditure via oxidative phosphorylation, and sympathetically mediated adaptive thermogenesis (38, 44-46). They also express myogenic factors, such as myf5, and may derive from a common lineage in the paraxial mesoderm (46, 47). Further support for a muscle-BAT link comes from a landmark investigation indicating that exercise-induced gains in muscle lead to an increase in the amount of cells with a brown fat phenotype (48). This study identifies a new hormone, irisin, as an exercise-induced myokine that allegedly activates the induction of brown adipocytes in white adipose tissue depots, a process known as white fat “browning.”
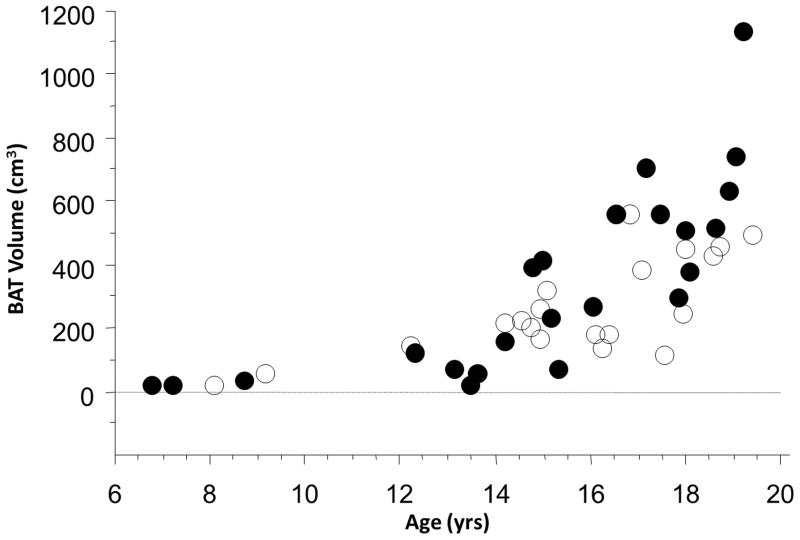
Skeletal musculature increases substantially during puberty. Gains in musculature associated with sexual development closely equal the growth of all other organs, systems, and tissues combined. Concurrently, a higher prevalence and large amounts of BAT are also present during adolescence. Whereas less than 20% of PET/CT exams in pre-pubertal girls or boys exhibit metabolically active BAT, more than 75% of such studies in pubertal teenagers display BAT uptake (4). Additionally, the volume of BAT increases during puberty in both sexes (Figure 2). The magnitude of the increase is substantially greater during the late stages of sexual development, higher in boys when compared to girls, and closely related to gains in muscle volume (18). While the reasons for the pubertal increase in BAT are unknown, data suggest that sex steroids and growth hormone have a marked effect on BAT activity (49, 50). Interestingly, past postmortem studies suggests UCP1 activity to be higher in teenagers compared to neonates (51).
Figure 2.
Gains in brown adipose tissue during adolescence. Regardless of sex, BAT volume increased with age in boys (black) and girls (white) (r = 0.77 for boys and r = 0.72 for girls; both P’s < .001). (Adapted from Journal of Pediatrics, Vol. 160, Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH, Changes in brown adipose tissue in boys and girls during childhood and puberty, pp. 604-609.e1, Copyright 2012, with permission from Elsevier.)
Association of BAT to Skeletal Development in Children
Two recent clinical studies suggest that BAT might be involved in the regulation of bone mass in humans. The first in young women with anorexia nervosa reported a positive relation between BAT and bone density in the axial skeleton, which was independent of disease status and body mass (52). The second study in children found the volume of BAT to be positively related to the amount of bone in the appendicular skeleton – a relation that was also independent of known major determinants of bone acquisition, such as height, weight, and gender (53).
