Abstract
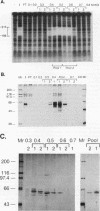
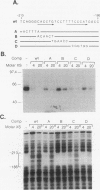
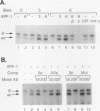
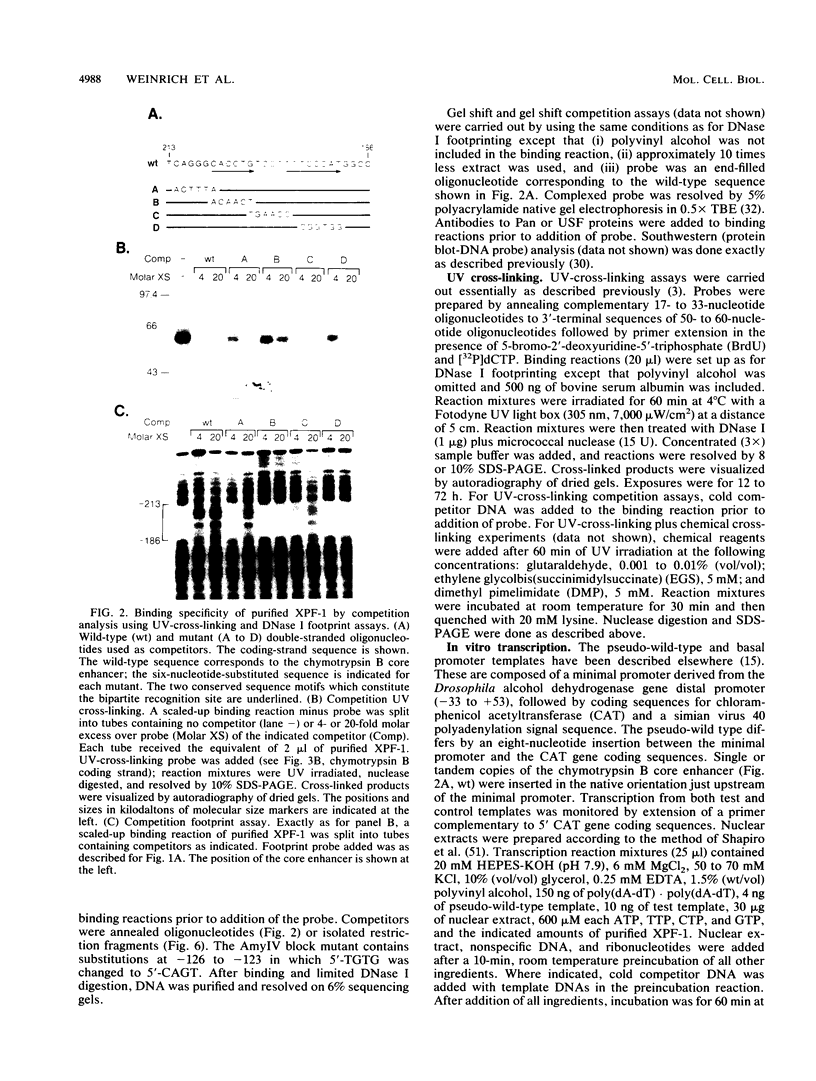
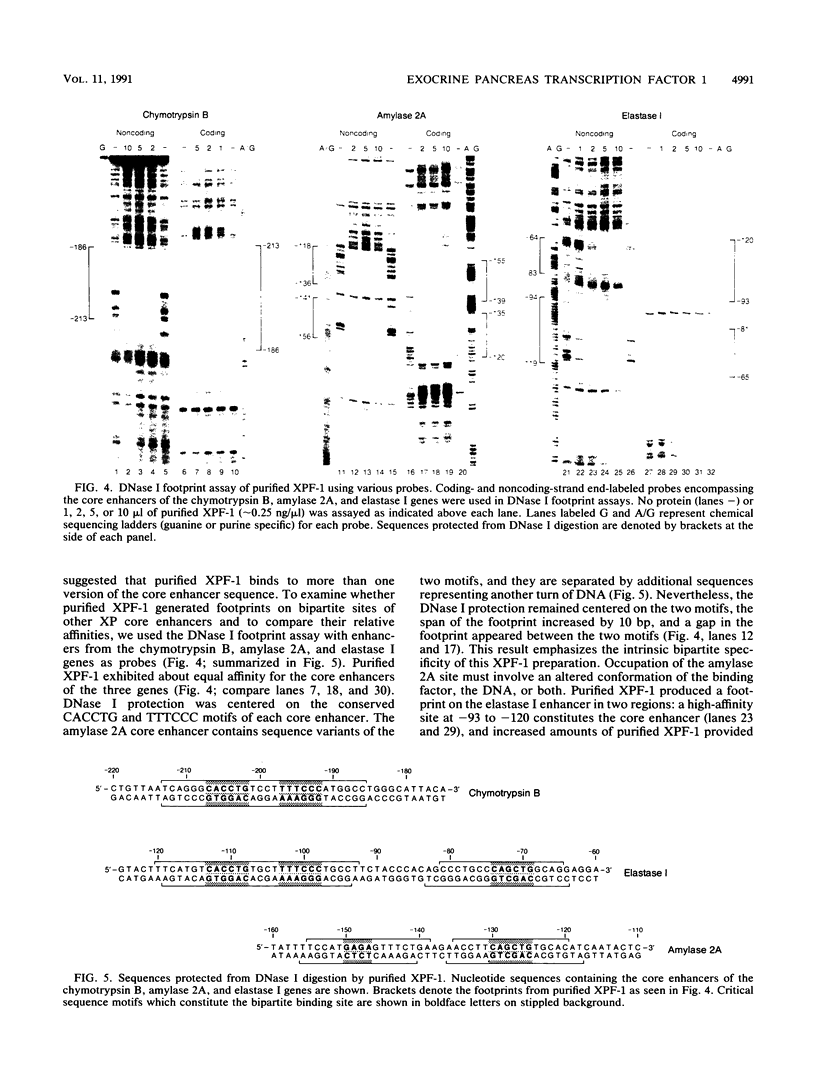
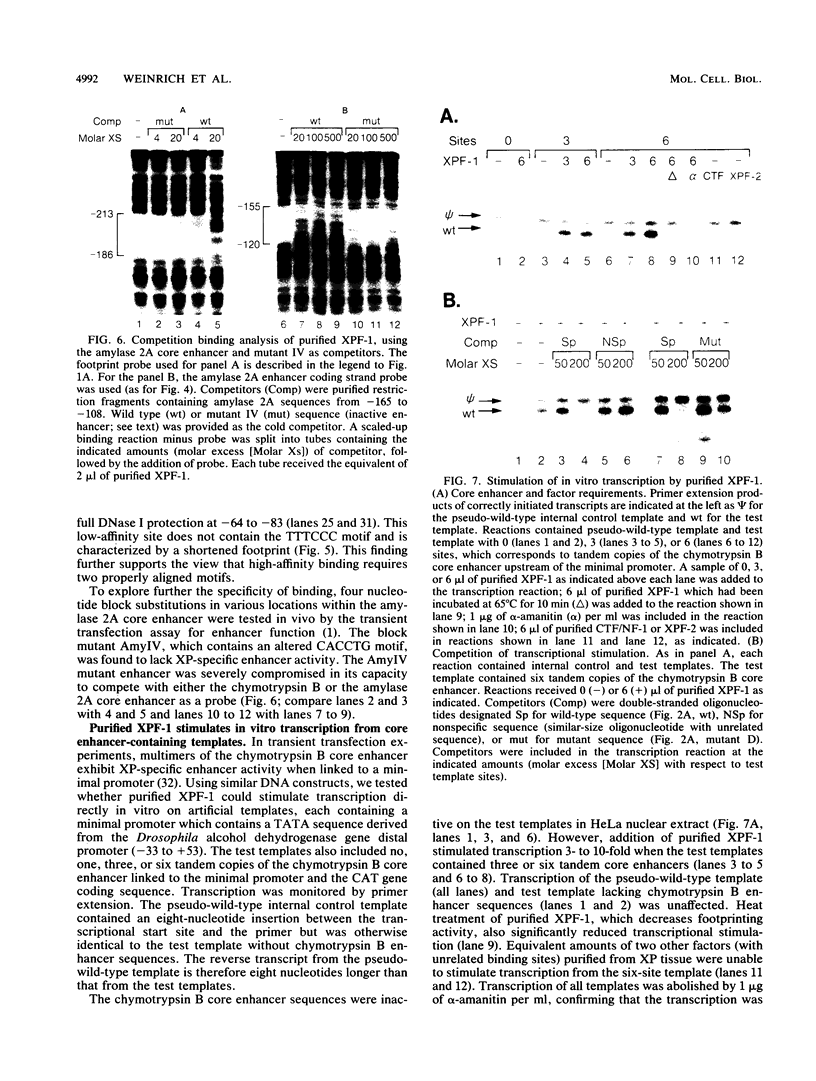
Exocrine pancreas (XP) enhancers, which contain a conserved core sequence, are active only in XP cells. A core enhancer-binding activity also appears to be restricted to XP nuclei. Here we describe the properties of a factor, purified approximately 100,000-fold from pancreas nuclei, which displays core enhancer-binding activity. It is not identical to previously characterized factors and is termed exocrine pancreas transcription factor 1 (XPF-1). In the highly purified preparation, only a single major protein of 60 kDa was detected by silver staining on sodium dodecyl sulfate-gels and by UV cross-linking. XPF-1 binds to the core enhancer of all tested XP genes and not to a mutant sequence which is inactive in vivo. High-affinity binding sites are bipartite. The results of competition binding and UV-cross-linking assays suggest that XPF-1 interacts with both motifs. XPF-1 selectively stimulates transcription of core enhancer templates in an in vitro transcription system. We hypothesize that XPF-1 plays a role in activation of the transcription of XP-specific genes.
Full text
PDF

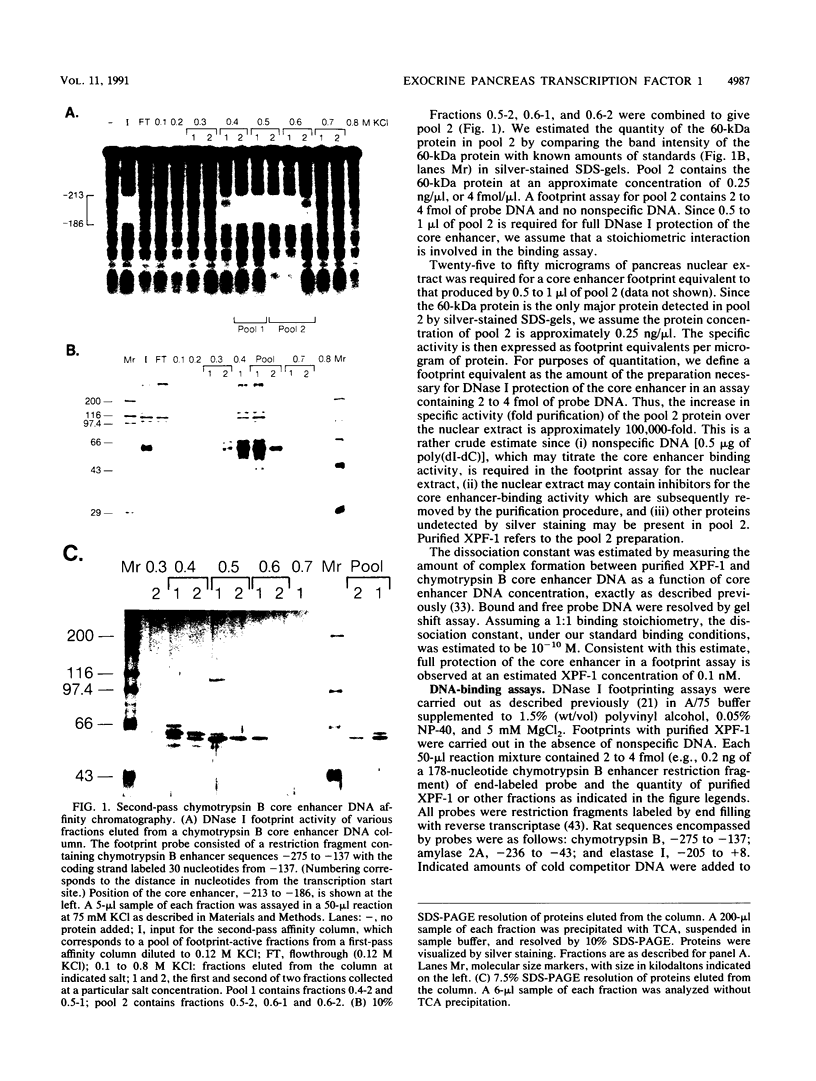







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulet A. M., Erwin C. R., Rutter W. J. Cell-specific enhancers in the rat exocrine pancreas. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3599–3603. doi: 10.1073/pnas.83.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Sharp P. A. A single polypeptide possesses the binding and transcription activities of the adenovirus major late transcription factor. Mol Cell Biol. 1986 Dec;6(12):4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell M., Stevenson B. J., Strubin M., Hagenbüchle O., Wellauer P. K. Identification of a cell-specific DNA-binding activity that interacts with a transcriptional activator of genes expressed in the acinar pancreas. Mol Cell Biol. 1989 Jun;9(6):2464–2476. doi: 10.1128/mcb.9.