Abstract
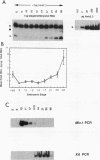
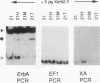
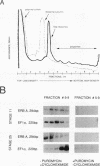
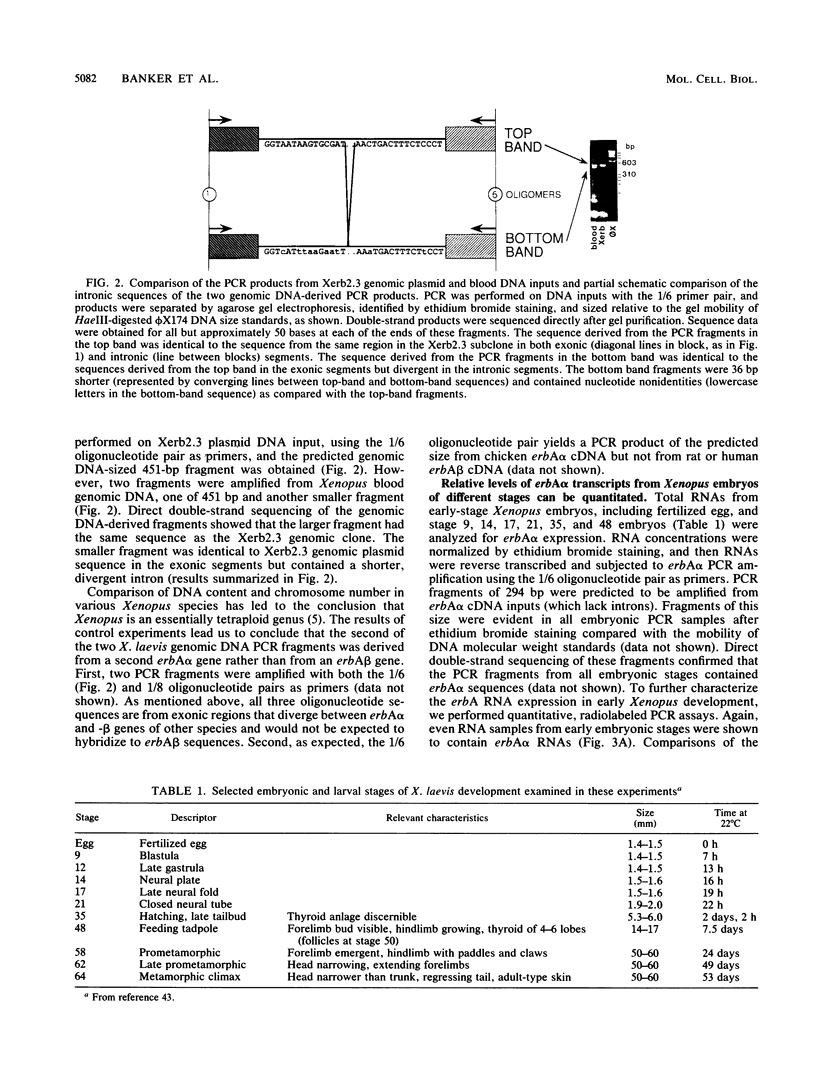
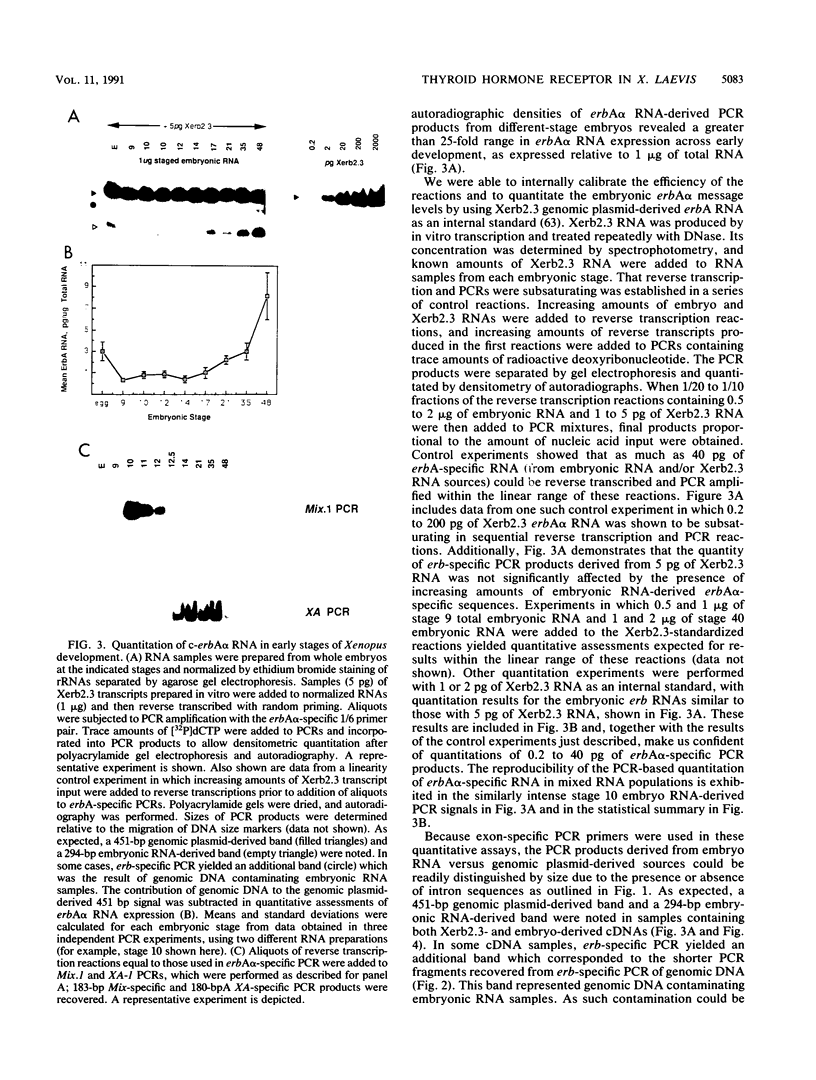
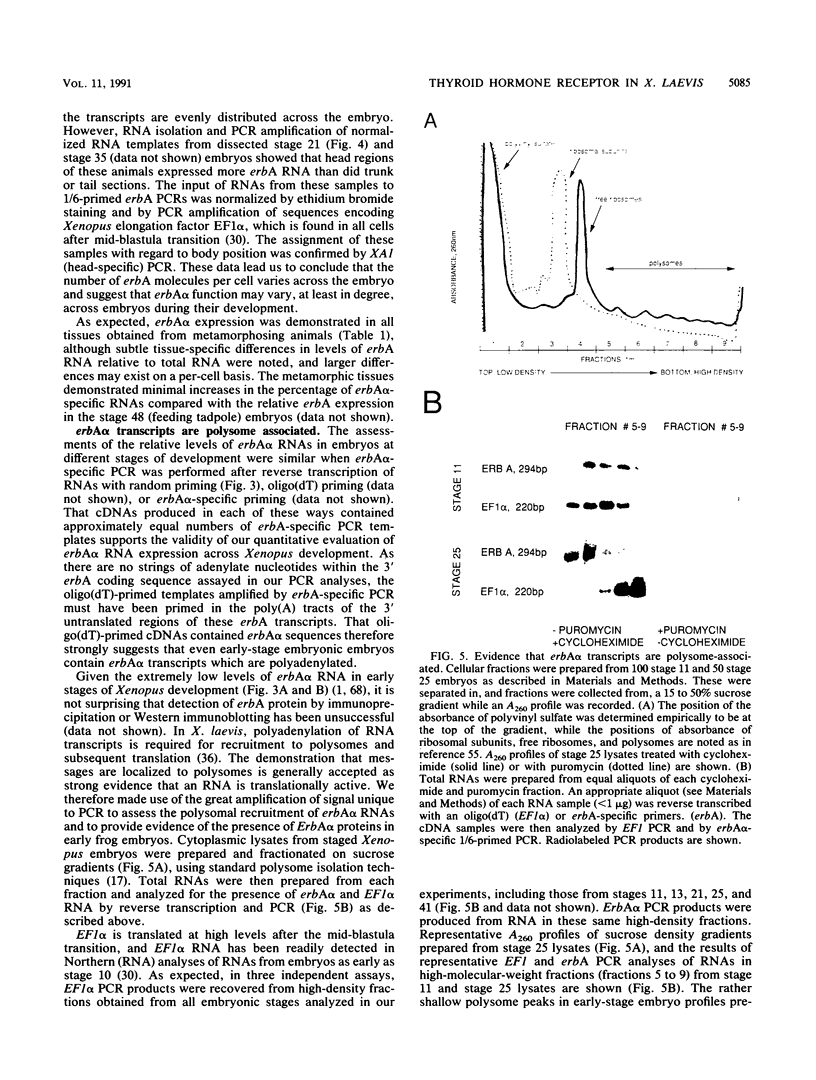
The c-erbA proto-oncogene encodes the thyroid hormone receptor, a ligand-dependent transcription factor which plays an important role in vertebrate growth and development. To define the role of the thyroid hormone receptor in developmental processes, we have begun studying c-erbA gene expression during the ontogeny of Xenopus laevis, an organism in which thyroid hormone has well-documented effects on morphogenesis. Using polymerase chain reactions (PCR) as a sensitive assay of specific gene expression, we found that polyadenylated erbA alpha RNA is present in Xenopus cells at early developmental stages, including the fertilized egg, blastula, gastrula, and neurula. By performing erbA alpha-specific PCR on reverse-transcribed RNAs from high-density sucrose gradient fractions prepared from early-stage embryos, we have demonstrated that these erbA transcripts are recruited to polysomes. Therefore, erbA is expressed in Xenopus development prior to the appearance of the thyroid gland anlage in tailbud-stage embryos. This implies that erbA alpha/thyroid hormone receptors may play ligand-independent roles during the early development of X. laevis. Quantitative PCR revealed a greater than 25-fold range in the steady-state levels of polyadenylated erbA alpha RNA across early stages of development, as expressed relative to equimolar amounts of total embryonic RNA. Substantial increases in the levels of erbA alpha RNA were noted at stages well after the onset of zygotic transcription at the mid-blastula transition, with accumulation of erbA alpha transcripts reaching a relative maximum in advance of metamorphosis. We also show that erbA alpha RNAs are expressed unequally across Xenopus neural tube embryos. This differential expression continues through later stages of development, including metamorphosis. This finding suggests that erbA alpha/thyroid hormone receptors may play roles in tissue-specific processes across all of Xenopus development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. S., Tata J. R. Accumulation of proto-oncogene c-erb-A related transcripts during Xenopus development: association with early acquisition of response to thyroid hormone and estrogen. EMBO J. 1990 Mar;9(3):879–885. doi: 10.1002/j.1460-2075.1990.tb08185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. J., Yoshihara C. M., Blackmer K., Kintner C. R., Burden S. J. Regulation of acetylcholine receptor transcript expression during development in Xenopus laevis. J Cell Biol. 1988 Feb;106(2):469–478. doi: 10.1083/jcb.106.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Steiner C., Köhne A. C., Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990 May 4;61(3):505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- Benbrook D., Pfahl M. A novel thyroid hormone receptor encoded by a cDNA clone from a human testis library. Science. 1987 Nov 6;238(4828):788–791. doi: 10.1126/science.3672126. [DOI] [PubMed] [Google Scholar]
