Abstract
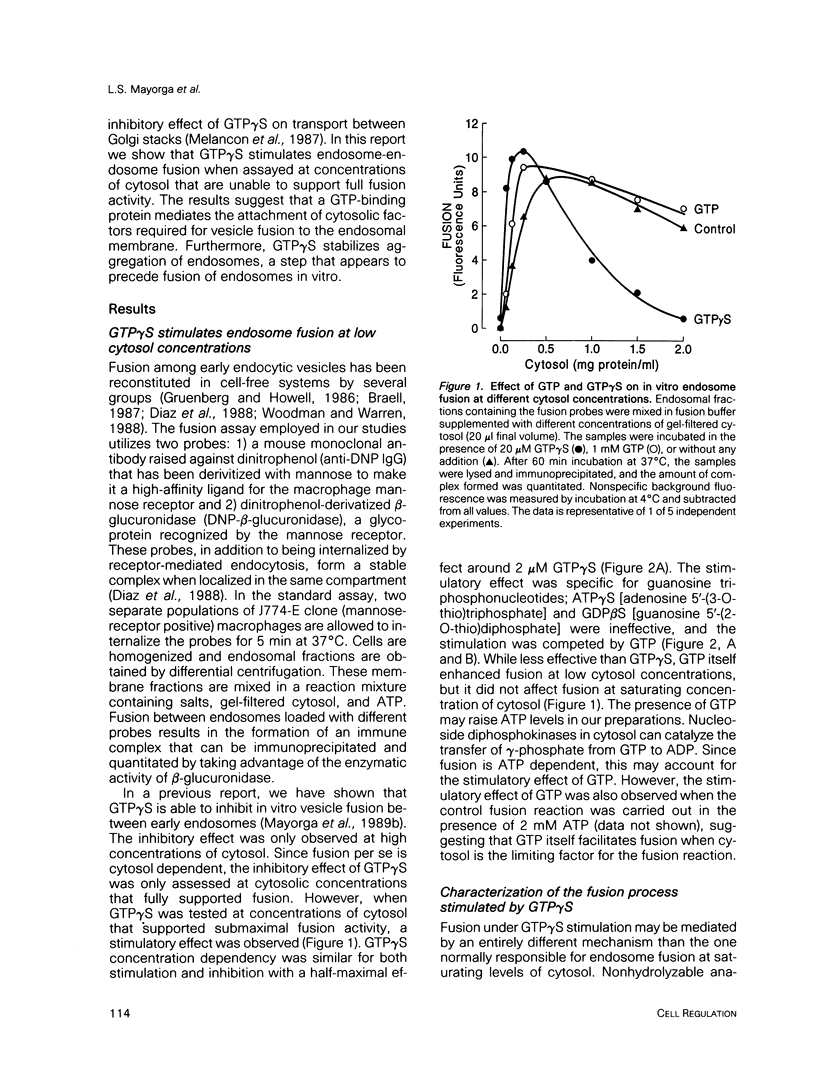
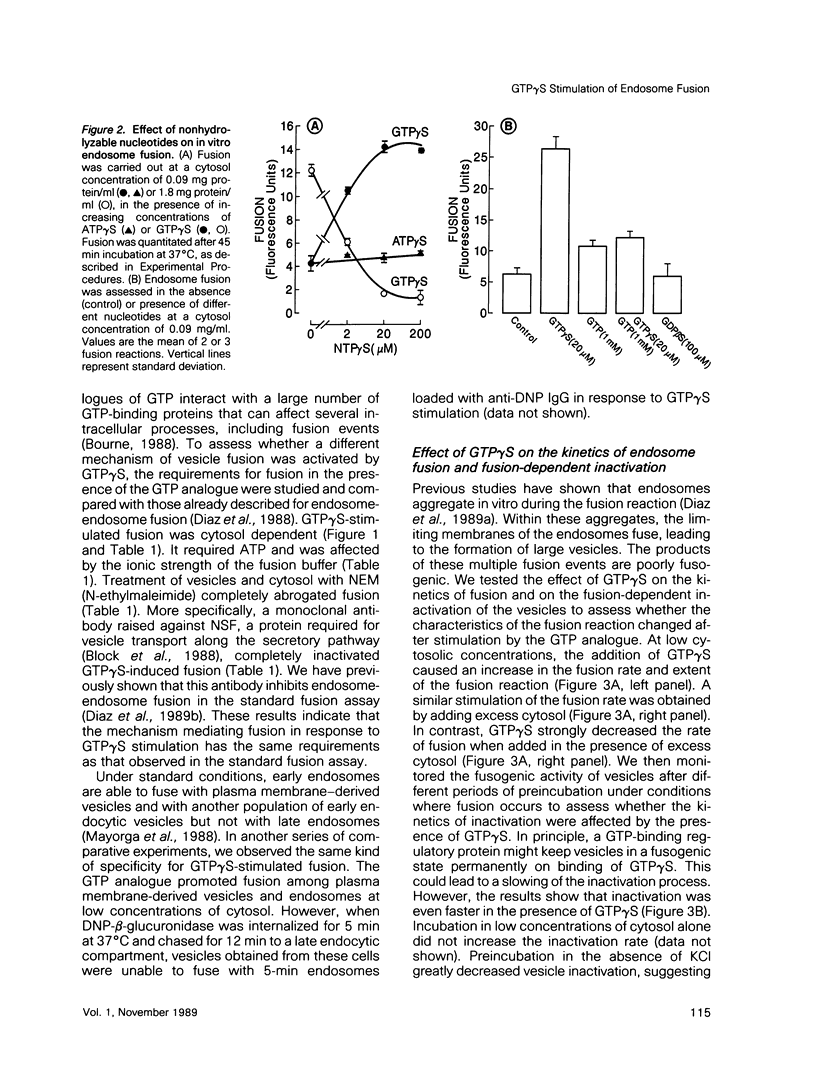
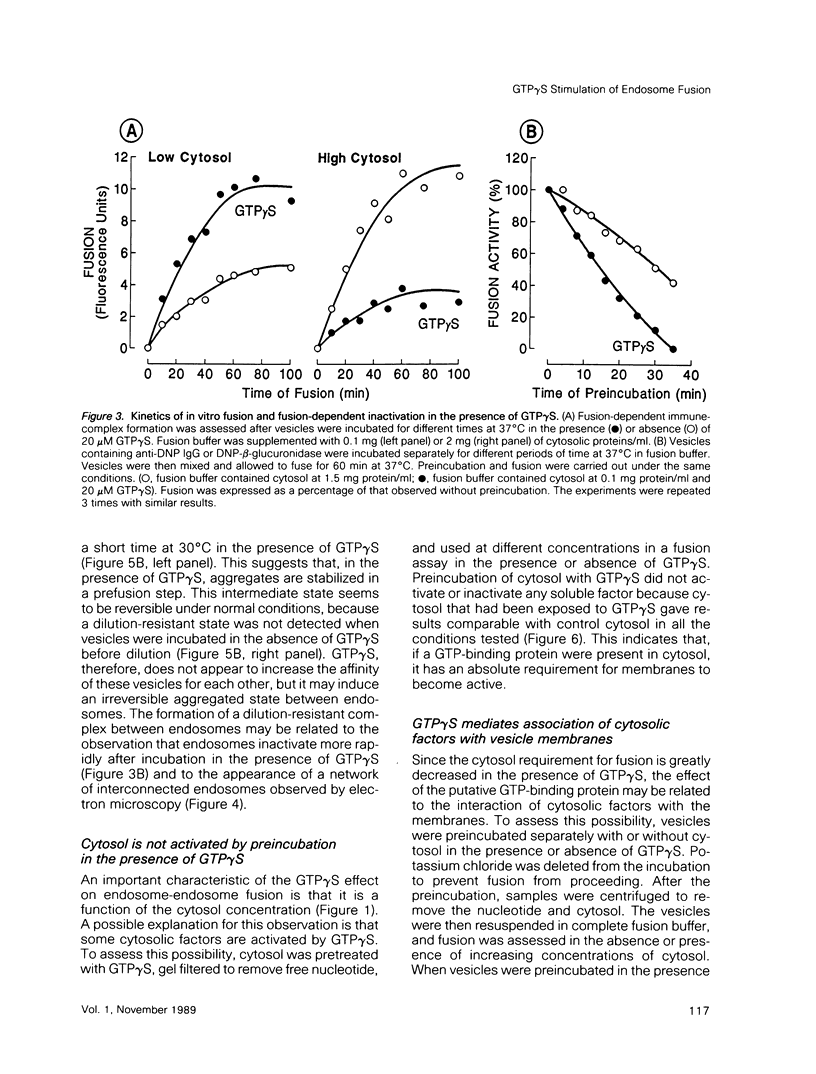
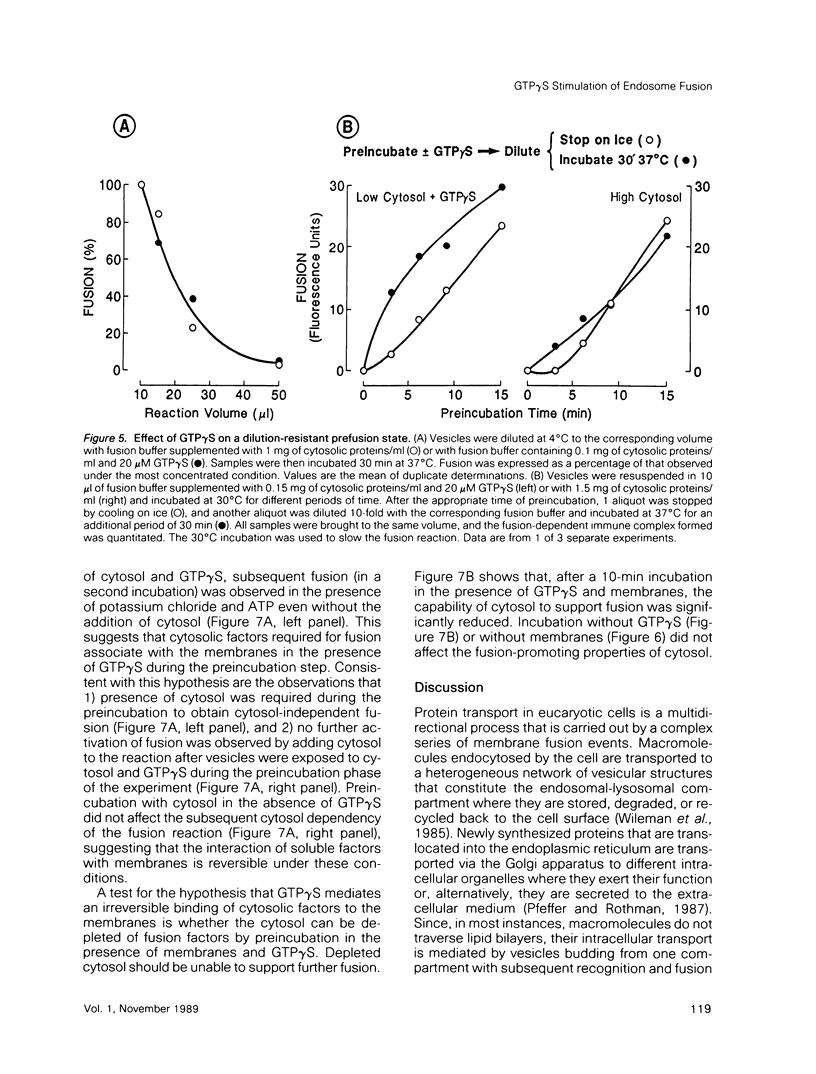
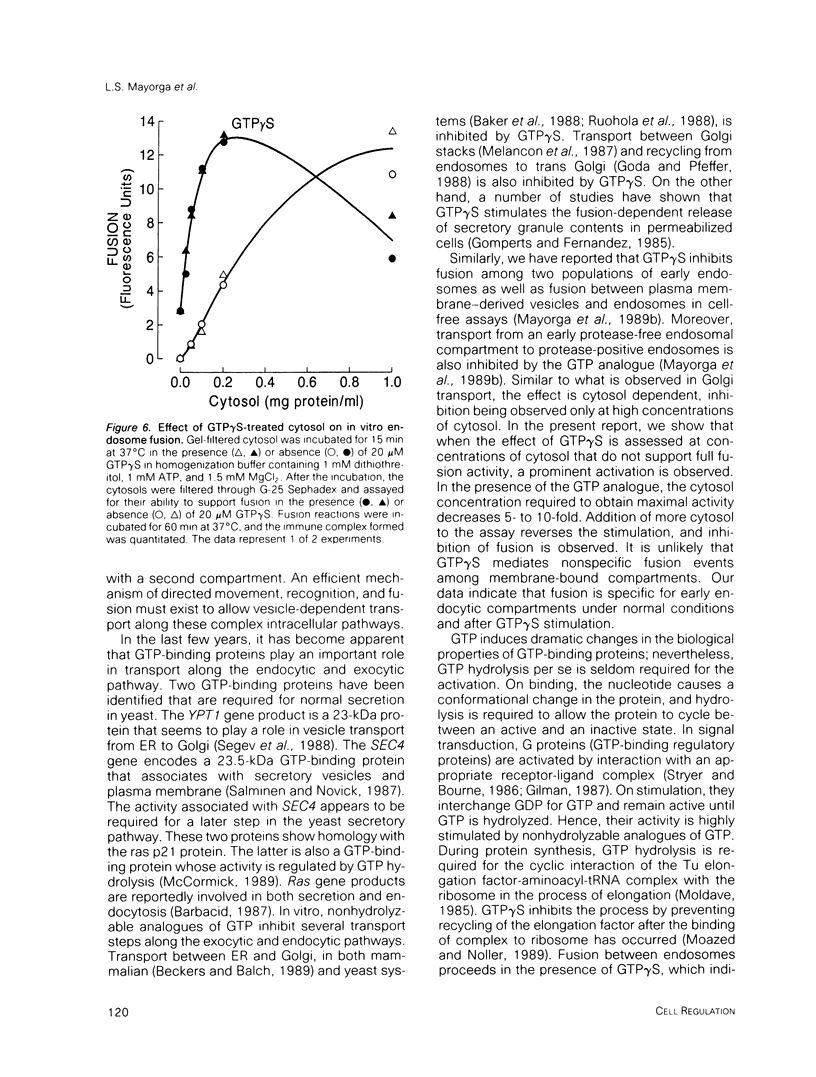
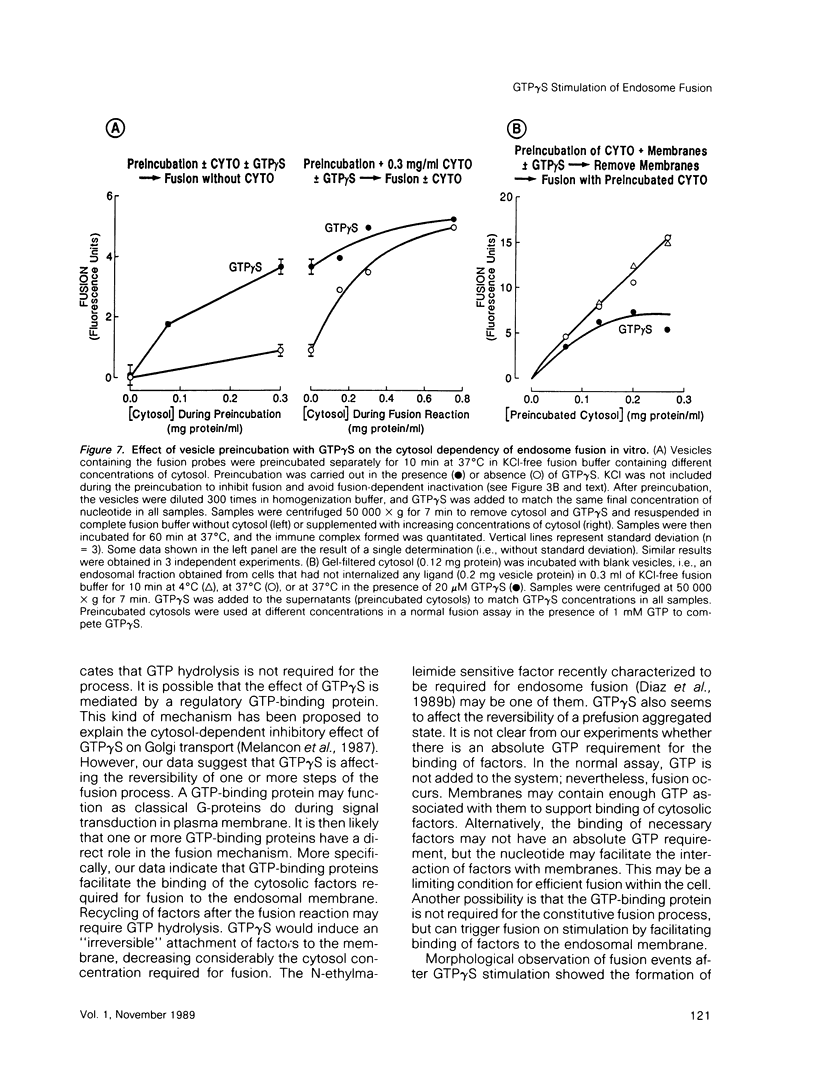
Guanosine 5'-(3-O-thio)triphosphate (GTP gamma S), a non-hydrolyzable analogue of GTP, inhibits in vitro fusion among early endocytic vesicles in the presence of high concentrations of cytosol. In this report we show that fusion is remarkably stimulated by GTP gamma S under conditions where cytosolic components are the limiting factors for the process. The amount of cytosolic factors required for maximal fusion activity is several-fold decreased by the presence of GTP gamma S. Moreover, preincubation of vesicles in the presence of cytosol and GTP gamma S allows fusion to proceed even in the absence of cytosol. Our results indicate that a GTP-binding protein facilitates the binding of cytosolic