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Choo Q. L., Swift G. H., Quinto C., MacDonald R. J., Rutter W. J. Structure of two related rat pancreatic trypsin genes. J Biol Chem. 1984 Nov 25;259(22):14255–14264. [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. A DNA-binding protein containing two widely separated zinc finger motifs that recognize the same DNA sequence. Genes Dev. 1990 Jan;4(1):29–42. doi: 10.1101/gad.4.1.29. [DOI] [PubMed] [Google Scholar]
- Gordon J. S., Bell G. I., Martinson H. C., Rutter W. J. Selective interaction of 5'-bromodeoxyuridine substituted DNA with different chromosomal proteins. Biochemistry. 1976 Nov 2;15(22):4778–4785. doi: 10.1021/bi00667a005. [DOI] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Wiebauer K., Caldwell R. M., Samuelson L. C., Meisler M. H. Concerted evolution of human amylase genes. Mol Cell Biol. 1988 Mar;8(3):1197–1205. doi: 10.1128/mcb.8.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Schibler U., Petrucco S., Van Tuyle G. C., Wellauer P. K. Expression of mouse Amy-2a alpha-amylase genes is regulated by strong pancreas-specific promoters. J Mol Biol. 1985 Sep 20;185(2):285–293. doi: 10.1016/0022-2836(85)90404-8. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Swift G. H., Ornitz D. M., Quaife C. J., Palmiter R. D., Brinster R. L., MacDonald R. J. The rat elastase I regulatory element is an enhancer that directs correct cell specificity and developmental onset of expression in transgenic mice. Mol Cell Biol. 1987 Aug;7(8):2956–2967. doi: 10.1128/mcb.7.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Rall L., Rutter W. J. Selective expression of rat pancreatic genes during embryonic development. Proc Natl Acad Sci U S A. 1986 Jan;83(1):110–114. doi: 10.1073/pnas.83.