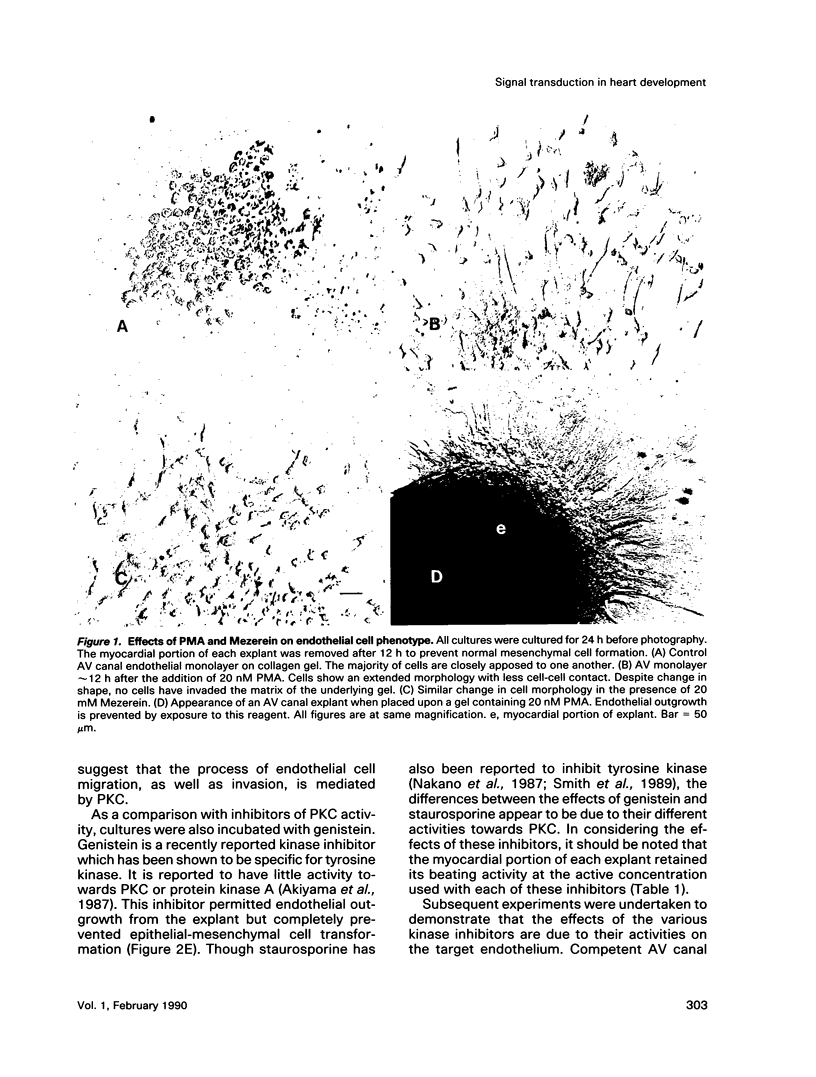
Abstract
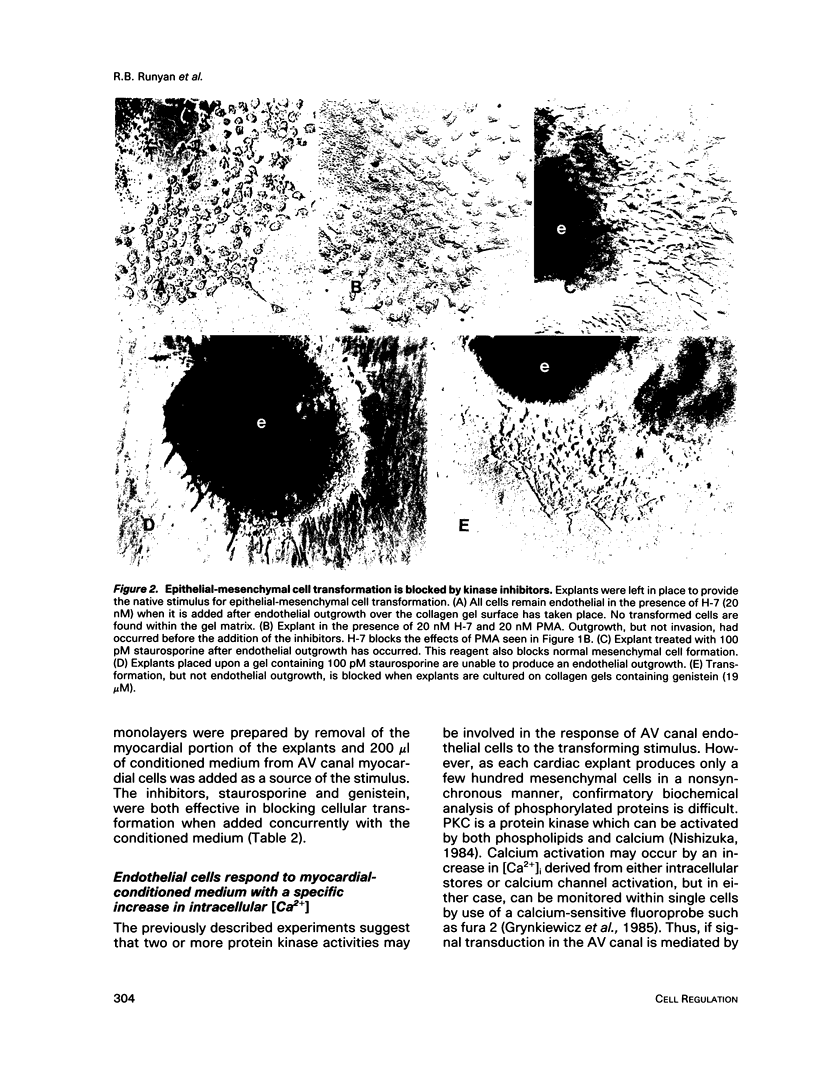
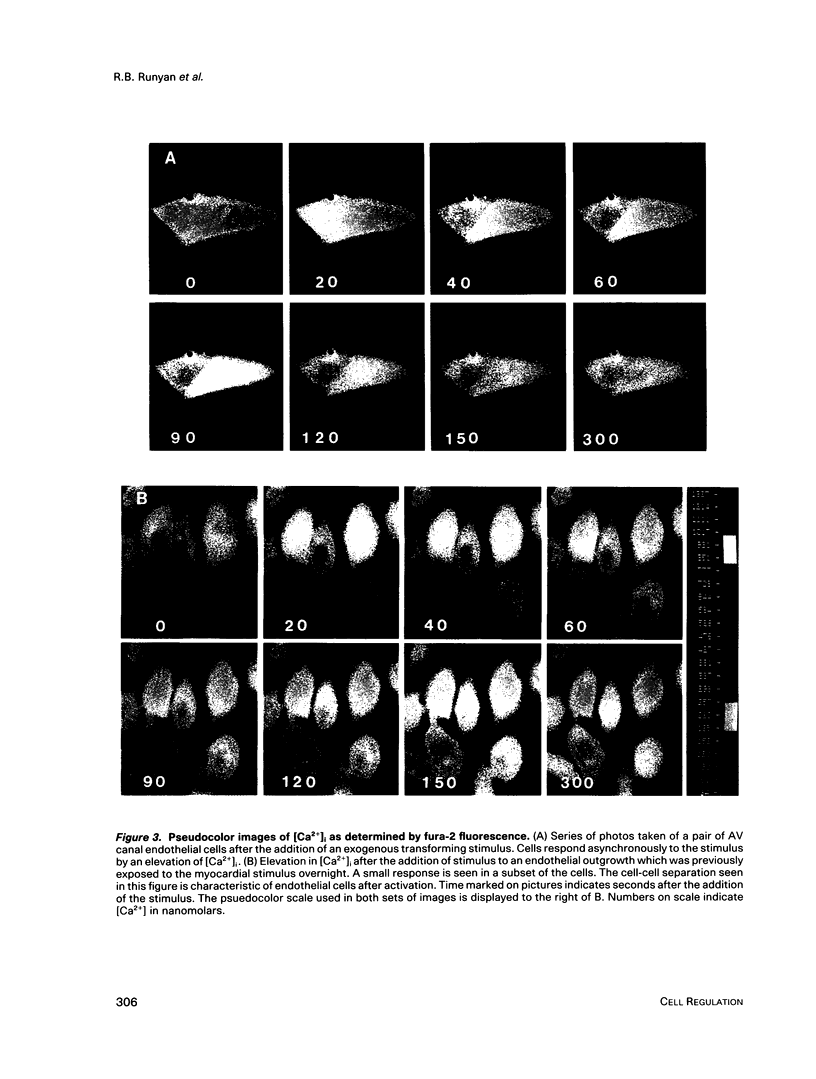
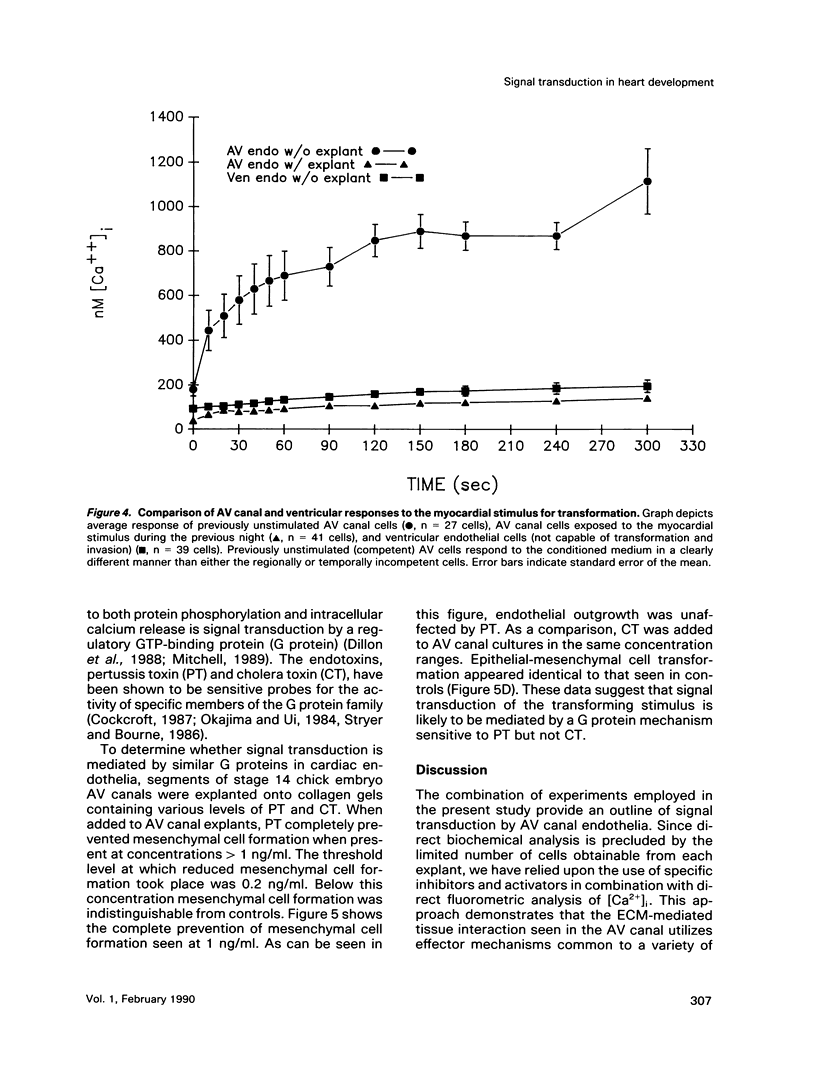
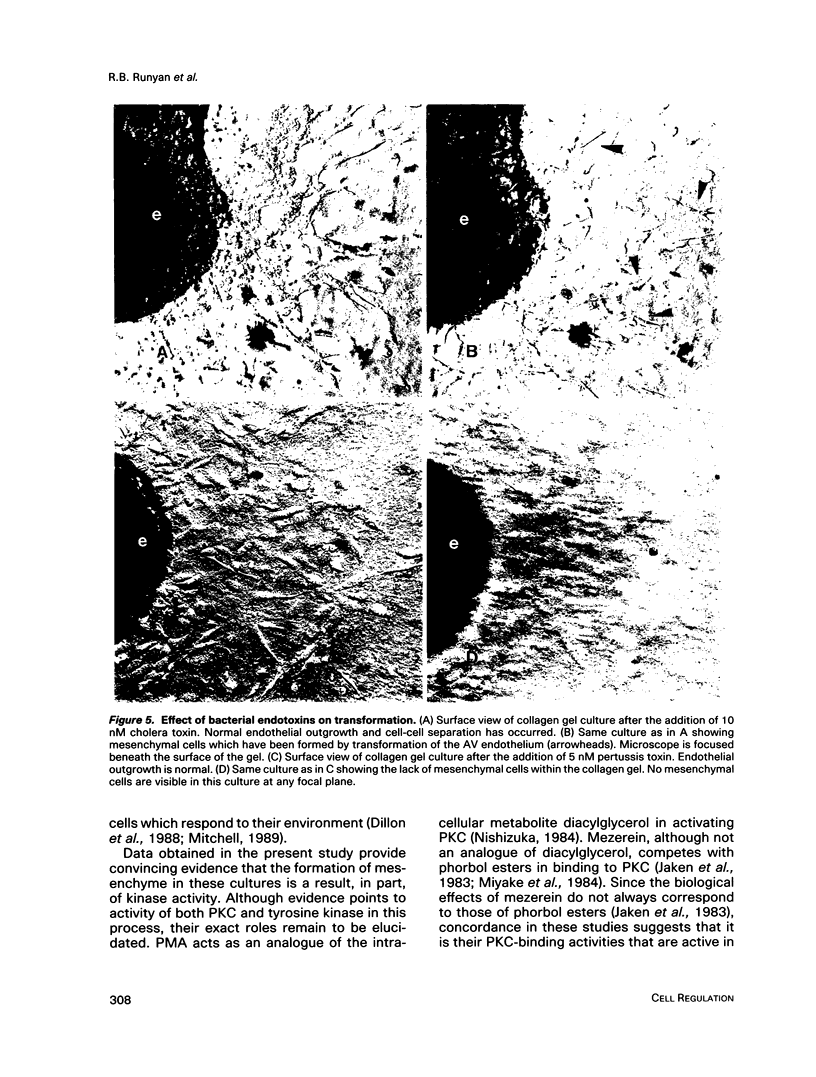
During early cardiac development, progenitors of the valves and septa of the heart are formed by an epithelial-mesenchymal cell transformation of endothelial cells of the atrioventricular (AV) canal. We have previously shown that this event is due to an interaction between the endothelium and products of the myocardium found within the extracellular matrix. The present study examines signal transduction mechanisms governing this differentiation of AV canal endothelium. Activators of protein kinase C (PKC), phorbol myristate acetate (PMA) and mezerein, both produced an incomplete phenotypic transformation of endothelial cells in an in vitro bioassay for transformation. On the other hand, inhibitors of PKC (H-7 and staurosporine) and tyrosine kinase (genistein) blocked cellular transformation in response to the native myocardium or a myocardially-conditioned medium. Intracellular free calcium concentration ([Ca2+]i) was measured in single endothelial cells by microscopic digital analysis of fura 2 fluorescence. Addition of a myocardial conditioned medium containing the transforming stimulus produced a specific increase in [Ca2+]i in "competent" AV canal, but not ventricular, endothelial cells. Epithelial-mesenchymal cell transformation was inhibited by pertussis toxin but not cholera toxin. These data lead to the hypothesis that signal transduction of this