factor(s) required for endosome fusion to the endosomal membrane and stabilizes a dilution-resistant intermediate of the fusion process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D., Hicke L., Rexach M., Schleyer M., Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988 Jul 29;54(3):335–344. doi: 10.1016/0092-8674(88)90196-1. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Beckers C. J., Balch W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989 Apr;108(4):1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers C. J., Block M. R., Glick B. S., Rothman J. E., Balch W. E. Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989 Jun 1;339(6223):397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- Block M. R., Glick B. S., Wilcox C. A., Wieland F. T., Rothman J. E. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Braell W. A. Fusion between endocytic vesicles in a cell-free system. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Mayorga L., Stahl P. In vitro fusion of endosomes following receptor-mediated endocytosis. J Biol Chem. 1988 May 5;263(13):6093–6100. [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goda Y., Pfeffer S. R. Selective recycling of the mannose 6-phosphate/IGF-II receptor to the trans Golgi network in vitro. Cell. 1988 Oct 21;55(2):309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. E., Howell K. E. Reconstitution of vesicle fusions occurring in endocytosis with a cell-free system. EMBO J. 1986 Dec 1;5(12):3091–3101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983 Aug;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. K., Touster O. Physical and chemical properties of beta-glucuronidase from the preputial gland of the female rat. J Biol Chem. 1975 Jun 25;250(12):4765–4769. [PubMed] [Google Scholar]