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent G. A., Dunn M. K., Harney J. W., Gulick T., Larsen P. R., Moore D. D. Thyroid hormone aporeceptor represses T3-inducible promoters and blocks activity of the retinoic acid receptor. New Biol. 1989 Dec;1(3):329–336. [PubMed] [Google Scholar]
- Brooks A. R., Sweeney G., Old R. W. Structure and functional expression of a cloned Xenopus thyroid hormone receptor. Nucleic Acids Res. 1989 Nov 25;17(22):9395–9405. doi: 10.1093/nar/17.22.9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Littna E. Synthesis and accumulation of DNA-like RNA during embryogenesis of Xenopus laevis. J Mol Biol. 1966 Sep;20(1):81–94. doi: 10.1016/0022-2836(66)90119-7. [DOI] [PubMed] [Google Scholar]
- Carr F. E., Burnside J., Chin W. W. Thyroid hormones regulate rat thyrotropin beta gene promoter activity expressed in GH3 cells. Mol Endocrinol. 1989 Apr;3(4):709–716. doi: 10.1210/mend-3-4-709. [DOI] [PubMed] [Google Scholar]
- Chatterjee V. K., Lee J. K., Rentoumis A., Jameson J. L. Negative regulation of the thyroid-stimulating hormone alpha gene by thyroid hormone: receptor interaction adjacent to the TATA box. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9114–9118. doi: 10.1073/pnas.86.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crone D. E., Kim H. S., Spindler S. R. Alpha and beta thyroid hormone receptors bind immediately adjacent to the rat growth hormone gene TATA box in a negatively hormone-responsive promoter region. J Biol Chem. 1990 Jul 5;265(19):10851–10856. [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Shrutkowski A., Dworkin-Rastl E. Mobilization of specific maternal RNA species into polysomes after fertilization in Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7636–7640. doi: 10.1073/pnas.82.22.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Yang C. R., Stanley F., Casanova J., Samuels H. H. c-erbA protooncogenes mediate thyroid hormone-dependent and independent regulation of the rat growth hormone and prolactin genes. Mol Endocrinol. 1988 Oct;2(10):902–911. doi: 10.1210/mend-2-10-902. [DOI] [PubMed] [Google Scholar]
- Forrest D., Sjöberg M., Vennström B. Contrasting developmental and tissue-specific expression of alpha and beta thyroid hormone receptor genes. EMBO J. 1990 May;9(5):1519–1528. doi: 10.1002/j.1460-2075.1990.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton V. A. Binding of thyroid hormones in vivo by hepatic nuclei of Rana catesbeiana tadpoles at different stages of metamorphosis. Endocrinology. 1980 Dec;107(6):1910–1915. doi: 10.1210/endo-107-6-1910. [DOI] [PubMed] [Google Scholar]