tissue interaction is mediated by a G protein and one or more kinase activities. In response to receptor activation, competent AV canal endothelial cells demonstrate an increase in [Ca2+]i. Together, the data provide direct evidence for a regional and temporal regulation of signal transduction processes which mediate a specific extracellular matrix-mediated tissue interaction in the embryo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Bernanke D. H., Markwald R. R. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982 Jun;91(2):235–245. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bolender D. L., Markwald R. R. Epithelial-mesenchymal transformation in chick atrioventricular cushion morphogenesis. Scan Electron Microsc. 1979;(3):313–321. [PubMed] [Google Scholar]
- Coghlan J. G., Paul V. E., Mitchell A. G. Cardiac involvement by lymphoma: diagnostic difficulties. Eur Heart J. 1989 Aug;10(8):765–768. doi: 10.1093/oxfordjournals.eurheartj.a059562. [DOI] [PubMed] [Google Scholar]
- Dillon S. B., Verghese M. W., Snyderman R. Signal transduction in cells following binding of chemoattractants to membrane receptors. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55(2):65–80. doi: 10.1007/BF02896561. [DOI] [PubMed] [Google Scholar]
- Funderburg F. M., Markwald R. R. Conditioning of native substrates by chondroitin sulfate proteoglycans during cardiac mesenchymal cell migration. J Cell Biol. 1986 Dec;103(6 Pt 1):2475–2487. doi: 10.1083/jcb.103.