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Howard G., Keller P. R., Johnson T. M., Meisler M. H. Binding of a pancreatic nuclear protein is correlated with amylase enhancer activity. Nucleic Acids Res. 1989 Oct 25;17(20):8185–8195. doi: 10.1093/nar/17.20.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. F., Lüscher B., Admon A., Mermod N., Tjian R. Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev. 1990 Oct;4(10):1741–1752. doi: 10.1101/gad.4.10.1741. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Sundseth R., Hansen U. Transcription factor LSF binds two variant bipartite sites within the SV40 late promoter. Genes Dev. 1990 Feb;4(2):287–298. doi: 10.1101/gad.4.2.287. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. A., Rosenberg M. P., Johnson T. M., Howard G., Meisler M. H. Regulation of amylase gene expression in diabetic mice is mediated by a cis-acting upstream element close to the pancreas-specific enhancer. Genes Dev. 1990 Aug;4(8):1316–1321. doi: 10.1101/gad.4.8.1316. [DOI] [PubMed] [Google Scholar]
- Korc M., Owerbach D., Quinto C., Rutter W. J. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science. 1981 Jul 17;213(4505):351–353. doi: 10.1126/science.6166044. [DOI] [PubMed] [Google Scholar]
- Kristie T. M., Sharp P. A. Interactions of the Oct-1 POU subdomains with specific DNA sequences and with the HSV alpha-trans-activator protein. Genes Dev. 1990 Dec;4(12B):2383–2396. doi: 10.1101/gad.4.12b.2383. [DOI] [PubMed] [Google Scholar]
- Kruse F., Komro C. T., Michnoff C. H., MacDonald R. J. The cell-specific elastase I enhancer comprises two domains. Mol Cell Biol. 1988 Feb;8(2):893–902. doi: 10.1128/mcb.8.2.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D., Perot K. J., McDonald A. R. Mechanism of glucocorticoid-induced increase in pancreatic amylase gene transcription. J Biol Chem. 1987 Nov 15;262(32):15765–15769. [PubMed] [Google Scholar]