The reason(s) for the association between BAT and bone mass are yet to be defined. Available data, however, supports a link between BAT and bone formation. The retinoblastoma protein was recently identified as a mesenchymal cell-fate regulator that controlled differentiation into either the brown adipocyte or the osteoblast (54). Several reports have found this key regulator to be capable of both inhibiting adipogenic differentiation and promoting osteoblast maturation (54, 55). Studies in animal models also suggest that BAT may be involved in regulating osteoblastogenesis. Heterotrophic ossification modeled by the bone morphogenic protein-2 is known to induce the accumulation of brown adipocytes and subsequently trigger chondrocyte development and bone formation (56). Moreover, mice lacking functional BAT have very low bone mass, reduced osteoblast activity, and increased bone resorption (57).
Regardless of the mechanism by which BAT influences skeletal growth, maintaining optimal bone mass depends on sensing and transducing mechanical loading information derived from muscle contractions (58). Therefore, the possibility exists for muscle to mediate the relationship between BAT and bone development (53). Support for the notion that BAT is crucial to the maintenance of musculoskeletal integrity comes from two physiologic situations characterized by an abundance of BAT in which decreased skeletal loading and locomotion do not result in muscle or bone loss. Large hibernating mammals are remarkably able to maintain their muscle and bone mass despite losing a third of their weight and remaining immobile over a period of nearly seven months (58, 59). Similarly, infancy is a developmental stage associated with rapid increases in muscle and bone mass despite the lack of significant skeletal loading.
Studies are needed to determine the degree to which BAT contributes to the maintenance of muscle function in the absence of mechanical strains associated with loading or locomotion.
FUTURE DIRECTIONS
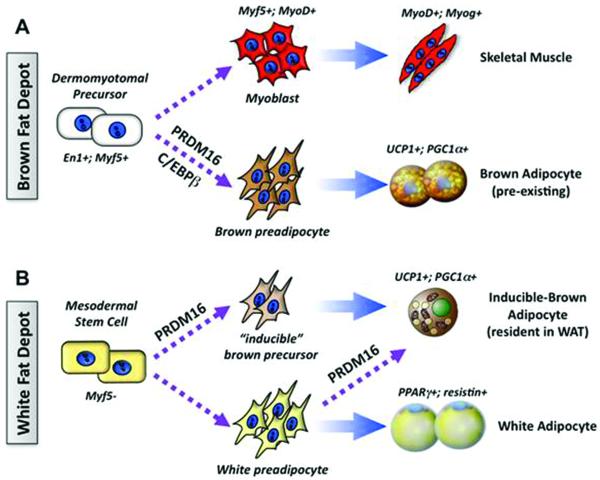
To date, most studies on the molecular regulation of BAT have been conducted in animal models and suggest that at least two types of brown adipocytes from distinct lineages exist – myoblast origin and adipocyte origin (45). Classical brown adipocytes (i.e., “pre-existing” brown adipocytes) that reside in the interscapular BAT depot form during the prenatal stage from myoblastic-like myf5 positive precursors and have a gene profile similar to that of skeletal muscle (46) (Figure 3A). These myf5 positive cells differentiate into brown adipocytes through the action of the transcriptional regulators PRDM16, PPARγ, and/or C/EBP-β. Additionally, pockets of a second, distinct type of brown adipocyte are found sporadically in the WAT of adult animals that have been exposed to chronic cold or to PPARγ agonists. While these inducible brown adipocytes, also known as brown-in white (BRITE) cells, possess many of the biochemical and morphological characteristics of brown adipocytes, including the presence of multilocular lipid droplets and UCP1 expression (60), they arise from a non-myf5 cell lineage (Figure 3B).
Figure 3.