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jaken S., Shupnik M. A., Blumberg P. M., Tashjian A. H., Jr Relationship between mezerein-mediated biological responses and phorbol ester receptor occupancy. Cancer Res. 1983 Jan;43(1):11–14. [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Kelvin D. J., Shreeve M., McAuley C., McLeod D. L., Simard G., Connolly J. A. Interleukin 3-stimulated proliferation is sensitive to pertussis toxin: evidence for a guanyl nucleotide regulatory protein-mediated signal transduction mechanism. J Cell Physiol. 1989 Feb;138(2):273–280. doi: 10.1002/jcp.1041380208. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Kozawa O., Kaibuchi K., Katada T., Ui M., Takai Y. Direct evidence for involvement of a guanine nucleotide-binding protein in chemotactic peptide-stimulated formation of inositol bisphosphate and trisphosphate in differentiated human leukemic (HL-60) cells. Reconstitution with Gi or Go of the plasma membranes ADP-ribosylated by pertussis toxin. J Biol Chem. 1986 Sep 5;261(25):11558–11562. [PubMed] [Google Scholar]
- Krug E. L., Mjaatvedt C. H., Markwald R. R. Extracellular matrix from embryonic myocardium elicits an early morphogenetic event in cardiac endothelial differentiation. Dev Biol. 1987 Apr;120(2):348–355. doi: 10.1016/0012-1606(87)90237-5. [DOI] [PubMed] [Google Scholar]
- Krug E. L., Runyan R. B., Markwald R. R. Protein extracts from early embryonic hearts initiate cardiac endothelial cytodifferentiation. Dev Biol. 1985 Dec;112(2):414–426. doi: 10.1016/0012-1606(85)90414-2. [DOI] [PubMed] [Google Scholar]