- Galton V. A. Putative nuclear triiodothyronine receptors in tadpole erythrocytes: regulation of receptor number by thyroid hormone. Endocrinology. 1984 Mar;114(3):735–742. doi: 10.1210/endo-114-3-735. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Franco R., Weinberger C., Albert V. R., Evans R. M., Rosenfeld M. G. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature. 1987 Oct 22;329(6141):738–741. doi: 10.1038/329738a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989 Aug 24;340(6235):653–656. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Jansson M., Philipson L., Vennström B. Isolation and characterization of multiple human genes homologous to the oncogenes of avian erythroblastosis virus. EMBO J. 1983;2(4):561–565. doi: 10.1002/j.1460-2075.1983.tb01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A., Kohara S., Amano M. Thyroid hormone directly induces hepatocyte competence for estrogen-dependent vitellogenin synthesis during the metamorphosis of Xenopus laevis. Dev Biol. 1989 Mar;132(1):73–80. doi: 10.1016/0012-1606(89)90206-6. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Warne R. L., Brent G. A., Harney J. W., Larsen P. R., Moore D. D. Isolation of a cDNA clone encoding a biologically active thyroid hormone receptor. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5031–5035. doi: 10.1073/pnas.85.14.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Varnum S. M., Wormington W. M., Melton D. A. The mRNA encoding elongation factor 1-alpha (EF-1 alpha) is a major transcript at the midblastula transition in Xenopus. Dev Biol. 1989 May;133(1):93–100. doi: 10.1016/0012-1606(89)90300-x. [DOI] [PubMed] [Google Scholar]
- Lavin T. N., Baxter J. D., Horita S. The thyroid hormone receptor binds to multiple domains of the rat growth hormone 5'-flanking sequence. J Biol Chem. 1988 Jul 5;263(19):9418–9426. [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988 Oct;2(10):893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- Mathisen P. M., Miller L. Thyroid hormone induction of keratin genes: a two-step activation of gene expression during development. Genes Dev. 1987 Dec;1(10):1107–1117. doi: 10.1101/gad.1.10.1107. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Tennyson G. E., Nikodem V. M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5804–5808. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi H., LaRochelle F. T., Jr, Suzuki M., Freeman M., Frieden E. Studies on thyroid hormones and their binding in bullfrog tadpole plasma during metamorphosis. Gen Comp Endocrinol. 1977 Oct;33(2):254–266. doi: 10.1016/0016-6480(77)90250-7. [DOI] [PubMed] [Google Scholar]
- Moriya T., Thomas C. R., Frieden E. Increase in 3,5,3'-triiodothyronine (T3)-binding sites in tadpole erythrocyte nuclei during spontaneous and T3-induced metamorphosis. Endocrinology. 1984 Jan;114(1):170–175. doi: 10.1210/endo-114-1-170. [DOI] [PubMed] [Google Scholar]