- MacDonald R. J. Expression of the pancreatic elastase I gene in transgenic mice. Hepatology. 1987 Jan-Feb;7(1 Suppl):42S–51S. doi: 10.1002/hep.1840070708. [DOI] [PubMed] [Google Scholar]
- Mangalam H. J., Albert V. R., Ingraham H. A., Kapiloff M., Wilson L., Nelson C., Elsholtz H., Rosenfeld M. G. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989 Jul;3(7):946–958. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Meister A., Weinrich S. L., Nelson C., Rutter W. J. The chymotrypsin enhancer core. Specific factor binding and biological activity. J Biol Chem. 1989 Dec 5;264(34):20744–20751. [PubMed] [Google Scholar]
- Meisterernst M., Gander I., Rogge L., Winnacker E. L. A quantitative analysis of nuclear factor I/DNA interactions. Nucleic Acids Res. 1988 May 25;16(10):4419–4435. doi: 10.1093/nar/16.10.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nelson C., Shen L. P., Meister A., Fodor E., Rutter W. J. Pan: a transcriptional regulator that binds chymotrypsin, insulin, and AP-4 enhancer motifs. Genes Dev. 1990 Jun;4(6):1035–1043. doi: 10.1101/gad.4.6.1035. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Hammer R. E., Brinster R. L., Swift G. H., MacDonald R. J. Specific expression of an elastase-human growth hormone fusion gene in pancreatic acinar cells of transgenic mice. Nature. 1985 Feb 14;313(6003):600–602. doi: 10.1038/313600a0. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Messing A., Hammer R. E., Pinkert C. A., Brinster R. L. Elastase I promoter directs expression of human growth hormone and SV40 T antigen genes to pancreatic acinar cells in transgenic mice. Cold Spring Harb Symp Quant Biol. 1985;50:399–409. doi: 10.1101/sqb.1985.050.01.050. [DOI] [PubMed] [Google Scholar]
