Abstract
Artesunate (ART) is widely used for the treatment of malaria, but the mechanisms of its effects on parasitized red blood cells (RBCs) are not fully understood. We investigated ART's influence on the dynamic deformability of red blood cells infected with ring-stage Plasmodium falciparum malaria (iRBCs) in order to elucidate its role in cellular mechanobiology. The dynamic deformability of red blood cells was measured by passing them through a microfluidic device with repeated bottleneck structures. The quasi-static deformability measurement was performed using micropipette aspiration. After ART treatment, microfluidic experiments showed 50% decrease in iRBC transit velocity whereas only small (~10%) velocity reduction was observed among uninfected RBCs (uRBCs). Micropipette aspiration also revealed ART-induced stiffening in RBC membranes. These results demonstrate, for the first time, that ART alters the dynamic and quasi-static red blood cell deformability, which may subsequently influence blood circulation through microvasculature and spleen cordal meshwork, thus adding a new aspect to artesunate's mechanism of action.
Introduction
Malaria is the most deadly parasitic disease which affects 200 million people worldwide and accounts for nearly one million annual deaths 1. The most virulent malarial parasite Plasmodium falciparum can lead to severe complications and has the highest mortality rate 2. During its asexual stage, P. falciparum infects red blood cells (RBCs), which then undergo notable morphological and rheological changes from the ring stage to trophozoite and finally schizont stage, constituting a 48 h asexual reproduction cycle 3. Cyclic febrile attack is a characteristic clinical feature of P. faciparum malaria, which corresponds to the release of merozoites in circulation following iRBC rupture in the late schizont stage.
Apart from cerebral malaria, malarial anemia is the most frequent and severe syndrome of falciparum malaria 4. Massive loss of RBCs cannot be entirely attributed to the destruction of infected RBCs (iRBCs), which usually constitute only a small fraction of total RBCs in malaria patients. Instead, the major cause of malarial anemia is believed to be the excessive loss of uninfected RBCs (uRBCs), mostly in the spleen and/or the liver 5. Malaria-related dyserythropoiesis is likely a minor factor because complete removal of erythropoiesis brings about only a minor decrease in RBC population 6. On the other hand, it has been suggested that uRBCs exposed to the parasites are slightly less deformable, and/or decorated with parasite molecules 7, both of which could potentially lead to splenic retention and clearance of a large number of uRBCs, exacerbating malarial anemia. However, the exact causes and mechanisms of malarial anemia are yet to be firmly established.
Several studies indicate that RBC filtration in the spleen is critical to influencing diverse pathophysiological outcomes of malaria 8. Splenomegaly (enlarged spleen) is a clinical consequence of malaria infection. The narrow splenic inter-endothelial slits (~1 2 μm in height) provide a stringent mechanical filter through which only RBCs with adequate deformability can pass. In the later (trophozoite and schizont) stages of malaria, iRBCs can be up to 50 times stiffer than uRBCs 9. However, in the earlier ring-stage (which occurs within the first 24 h of intra-erythrocytic invasion of the parasite), iRBCs are only moderately stiffer than uRBCs. Although the ring stage is the only asexual parasite stage that can be found in blood circulation, a substantial (~50%) proportion of the ring-stage iRBCs are retained by the human spleen, as demonstrated in the experiment using isolated human spleens 10. The Ring-infected Erythrocyte Surface Antigen (RESA), is one of the parasite derived-proteins responsible for the reduction of ring-stage iRBC membrane deformability 11.