- Morris S. M., Jr Thyroxine elicits divergent changes in mRNA levels for two urea cycle enzymes and one gluconeogenic enzyme in tadpole liver. Arch Biochem Biophys. 1987 Nov 15;259(1):144–148. doi: 10.1016/0003-9861(87)90479-6. [DOI] [PubMed] [Google Scholar]
- Murray M. B., Zilz N. D., McCreary N. L., MacDonald M. J., Towle H. C. Isolation and characterization of rat cDNA clones for two distinct thyroid hormone receptors. J Biol Chem. 1988 Sep 5;263(25):12770–12777. [PubMed] [Google Scholar]
- Nakai A., Seino S., Sakurai A., Szilak I., Bell G. I., DeGroot L. J. Characterization of a thyroid hormone receptor expressed in human kidney and other tissues. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2781–2785. doi: 10.1073/pnas.85.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa A., Kaiho M., Yoshizato K. Cell death in the anuran tadpole tail: thyroid hormone induces keratinization and tail-specific growth inhibition of epidermal cells. Dev Biol. 1989 Feb;131(2):337–344. doi: 10.1016/s0012-1606(89)80007-7. [DOI] [PubMed] [Google Scholar]
- Norman M. F., Lavin T. N., Baxter J. D., West B. L. The rat growth hormone gene contains multiple thyroid response elements. J Biol Chem. 1989 Jul 15;264(20):12063–12073. [PubMed] [Google Scholar]
- Petty K. J., Desvergne B., Mitsuhashi T., Nikodem V. M. Identification of a thyroid hormone response element in the malic enzyme gene. J Biol Chem. 1990 May 5;265(13):7395–7400. [PubMed] [Google Scholar]
- Regard E., Taurog A., Nakashima T. Plasma thyroxine and triiodothyronine levels in spontaneously metamorphosing Rana catesbeiana tadpoles and in adult anuran amphibia. Endocrinology. 1978 Mar;102(3):674–684. doi: 10.1210/endo-102-3-674. [DOI] [PubMed] [Google Scholar]
- Rosa F. M. Mix.1, a homeobox mRNA inducible by mesoderm inducers, is expressed mostly in the presumptive endodermal cells of Xenopus embryos. Cell. 1989 Jun 16;57(6):965–974. doi: 10.1016/0092-8674(89)90335-8. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Schmitt J., Stunnenberg H., Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989 Jul 20;340(6230):242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Sap J., de Magistris L., Stunnenberg H., Vennström B. A major thyroid hormone response element in the third intron of the rat growth hormone gene. EMBO J. 1990 Mar;9(3):887–896. doi: 10.1002/j.1460-2075.1990.tb08186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueler P. A., Schwartz H. L., Strait K. A., Mariash C. N., Oppenheimer J. H. Binding of 3,5,3'-triiodothyronine (T3) and its analogs to the in vitro translational products of c-erbA protooncogenes: differences in the affinity of the alpha- and beta-forms for the acetic acid analog and failure of the human testis and kidney alpha-2 products to bind T3. Mol Endocrinol. 1990 Feb;4(2):227–234. doi: 10.1210/mend-4-2-227. [DOI] [PubMed] [Google Scholar]