- Osborn L., Rosenberg M. P., Keller S. A., Meisler M. H. Tissue-specific and insulin-dependent expression of a pancreatic amylase gene in transgenic mice. Mol Cell Biol. 1987 Jan;7(1):326–334. doi: 10.1128/mcb.7.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Rosenberg M. P., Keller S. A., Ting C. N., Meisler M. H. Insulin response of a hybrid amylase/CAT gene in transgenic mice. J Biol Chem. 1988 Nov 15;263(32):16519–16522. [PubMed] [Google Scholar]
- Oyama F., Kikuchi R., Omori A., Uchida T. Avian myeloblastosis virus reverse transcriptase is easier to use than the Klenow fragment of DNA polymerase I for labeling the 3'-end of a DNA fragment. Anal Biochem. 1988 Aug 1;172(2):444–450. doi: 10.1016/0003-2697(88)90467-8. [DOI] [PubMed] [Google Scholar]
- Petrucco S., Wellauer P. K., Hagenbüchle O. The DNA-binding activity of transcription factor PTF1 parallels the synthesis of pancreas-specific mRNAs during mouse development. Mol Cell Biol. 1990 Jan;10(1):254–264. doi: 10.1128/mcb.10.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer K., Arcangioli B., Guarente L. Yeast HAP1 activator competes with the factor RC2 for binding to the upstream activation site UAS1 of the CYC1 gene. Cell. 1987 Apr 10;49(1):9–18. doi: 10.1016/0092-8674(87)90750-1. [DOI] [PubMed] [Google Scholar]
- Pfeifer K., Prezant T., Guarente L. Yeast HAP1 activator binds to two upstream activation sites of different sequence. Cell. 1987 Apr 10;49(1):19–27. doi: 10.1016/0092-8674(87)90751-3. [DOI] [PubMed] [Google Scholar]
- Przybyla A. E., MacDonald R. J., Harding J. D., Pictet R. L., Rutter W. J. Accumulation of the predominant pancreatic mRNAs during embryonic development. J Biol Chem. 1979 Mar 25;254(6):2154–2159. [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Roux E., Strubin M., Hagenbüchle O., Wellauer P. K. The cell-specific transcription factor PTF1 contains two different subunits that interact with the DNA. Genes Dev. 1989 Oct;3(10):1613–1624. doi: 10.1101/gad.3.10.1613. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Stevenson B. J., Hagenbüchle O., Wellauer P. K. Sequence organisation and transcriptional regulation of the mouse elastase II and trypsin genes. Nucleic Acids Res. 1986 Nov 11;14(21):8307–8330. doi: 10.1093/nar/14.21.8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Helix-turn-helix, zinc-finger, and leucine-zipper motifs for eukaryotic transcriptional regulatory proteins. Trends Biochem Sci. 1989 Apr;14(4):137–140. doi: 10.1016/0968-0004(89)90145-X. [DOI] [PubMed] [Google Scholar]
- Swift G. H., Craik C. S., Stary S. J., Quinto C., Lahaie R. G., Rutter W. J., MacDonald R. J. Structure of the two related elastase genes expressed in the rat pancreas. J Biol Chem. 1984 Nov 25;259(22):14271–14278. [PubMed] [Google Scholar]
- Swift G. H., Hammer R. E., MacDonald R. J., Brinster R. L. Tissue-specific expression of the rat pancreatic elastase I gene in transgenic mice. Cell. 1984 Oct;38(3):639–646. doi: 10.1016/0092-8674(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Swift G. H., Kruse F., MacDonald R. J., Hammer R. E. Differential requirements for cell-specific elastase I enhancer domains in transfected cells and transgenic mice. Genes Dev. 1989 May;3(5):687–696. doi: 10.1101/gad.3.5.687. [DOI] [PubMed] [Google Scholar]
- Treisman J., Harris E., Desplan C. The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 1991 Apr;5(4):594–604. doi: 10.1101/gad.5.4.594. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., van der Vliet P. C. The oct-1 homeo domain contacts only part of the octamer sequence and full oct-1 DNA-binding activity requires the POU-specific domain. Genes Dev. 1990 Nov;4(11):1964–1974. doi: 10.1101/gad.4.11.1964. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Matthes H., Garnier J. M., Chambon P. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell. 1991 May 17;65(4):551–568. doi: 10.1016/0092-8674(91)90088-g. [DOI] [PubMed] [Google Scholar]