Differential developmental origins of brown and white adipocytes. Brown adipose tissue (A) and white adipose tissue (B) have separate developmental origins in the embryo. (A) BAT and skeletal muscle originate during the prenatal stage from precursors in the dermomyotome that express engrailed-1 (En1) and myf5. PRDM16, PPARγ, PGC-1α and UCP1 are functional markers of brown adipose cells in the developmental, homogenous deposits of BAT. (B) The embryonic stem cells of the white adipose lineage remain to be well defined. The “inducible”-brown adipocytes in WAT develop in response to cold, β-adrenergic stimulation or PPARγ agonists. These cells may be derived from myf5(−) brown precursors or directed differentiation from white preadipocytes or from mature white adipocytes. (Adapted from Cell Metabolism, Vol. 11, Kajimura S, Seale P, Spiegelman BM, Transcriptional control of brown fat development, pp. 254-62, Copyright 2010, with permission from Elsevier.)
There should ultimately be increased scope for studies decoding the transcriptional control of human brown fat development. This is especially pertinent as the gene profile of specialized tissues, such as BAT, has a very different molecular signature in humans compared with mice (61). Recent data reports differences in the response of UCP1 mRNA to hormonal stimulation even between rat and mouse brown adipocytes (62). Emerging questions that must be address regarding the biological significance of the two types of brown adipocytes in humans include: What are the molecular or functional differences between the two types of brown adipocytes? Are the molecular signatures of these cells in humans closer to that of myocytes or white adipocytes? How relevant is the inducible-brown adipocyte for the control of energy homeostasis as well as for obesity and metabolic disease? Of note are data indicating that preadipocytes isolated from supraclavicular fat in humans, ages 35-64 years, are capable of differentiating into brown adipocytes in vitro, regardless of PET status (63). Hence, molecular pathways of brown fat development should be intact and can be reactivated in adult humans. In this regard, synthetic PPARγ ligands such as thiazolidinedione, widely used in drugs to treat type 2 diabetes, can direct white preadipocytes into mature brown adipocytes (64-66); this PPARγ ligand-induced browning effect is reported to be mediated through stabilization of the PRDM16 protein (67).
Recently, we examined the relevance of BRITE cells in humans by analyzing the molecular signature of human BAT isolated from infants and adolescents. To our surprise, BAT in children predominantly expressed BRITE cell-selective genes rather than pre-existing brown fat-selective genes. This data indicate that human BAT possesses molecular signatures that resemble beige cells (82).
Obesity has become the leading cause of preventable death. Since lean children appear to have greater BAT activity than obese subjects, there is considerable interest in defining the mechanisms responsible for relations between BAT and body composition. As it also seems that BAT and muscle mass are positively related and higher muscle mass is associated with better insulin sensitivity and lower risk of pre-diabetes (68), it is imperative that we examine the influence that BAT has on metabolic health. Specifically, there is a need to establish whether a deficiency in BAT in early postnatal life permanently increases the risk of obesity and its comorbidities throughout life.
Of equal importance is to determine how the transcriptional and epigenetic regulatory networks that govern human brown adipocytes respond to early stages of human postnatal growth. Post-mortem studies indicate that BAT is established in fetuses within the fifth month of gestation (69). At the time of birth, BAT abundance peaks as reflected by levels of UCP1, before declining over the next 9 months (51). Studies are needed to examine the degree to which BAT accounts for phenotypic differences among infants, and the degree to which BAT is influenced by maternal health and diet, gestational age, birth weight, and feeding practice. For example, current data shows that compared to formula-fed infants, breast-fed (BF) infants are leaner and grow more slowly (52). Not only do BF infants grow at different rates during infancy, but breastfeeding appears to have a profound long-term influence on metabolism and disease risk later in life (70). The notion that BAT accounts for the leaner phenotype of BF infants is supported by observations that leptin, ghrelin, adiponectin, resistin, and obestatin, all hormones involved in energy balance regulation, are identified in breast milk (71). Indeed, both leptin and adiponectin concentrations in breast milk have recently been found to influence UCP1 expression in BAT and negatively correlate with infant body weight (72).