- Schultz J. J., Price M. P., Frieden E. Triiodothyronine increases translatable albumin messenger RNA in Rana catesbeiana tadpole liver. J Exp Zool. 1988 Jul;247(1):69–76. doi: 10.1002/jez.1402470110. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Brown D. D. Developmental and thyroid hormone-dependent regulation of pancreatic genes in Xenopus laevis. Genes Dev. 1990 Jul;4(7):1107–1113. doi: 10.1101/gad.4.7.1107. [DOI] [PubMed] [Google Scholar]
- Shiokawa K., Tashiro K., Misumi Y. Association of maternal and newly synthesized ribosomes with membranous noncytoskeletal structures in Xenopus laevis embryonic cells. J Exp Zool. 1985 Aug;235(2):227–236. doi: 10.1002/jez.1402350209. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Draper B. W., Harland R. M., Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990 Jun;4(6):932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Hattori K., Weintraub H. Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell. 1989 Jul 14;58(1):171–180. doi: 10.1016/0092-8674(89)90413-3. [DOI] [PubMed] [Google Scholar]
- Strait K. A., Schwartz H. L., Perez-Castillo A., Oppenheimer J. H. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J Biol Chem. 1990 Jun 25;265(18):10514–10521. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. Early metamorphic competence of Xenopus larvae. Dev Biol. 1968 Nov;18(5):415–440. doi: 10.1016/0012-1606(68)90050-x. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Weinberger C., Lebo R., Evans R. M. Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science. 1987 Sep 25;237(4822):1610–1614. doi: 10.1126/science.3629259. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangh L. J., Schneider W. Thyroid hormones are corequisites for estradiol-17beta in vitro induction of Xenopus vitellogenin synthesis and secretion. Dev Biol. 1982 Feb;89(2):287–293. doi: 10.1016/0012-1606(82)90317-7. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Wight P. A., Crew M. D., Spindler S. R. Discrete positive and negative thyroid hormone-responsive transcription regulatory elements of the rat growth hormone gene. J Biol Chem. 1987 Apr 25;262(12):5659–5663. [PubMed] [Google Scholar]
- Yaoita Y., Brown D. D. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990 Nov;4(11):1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Ye Z. S., Forman B. M., Aranda A., Pascual A., Park H. Y., Casanova J., Samuels H. H. Rat growth hormone gene expression. Both cell-specific and thyroid hormone response elements are required for thyroid hormone regulation. J Biol Chem. 1988 Jun 5;263(16):7821–7829. [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- Zilz N. D., Murray M. B., Towle H. C. Identification of multiple thyroid hormone response elements located far upstream from the rat S14 promoter. J Biol Chem. 1990 May 15;265(14):8136–8143. [PubMed] [Google Scholar]