- McArdle C. A., Conn P. M. Use of protein kinase C-depleted cells for investigation of the role of protein kinase C in stimulus-response coupling in the pituitary. Methods Enzymol. 1989;168:287–301. doi: 10.1016/0076-6879(89)68020-2. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Stevens V. L. Modulation of protein kinase C and diverse cell functions by sphingosine--a pharmacologically interesting compound linking sphingolipids and signal transduction. Biochim Biophys Acta. 1989 Feb 9;1010(2):131–139. doi: 10.1016/0167-4889(89)90152-3. [DOI] [PubMed] [Google Scholar]
- Miyake R., Tanaka Y., Tsuda T., Kaibuchi K., Kikkawa U., Nishizuka Y. Activation of protein kinase C by non-phorbol tumor promoter, mezerein. Biochem Biophys Res Commun. 1984 Jun 15;121(2):649–656. doi: 10.1016/0006-291x(84)90231-6. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C. H., Lepera R. C., Markwald R. R. Myocardial specificity for initiating endothelial-mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev Biol. 1987 Jan;119(1):59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L., Rodland K. D., Magun B. E. Transforming growth factor beta modulates epidermal growth factor-induced phosphoinositide metabolism and intracellular calcium levels. J Biol Chem. 1988 Apr 15;263(11):5030–5033. [PubMed] [Google Scholar]
- Nakano H., Kobayashi E., Takahashi I., Tamaoki T., Kuzuu Y., Iba H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J Antibiot (Tokyo) 1987 May;40(5):706–708. doi: 10.7164/antibiotics.40.706. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Otte A. P., Koster C. H., Snoek G. T., Durston A. J. Protein kinase C mediates neural induction in Xenopus laevis. Nature. 1988 Aug 18;334(6183):618–620. doi: 10.1038/334618a0. [DOI] [PubMed] [Google Scholar]