Given that BAT is a highly dynamic and elusive tissue that can exist in a variety of states depending on a wide spectrum of environmental factors, other non-invasive approaches beyond PET/CT are needed to assess the relevance of this tissue in humans. Thermal imaging is a rapid, nonionizing, and acceptable technique that can reliably quantify thermogenesis within the supraclavicular region in humans (73, 74). Increases in BAT activity are closely related to a rise in depot temperature. However, the accuracy of these measures decreases in obese subjects since it is influenced by the amount of subcutaneous tissue in the supraclavicular fossa (75).
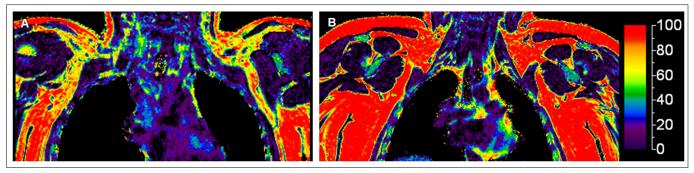
MRI developments will likely be a major driving force in deciphering the relevance of BAT in children. Based on the cytological differences in lipid content and degree of vascularization between BAT and WAT, fast magnetic resonance techniques are being developed that provide reliable BAT measures which can be applied even to infants without the need for sedation (76, 77) (Figure 4). It should be noted that BAT depots contain a mixture of multilocular brown adipocytes interspersed within unilocular white adipocytes, and that no imaging modality currently has sufficient resolution to localize microscopic deposits of brown adipocytes within a mixed cell population.
Figure 4.
Imaging characteristics of brown and white adipose tissue in infancy. Axial (A, B) and coronal (C, D) MR views of a 4 month infant depicting both white fat (white arrow) and brown fat (brown arrow) at the level of thoracic inlet. Compared to white fat, brown fat is hypointense/darker in the fat images (A, C), and has a lower fat fraction (green versus red) in the co-registered fat and water images (B, D). (Adapted from Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, Kajimura S. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7(11):e49452.)
Lastly, emerging evidence indicates circadian rhythms in BAT activity (78). Data in mice using PET/CT shows a diurnal rhythm in glucose uptake by BAT (79). Studies are needed to establish the rhythms in clock gene expression in BAT in humans and whether the activity of this tissue increases during night. Given the close association between exercise-induced muscle function, the release of irisin, and the formation of brown fat-like adipocytes (48), it is essential to investigate the possible correlate to complete the cycle: Does sleep-induced BAT activity promote structural and metabolic changes in skeletal muscle? And which brown adipokine(s) governs this adaptive response? Only then will we be in a position to begin to understand the mechanism(s) by which musculoskeletal development progresses during periods of inactivity.
CONCLUSIONS
Brown fat is known to have been present in mammals over 150 million years ago, and was considered an evolutionary advantage due solely to its unique ability to enhance survival in cold environments (7, 80). However, it is becoming increasingly clear that this tissue may have greater relevance to human health. The main areas of progress in BAT research during the last decade have been: 1) the general acceptance that this tissue is present in humans of all ages and especially abundant during adolescence, 2) the recognition that BAT may not only dissipate energy in the form of heat, but may also be a key determinant of weight and musculoskeletal development during childhood, and 3) insights into the complex transcriptional controls of brown fat development. Although much more work is needed, it is tempting to think that our challenge for the next decade lies in delineating the molecular regulation of BAT in humans and the crosstalk between brown and white adipocytes and myocytes. Defining the implications that BAT has for early human growth and how it influences health as we age is a most attractive and promising field of research.
Acknowledgments
Statement of financial support: National Institutes of Health grant R21DK090778-01.