Clinical studies show that malaria patients with artesunate (ART) treatment exhibit a more rapid decline in the parasitemia and also that the accelerated parasite clearance is delayed in splenectomized patients 12. The involvement of spleen is therefore believed to be responsible for rapid parasite clearance after ART drug treatment 5. The in vivo parasite clearance following ART treatment has often been attributed to a process known as ‘pitting’, whereby spleen removes intraerythrocytic parasites without destructing the host RBCs13, 14. However, it is unclear whether pitting is the main mechanism of splenic parasite clearance. Studies by Newton et al. noted that the average lifespan of pitted RBCs (i.e. RESA RBC) is only 183 hours, significantly shorter compared to normal RBC life of 1027 hours 15. Furthermore, ART treatment was found to further shorten the pitted RBC life, suggesting that other mechanisms facilitating splenic parasite clearance may exist. Since spleen is also known as a “mechanical filter” that removes old and stiffened RBCs from microcirculation 5, 16, the rapid splenic parasite clearance after ART treatment might be attributed to ART altering the mechanical properties of iRBCs and possibly of uRBCs. However, there is currently a lack of experimental evidence to confirm such an effect of ART on RBC deformability 14.
Experiments show that subtle changes in RBC deformability may lead to a significant shift in splenic RBC retention efficiency 5, 17. A sensitive single cell deformability cytometry for characterizing the dynamic deformability of both uRBCs and iRBCs is therefore essential for understanding malaria pathophysiology. Bulk cell measurements such as ektacytometry 18, 19, which describe the averaged values of RBC deformability through geometric estimates, do not provide reliable measures of individual cells’ mechanical properties. On the other hand, most of the established tools for single cell deformability measurements including micropipette aspiration 20, 21, atomic force microscopy 22, and optical tweezers 11 require extensive experimental effort to extract reliable information for large populations of cells. More importantly, such measurements impose quasi-static loads to attain notable deformation, and the resulting information may not be directly applicable to the more realistic situation involving dynamic cell deformation. Furthermore, static deformability of RBCs is characterized by the shear and bending moduli of the cell membrane, whereas dynamic deformability can be influenced by additional factors such as membrane viscosity and cell shape. When RBCs circulate in blood capillaries and spleen cordal meshwork, they undergo a complicated, time-dependent deformation process. Therefore, a more straightforward and direct way to evaluate the amenability of RBCs to pass through constrictions in the spleen and microcapillary blood vessels is to simulate such in vivo RBC deformations during circulation using microfluidic artificial filter structures 23. In contrast to conventional filtration system, in which the RBC “filterability” is evaluated directly by the percentage of cells that pass through, the microfluidic system employed in this work characterizes single RBC deformability via its transit velocity through repeated bottleneck structures while facilitating such measurements on a large population of cells.
In this paper, the influence of ART on RBC deformability in the pathology of P. falciparum malaria is evaluated using two approaches: a microfabricated deformability cytometer that mimics in vivo RBC microcirculation24 and micropipette aspiration that measures RBC membrane stiffness. Besides, ART's effects on the mechanical properties of iRBCs were further assessed by treating the iRBCs in combination with pentoxifylline (PTX), a possible adjunctive therapy for cerebral malaria25,27.
Materials and Methods
Microfluidic device
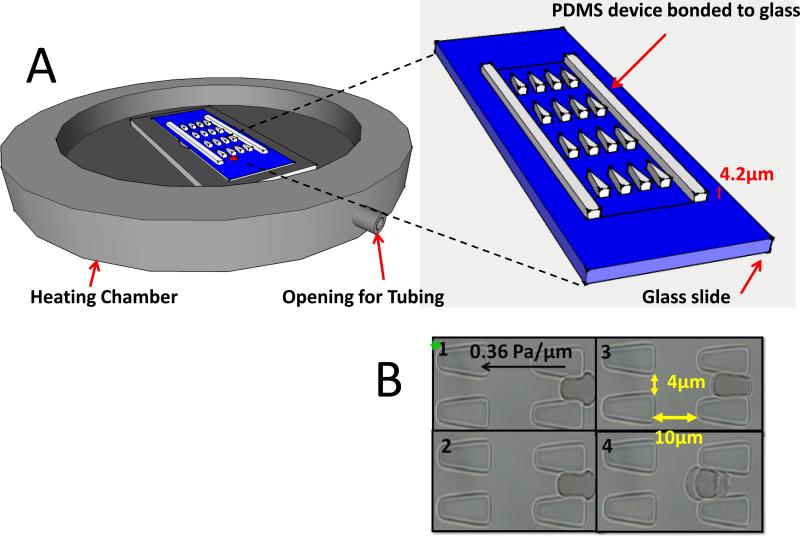
The microfluidic device was designed with layout program and it consists of 500 μm × 500 μm inlet/outlet reservoirs and parallel capillary channels with triangular pillar arrays (Figure 1A). Two different inter-pillar gap sizes of 3.0 and 4.0 μm were tested for optimum deformation condition. Detailed fabrication steps was described by Bow et al 24.