- Potts J. D., Runyan R. B. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989 Aug;134(2):392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Runyan R. B., Markwald R. R. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Dev Biol. 1983 Jan;95(1):108–114. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Nakamura T., Porter K. R., Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984 Sep;99(3):1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. V., Bhalla R. C. Regulation of cytosolic free Ca2+ concentration in vascular smooth muscle cells by A- and C-kinases. Hypertension. 1989 Jun;13(6 Pt 2):845–850. doi: 10.1161/01.hyp.13.6.845. [DOI] [PubMed] [Google Scholar]
- Sinning A. R., Lepera R. C., Markwald R. R. Initial expression of type I procollagen in chick cardiac mesenchyme is dependent upon myocardial stimulation. Dev Biol. 1988 Nov;130(1):167–174. doi: 10.1016/0012-1606(88)90423-x. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Glickman J. F., Chang K. J. The antiproliferative effects of staurosporine are not exclusively mediated by inhibition of protein kinase C. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1250–1256. doi: 10.1016/s0006-291x(88)80767-8. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Zhao F. K., Chuang L. F., Israel M., Chuang R. Y. Cremophor EL, a widely used parenteral vehicle, is a potent inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1359–1367. doi: 10.1016/0006-291x(89)92260-2. [DOI] [PubMed] [Google Scholar]