REFERENCES
- 1.Gessner K. History of Animals. 1551:842. Conradi Gesneri medici Tiguine Historiae Animalium Lib I de Quadripedibus uiuparis. Book 1 of Four-Legged Uniparous. [Google Scholar]
- 2.Smith RE. Thermogenic activity of the hibernating gland in the cold-acclimated rat. Physiologist. 1961;4:113. [Google Scholar]
- 3.Lucas L, Rappeport Jack, A 2011. Obesity: An even heftier problem. Financial Times [Google Scholar]
- 4.Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. 2012;160:604–609 e601. doi: 10.1016/j.jpeds.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 8.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 9.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–606. doi: 10.1152/ajpendo.00298.2010. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 11.Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 12.Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp Physiol. 2003;88:141–148. doi: 10.1113/eph8802508. [DOI] [PubMed] [Google Scholar]
- 13.Ouellet V, Labbe SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stillway LW. Bioenergetics and oxidative metabolism. In: Baynes JW, Dominiczak MH, editors. Medical Biochemistry. Elsevier Mosby; Philadelphia: 2008. p. 106. [Google Scholar]
- 15.Furlong MA, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25:809–814. doi: 10.1097/00000478-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Ichimiya H, Arakawa S, Sato T, et al. Involvement of brown adipose tissue in subcutaneous fat necrosis of the newborn. Dermatology. 2011;223:207–210. doi: 10.1159/000331810. [DOI] [PubMed] [Google Scholar]
- 17.Hatai S. Annals of Anatomy. 1902 Anatomischer Anzeiger. [Google Scholar]
- 18.Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH. Functional brown adipose tissue is related to muscle volume in children and adolescents. J Pediatr. 2011;158:722–726. doi: 10.1016/j.jpeds.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelfand MJ, O’Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35:984–990. doi: 10.1007/s00247-005-1505-8. [DOI] [PubMed] [Google Scholar]
- 20.Drubach LA, Palmer EL, 3rd, Connolly LP, Baker A, Zurakowski D, Cypess AM. Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J Pediatr. 2011;159:939–944. doi: 10.1016/j.jpeds.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Zukotynski KA, Fahey FH, Laffin S, et al. Constant ambient temperature of 24 degrees C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. Eur J Nucl Med Mol Imaging. 2009;36:602–606. doi: 10.1007/s00259-008-0983-y. [DOI] [PubMed] [Google Scholar]
- 22.Truong MT, Erasmus JJ, Munden RF, et al. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol. 2004;183:1127–1132. doi: 10.2214/ajr.183.4.1831127. [DOI] [PubMed] [Google Scholar]
- 23.Garcia CA, Van Nostrand D, Atkins F, et al. Reduction of brown fat 2-deoxy-2-[F-18]fluoro-D-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imaging Biol. 2006;8:24–29. doi: 10.1007/s11307-005-0030-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee P, Zhao JT, Swarbrick MM, et al. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab. 2011;96:2450–2455. doi: 10.1210/jc.2011-0487. [DOI] [PubMed] [Google Scholar]
- 25.Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- 26.Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–2587. doi: 10.2337/db09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilsanz V, Hu HH, Smith ML, et al. The depiction of brown adipose tissue is related to disease status in pediatric patients with lymphoma. AJR Am J Roentgenol. 2012;198:909–913. doi: 10.2214/AJR.11.7488. [DOI] [PubMed] [Google Scholar]
- 28.Chalfant JS, Smith ML, Hu HH, et al. Inverse association between brown adipose tissue activation and white adipose tissue accumulation in successfully treated pediatric malignancy. Am J Clin Nutr. 2012;95:1144–1149. doi: 10.3945/ajcn.111.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai N, Sakane N, Ueno LM, Hamada T, Moritani T. The −3826 A-->G variant of the uncoupling protein-1 gene diminishes postprandial thermogenesis after a high fat meal in healthy boys. J Clin Endocrinol Metab. 2003;88:5661–5667. doi: 10.1210/jc.2003-030672. [DOI] [PubMed] [Google Scholar]