Figure 1. Experimental schematics.
A. A heating chamber (Olympus) was mounted to the inverted microscope stage. The PDMS-glass bonded device consists of the inlet and outlet reservoirs and main channels with triangular pillar arrays. B. Experimental images of uRBCs passing through the gaps.
Parasite culture
P. falciparum 3D7A parasites used in this study were obtained from Malaria Research and Reference Reagent Source, American Type Culture Collection. Parasites were cultured as previously described 28. Cultures were synchronized at the ring-stage by sorbitol lysis 29 two hours after merozoite invasion.
Artesunate drug treatment
The stock ART solution (1.25 mg/ml) was prepared by dissolving ART powder (Sigma A3731) in aqueous sodium bicarbonate. A highly synchronized culture of 6 hour old rings with ~ 15% parasitemia was resuspended at 0.1% hematocrit in malaria culture medium containing various concentration of ART drug. In the control group, sodium bicarbonate solution without ART was added. Both drug and control groups were incubated at 37 °C for 2-6 h.
Solution preparation
Phosphate buffered saline (PBS) with 1% w/v Bovine Serum Albumin (BSA) (Sigma-Aldrich, St. Louis, MO) was fresh made on every experimental day as stock solution. For experiments measuring healthy RBC deformability, fresh whole blood (Research Blood Components, Brighton, MA) was diluted 100 times with PBS/BSA buffer. For experiments measuring malaria infected cells, 1ml of cultured cells were centrifuged at 350g for 5 min; 1 μl of the pellet was then aliquot to 200 μl stock solution.
To distinguish infected cells from uninfected RBCs, 10 μl of 1 × 10-6 M thiazole orange (Invitrogen, Carlsbad, CA), which stains the RNA of the cell, was added to the aforementioned 200 μl iRBC containing solution 20 min before the experiment. The iRBCs would appear fluorescent under the GFP filter set whereas the uRBCs were seen as dark shadows.
Experimental protocol for microfluidics
To accurately control the ambient temperature, the microscope surface was replaced by a heating chamber (Olympus), which was preheated to a desired temperature for 30 min before the beginning of every experiment. Meanwhile, the PBS/BSA stock solution was injected into the device to coat the PDMS walls to prevent adhesion. This filling step needs not to be done inside the heating chamber, but the PBS/BSA filled device needed to be placed into the heating chamber at least 5 min before loading 5 μL of diluted blood sample. During temperature calibration phase, a thermal meter was used to probe the exact temperature inside the heating chamber. When the temperature needed to be adjusted to a different value, at least 5 min of waiting time was required to ensure a new stable ambient temperature.
The microfluidic device was driven by a constant pressure gradient across. The inlet reservoir was connected to a vertically held 60-ml syringe which was partially filled with PBS/BSA buffer solution. A CCD camera (Hamamatsu Photonics, C4742-80-12AG, Japan) was connected to the inverted fluorescent microscope (Olympus IX71, Center Valley, PA) to capture the movement of the RBCs in the microchannels. Images were automatically acquired by IPLab (Scanalytics, Rockville, MD) at 100 ms time interval and the post-imaging analysis was done using imageJ. The velocity of individual RBCs was defined as the distance the cells moved divide by the time in seconds. To better illustrate drug-induced percentage change in iRBC/uRBC velocity, the term “normalized velocity” was adopted. Normalized velocity was obtained by dividing the measured velocity of individual RBCs from various experimental conditions by the average uRBC velocity of the control group on the same experimental day.