- Malhotra V., Orci L., Glick B. S., Block M. R., Rothman J. E. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988 Jul 15;54(2):221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Plasma membrane-derived vesicles containing receptor-ligand complexes are fusogenic with early endosomes in a cell-free system. J Biol Chem. 1988 Nov 25;263(33):17213–17216. [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Regulatory role for GTP-binding proteins in endocytosis. Science. 1989 Jun 23;244(4911):1475–1477. doi: 10.1126/science.2499930. [DOI] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989 May 19;57(4):585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Ohmori T., Kikuchi A., Yamamoto K., Kim S., Takai Y. Small molecular weight GTP-binding proteins in human platelet membranes. Purification and characterization of a novel GTP-binding protein with a molecular weight of 22,000. J Biol Chem. 1989 Jan 25;264(3):1877–1881. [PubMed] [Google Scholar]
- Orci L., Malhotra V., Amherdt M., Serafini T., Rothman J. E. Dissection of a single round of vesicular transport: sequential intermediates for intercisternal movement in the Golgi stack. Cell. 1989 Feb 10;56(3):357–368. doi: 10.1016/0092-8674(89)90239-0. [DOI] [PubMed] [Google Scholar]
- Otsuka F. L., Welch M. J., McElvany K. D., Nicolotti R. A., Fleischman J. B. Development of a model system to evaluate methods for radiolabeling monoclonal antibodies. J Nucl Med. 1984 Dec;25(12):1343–1349. [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Ruohola H., Kabcenell A. K., Ferro-Novick S. Reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex in yeast: the acceptor Golgi compartment is defective in the sec23 mutant. J Cell Biol. 1988 Oct;107(4):1465–1476. doi: 10.1083/jcb.107.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- Woodman P. G., Warren G. Fusion between vesicles from the pathway of receptor-mediated endocytosis in a cell-free system. Eur J Biochem. 1988 Apr 5;173(1):101–108. doi: 10.1111/j.1432-1033.1988.tb13972.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]