- 31.Warzocha K, Salles G, Bienvenu J, et al. Tumor necrosis factor ligand-receptor system can predict treatment outcome in lymphoma patients. J Clin Oncol. 1997;15:499–508. doi: 10.1200/JCO.1997.15.2.499. [DOI] [PubMed] [Google Scholar]
- 32.Nisoli E, Briscini L, Tonello C, De Giuli-Morghen C, Carruba MO. Tumor necrosis factor-alpha induces apoptosis in rat brown adipocytes. Cell Death Differ. 1997;4:771–778. doi: 10.1038/sj.cdd.4400292. [DOI] [PubMed] [Google Scholar]
- 33.Romanatto T, Roman EA, Arruda AP, et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem. 2009;284:36213–36222. doi: 10.1074/jbc.M109.030874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Rousseau C, Bourbouloux E, Campion, et al. Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging. 2006;33:785–791. doi: 10.1007/s00259-006-0066-x. [DOI] [PubMed] [Google Scholar]
- 35.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Cannon B, Nedergaard J. Thermogenesis challenges the adipostat hypothesis for body-weight control. Proc Nutr Soc. 2009;68:401–407. doi: 10.1017/S0029665109990255. [DOI] [PubMed] [Google Scholar]
- 37.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 38.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- 39.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 40.Sturkenboom MG, Franssen EJ, Berkhof J, Hoekstra OS. Physiological uptake of [18F]fluorodeoxyglucose in the neck and upper chest region: are there predictive characteristics? Nucl Med Commun. 2004;25:1109–1111. doi: 10.1097/00006231-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Yoneshiro T, Aita S, Matsushita M, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19:13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 42.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cartier A, Lemieux I, Almeras N, Tremblay A, Bergeron J, Despres JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab. 2008;93:1931–1938. doi: 10.1210/jc.2007-2191. [DOI] [PubMed] [Google Scholar]
- 44.Farmer SR. Brown fat and skeletal muscle: unlikely cousins? Cell. 2008;134:726–727. doi: 10.1016/j.cell.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Cuenca S, Monjo M, Frontera M, Gianotti M, Proenza AM, Roca P. Sex steroid receptor expression profile in brown adipose tissue. Effects of hormonal status. Cell Physiol Biochem. 2007;20:877–886. doi: 10.1159/000110448. [DOI] [PubMed] [Google Scholar]
- 50.Hioki C, Yoshida T, Kogure A, et al. Effects of growth hormone (GH) on mRNA levels of uncoupling proteins 1, 2, and 3 in brown and white adipose tissues and skeletal muscle in obese mice. Horm Metab Res. 2004;36:607–613. doi: 10.1055/s-2004-825905. [DOI] [PubMed] [Google Scholar]
- 51.Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond) 1986;71:291–297. doi: 10.1042/cs0710291. [DOI] [PubMed] [Google Scholar]
- 52.Bredella MA, Fazeli PK, Freedman LM, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97:E584–590. doi: 10.1210/jc.2011-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponrartana S, Aggabao PC, Hu HH, Aldrovandi GM, Wren TA, Gilsanz V. Brown adipose tissue and its relationship to bone structure in pediatric patients. J Clin Endocrinol Metab. 2012;97:2693–2698. doi: 10.1210/jc.2012-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas DM, Carty SA, Piscopo DM, et al. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell. 2001;8:303–316. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 56.Olmsted-Davis E, Gannon FH, Ozen M, et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–632. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zanotti S, Stadmeyer L, Smerdel-Ramoya A, Durant D, Canalis E. Misexpression of CCAAT/enhancer binding protein beta causes osteopenia. J Endocrinol. 2009;201:263–274. doi: 10.1677/JOE-08-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seger RL, Cross RA, Rosen CJ, et al. Investigating the mechanism for maintaining eucalcemia despite immobility and anuria in the hibernating American black bear (Ursus americanus) Bone. 2011;49:1205–1212. doi: 10.1016/j.bone.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Egginton S, Fairney J, Bratcher J. Differential effects of cold exposure on muscle fibre composition and capillary supply in hibernator and non-hibernator rodents. Exp Physiol. 2001;86:629–639. doi: 10.1113/eph8602260. [DOI] [PubMed] [Google Scholar]