Experimental protocol for micropipette aspiration
Micropipette Aspiration was carried out using an inverted optical microscope (Olympus IX71) equipped with a micromanipulator (Eppendorf Transferman NK 2), a heating stage (Linkam Scientific Instruments) and an objective heater (Tokai Hit thermo plate). Pipettes were drawn from borosilicate glass tubing (Sutter Instrument Model P-2000) and cut open (Narishige MF-900) prior to mounting to the micromanipulator. The pipette diameter was kept at 1.0 μm throughout the experiments. Prior to the measurements, the cell chamber was preheated to 37 °C and flushed with the PBS-BSA stock solution with the glass pipette readily mounted and inserted. After the temperature of the stock solution equalized to 37 °C, 1 μl of cells were injected into the cell chamber. Subsequently, the aspiration pressure was increased to 100Pa at a rate of 0.5 Pa/s measuring one cell at a time. Images of each aspirated cell were captured at a rate of 1/s using a high-resolution digital camera (Olympus DP72). The maximum time span before the whole sample was replaced by a fresh sample was 1 h. From the high-resolution recordings, the leading edge of the aspired RBC membrane was tracked manually for calculating the elastic shear modulus using the spherical cap model 21.
Statistical analysis
Two-tailed Student's t-tests were used to compare the microfluidic results and the shear modulus values at various test conditions.
Results
The microfluidic device comprises a series of equally spaced triangular pillar arrays with gap sizes ranging from 2.5 to 4 μm (Figure 1 illustrates the system with 4 μm gap size). The gap sizes were designed to impose mechanical constraints similar to those encountered by the RBCs (with an average diameter of about 8 μm) when traversing blood capillaries and splenic meshwork. Driven by a constant pressure gradient that is smaller than the Pa/μm level, RBCs undergo large elastic deformation at each constriction while traversing the channel 24. The dynamic deformability of RBCs is then characterized by their velocity at a given pressure gradient which signifies their ability to deform repeatedly in order to pass through many successive constrictions.
Figure 1B presents sequential freeze-frame images of an uRBC moving inside the microchannel. The velocity of individual RBCs can then be derived by recording the time of passage of each cell through 10 constrictions in series (i.e. for a total travel distance of 200 μm). The typical pressure gradient (~0.5 Pa/μm) and shear rate (~100 s-1) applied in this device as well as the resulting RBC flow rate (20 - 200 μm/s) are comparable to those of physiological flows 30; they are also of the same order of magnitude as RBCs passing through splenic inter-endothelial slits (IES) 31.
Time-dependent effects of ART on the dynamic deformability of iRBCs
Ring-stage P. falciparum cultures were exposed to ART, an artemisinin derivative. The deformability of both iRBCs and co-cultured uRBCs was measured 2, 4 and 6 h after ART drug treatment. Cultures of iRBCs and uRBCs without ART treatment were used as control. All experiments were performed at 37 °C.
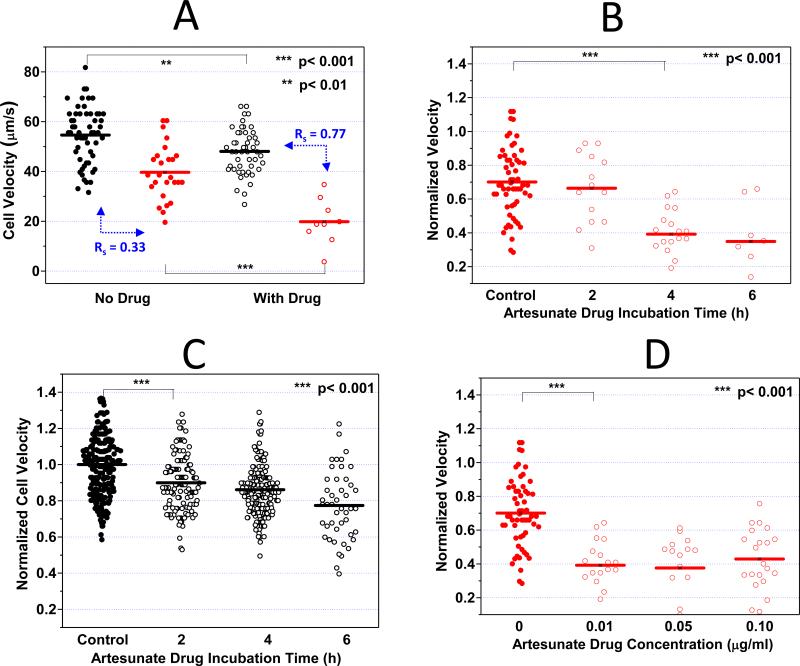
Figure 2A demonstrates pronounced decrease in iRBC velocity after 4 h of ART exposure. Compared to the control iRBCs (40 μm/s), the average transit velocity of ART-exposed iRBCs was halved (20 μm/s), indicating a statistically significant reduction of the dynamic deformability induced by ART (p < 0.001). On the other hand, a much smaller decrease in the average transit velocity of uRBCs was observed after ART exposure.
Figure 2. Artesunate (ART) effect on iRBC/uRBC dynamic deformabilities.
A. 0.01 μg/ml ART was added to the RPMI cell culture medium containing 0.1 % hematocrit. After 4 h ART incubation, the iRBC velocities with and without ART treament were compared. B. 0.01 μg/ml ART was added to the RPMI cell culture medium containing 0.1 % hematocrit. After 2, 4, and 6 h ART incubation, the iRBC velocities were compared. C. Time dependent ART effects on uRBC velocities were studied. D. 0.01, 0.05 and 0.10 μg/ml ART was added to the RPMI cell culure medium containing 0.1% hematocrit. After 4 h ART incubation, the concentration dependent ART effects on iRBC velocities were compared. Black and red dots denote uRBCs and iRBCs without ART treatment whereas black and red circles denote uRBCs and iRBCs with ART treatment respectively. Velocity measurement on every experimental day is normalized against the average uRBC velocity in the control sample.
To assess the time-dependent effects of ART treatment, the dynamic deformability measurements were performed after 2, 4, and 6 h of ART exposure. The results are given in Figure 2B. While no significant difference in the average iRBC velocity could be observed after 2 h ART incubation, iRBC velocity dropped significantly by approximately 40% after 4 h of ART exposure (p < 0.001).
The differential deformability between uRBCs and iRBCs was previously noted as the key parameter for efficient splenic filtration of iRBCs 5, 10, 32, 33. The deformability difference between uRBCs and iRBCs can be assessed through the index, Rs:
Here X1 and X2 are the mean values of velocities of iRBCs and uRBCs, respectively, and σ1 andσ2 denote the standard deviations of velocities of iRBCs and uRBCs, respectively. A higher Rs value implies larger separation between the velocities of iRBCs and uRBCs.
While in both control and ART-exposed groups the iRBC velocities are significantly slower than that of cocultured uRBCs (p < 0.001), the velocity separation resolution Rs was enhanced by 2.3 times from 0.33 to 0.77 after ART exposure. Additionally, the average iRBC velocity after ART exposure was 3.13σ2 (σ2: standard deviation of uRBC velocity distribution) away from the average uRBC velocity. This result suggests a more specific dynamic deformability differentiation between uRBCs and iRBCs after ART exposure, which may consequently lead to more efficient splenic parasite clearance.