- 60.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Svensson PA, Jernas M, Sjoholm K, et al. Gene expression in human brown adipose tissue. Int J Mol Med. 2011;27:227–232. doi: 10.3892/ijmm.2010.566. [DOI] [PubMed] [Google Scholar]
- 62.Hernandez A, Martinez de Mena R, Martin E, Obregon MJ. Differences in the response of UCP1 mRNA to hormonal stimulation between rat and mouse primary cultures of brown adipocytes. Cell Physiol Biochem. 2011;28:969–980. doi: 10.1159/000335810. [DOI] [PubMed] [Google Scholar]
- 63.Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology. 2011;152:3597–3602. doi: 10.1210/en.2011-1349. [DOI] [PubMed] [Google Scholar]
- 64.Wilson-Fritch L, Nicoloro S, Chouinard M, et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vernochet C, Peres SB, Davis KE, et al. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–4728. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2009;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58:1013–1022. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 69.Merklin RJ. Growth and distribution of human fetal brown fat. Anat Rec. 1974;178:637–645. doi: 10.1002/ar.1091780311. [DOI] [PubMed] [Google Scholar]
- 70.Oddy WH. Long-term health outcomes and mechanisms associated with breastfeeding. Expert Rev Pharmacoecon Outcomes Res. 2002;2:161–177. doi: 10.1586/14737167.2.2.161. [DOI] [PubMed] [Google Scholar]
- 71.Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. Int J Pediatr Endocrinol. 2009;2009:327505. doi: 10.1155/2009/327505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Matheny M, Zolotukhin S, Tumer N, Scarpace PJ. Regulation of adiponectin and leptin gene expression in white and brown adipose tissues: influence of beta3-adrenergic agonists, retinoic acid, leptin and fasting. Biochim Biophys Acta. 2002;1584:115–122. doi: 10.1016/s1388-1981(02)00298-6. [DOI] [PubMed] [Google Scholar]
- 73.Lee P, Ho KK, Greenfield JR. Hot fat in a cool man: infrared thermography and brown adipose tissue. Diabetes Obes Metab. 2011;13:92–93. doi: 10.1111/j.1463-1326.2010.01318.x. [DOI] [PubMed] [Google Scholar]
- 74.Rylander E. Age dependent reactions of rectal and skin temperatures of infants during exposure to cold. Acta Paediatr Scand. 1972;61:597–605. doi: 10.1111/j.1651-2227.1972.tb15952.x. [DOI] [PubMed] [Google Scholar]
- 75.Symonds ME, Henderson K, Elvidge L, et al. Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.04.056. doi:10.1016/j.jpeds.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 76.Hu HH, Tovar JP, Pavlova Z, Smith ML, Gilsanz V. Unequivocal identification of brown adipose tissue in a human infant. J Magn Reson Imaging. 2012;35:938–942. doi: 10.1002/jmri.23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu HH, Perkins TG, Chia JM, Gilsanz V. Characterization of human brown adipose tissue by chemical-shift water-fat MRI. Am J Roentgenol. 2013 Jan;200(1):177–83. doi: 10.2214/AJR.12.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zvonic S, Ptitsyn AA, Conrad SA, et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 79.van der Veen DR, Shao J, Chapman S, Leevy WM, Duffield GE. A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity (Silver Spring) 2012;20:1527–1529. doi: 10.1038/oby.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jastroch M, Withers KW, Taudien S, et al. Marsupial uncoupling protein 1 sheds light on the evolution of mammalian nonshivering thermogenesis. Physiol Genomics. 2008;32:161–169. doi: 10.1152/physiolgenomics.00183.2007. [DOI] [PubMed] [Google Scholar]
- 81.Hu HH, Yin L, Aggabao PC, Perkins TG, Chia JM, Gilsanz V. Comparison of brown and white adipose tissues in infants and children with chemical-shift-encoded water-fat MRI. J Magn Reson Imaging. 2013 Feb 25; doi: 10.1002/jmri.24053. doi: 10.1002/jmri.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, Kajimura S. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7(11):e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]