Concentration-dependent ART effect on dynamic deformability of iRBCs
The in vivo serum concentration of ART varies with time after injection 34, 35. Typically, the half-life of ART in patients is approximately 3 h with peak serum concentration of 0.1 μg/ml. Therefore, the dynamic deformability assay was further expanded to different concentrations. Figure 2D indicates that from 0.01 μg/ml to 0.1 μg/ml, there is no statistically significant dose dependency on ART induced alteration of the RBCs deformability.
Time-dependent ART effect on the quasi-static deformability of iRBCs
In parallel with the microfluidic experiments, micropipette aspiration of iRBCs and uRBCs was carried out. The membrane elastic properties were determined before and after ART exposure of RBCs for 2, 4 and 6 h. As control, cultures of iRBCs and uRBCs without exposure to ART were measured. All measurements were performed at 37°C.
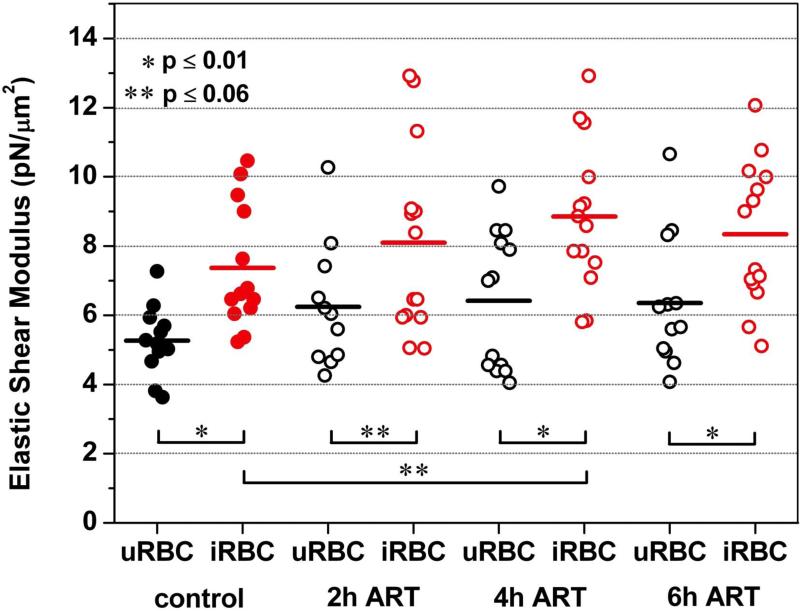
Figure 3 summarizes the membrane elastic shear moduli of uRBCs and iRBCs before and after exposure to ART. The mean values for the elastic shear moduli of the iRBCs were consistently 30% to 40% higher than the moduli of their uninfected counterparts. Solely after incubating with ART for 2 h, the mean elastic shear moduli for both iRBCs and uRBCs increased by 10% to 20% with p-value of 0.06, which is approximately at the threshold level of statistical significance of p = 0.05.
Figure 3. Artesunate effect on iRBC/uRBC membrane stiffness.
0.05 μg/ml of ART were added to the RPMI cell culture medium containing 0.1% hematocrit. After 2, 4 and 6 h of incubation with ART, micropipette aspiration of uRBCs (black circles) and iRBCs (red circles) was carried out at a suction rate of 0.5 Pa/s. Black dots (uRBCs) and red dots (iRBCs) represent the control without ART treatment. The mean elastic shear moduli of the iRBCs are 30% to 40% higher than the moduli of their uninfected counterparts. Exposure to ART results in a mild but consistent increase of the membrane elastic shear modulus of both uRBCs and iRBCs.
Exposure to ART results in an increase of the membrane elastic shear modulus of both uRBCs and iRBCs (Figure 3). For uRBCs the relative increase in mean elastic shear modulus is 20% ± 2% independent of the incubation time. For iRBCs the maximum relative increase was measured to be 20% after 4 h ART incubation.
Effect of Pentoxifylline on the dynamic deformability of iRBCs
Pentoxifylline (PTX), a hydroxyl radical scavenger 19, 36, 37, is believed to be able to improve impaired blood flow. Several studies also suggest it can be used as an ancillary treatment for severe falciparum malaria, in combination with ART. In this section, the effect of PTX alone, as well as the combined effect of PTX and ART on the dynamic deformability of P. falciparum-infected RBCs are studied in vitro. All experiments were performed at 37 °C.
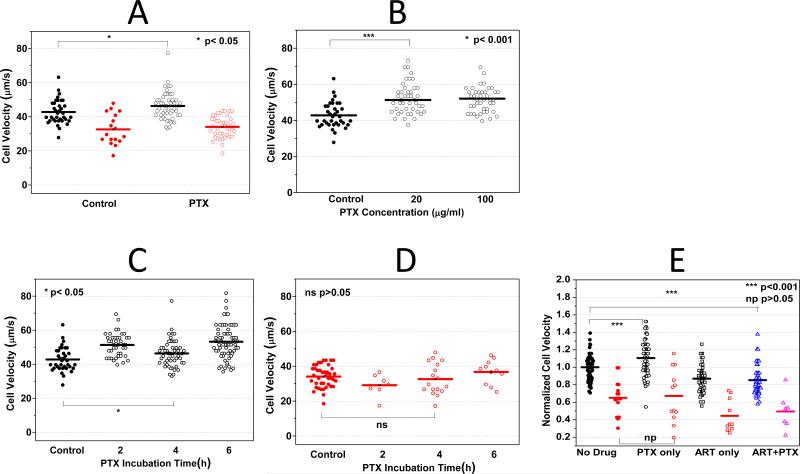
Figure 4A demonstrates that PTX has no statistically significant effect on the deformability of ring-stage iRBCs even after 4 h of incubation at a concentration of 100 μg/ml. The average transit velocity of iRBCs after PTX treatment was 33 μm/s, which was very similar to that of the control sample (34 μm/s, p = 0.54). Simultaneous measurements were also performed on co-cultured uRBCs. Whereas the average uRBC velocity in the control sample was 43 μm/s, the value after PTX treatment was statistically different (46 μm/s, p = 0.034), exhibiting a significant albeit mild increase on the dynamic deformability of uRBCs.
Figure 4. Pentoxifylline (PTX) effect on iRBC/uRBC dynamic deformabilities.
A. 100 μg/ml PTX was added to the RPMI cell culture medium containing 0.1 % hematocrit. After 4 h PTX incubation, the iRBC/uRBCs velocities with and without PTX treament were compared. B. 20 and 100 μg/ml PTX was added to the RPMI cell culure medium containing 0.1% hematocrit. After 4 h PTX incubation, the velocities of uRBCs treated with different PTX concentrations were compared. C. 100 μg/ml PTX was added to the RPMI cell culture medium containing 0.1% hematocrit. After 2, 4, and 6 h PTX incubation, the uRBC velocities at different test conditions were compared. D. iRBC velocities after 2, 4 and 6h PTX treatment were compared. No statistically significant change in iRBC velocity can be concluded. E. PTX-ART Combination effect on RBC deformability. No statistically significant difference can be concluded comparing RBCs treated with ART only versus cells treated with PTX-ART combinational therapy.
The effect of PTX on the difference in response between uRBC and iRBC was calculated in the similar way as introduced before. The Rs value for the control sample was 0.33 compared to 0.40 after PTX treatment. The result suggests a marginal (if any) effect of PTX drug on RBC deformability.
Typical clinical dosage of PTX is between 5 and 20 μg/ml. To investigate whether the effect of PTX on uRBCs is dose-dependent, the co-cultured uRBCs were exposed to 20 μg/ml and 100 μg/ml PTX for 2 h. Compared to the control sample (42.79 μm/s), the average uRBC velocity exhibited significant improvement at both concentrations (52.03 μm/s and 51.33 μm/s, p < 0.01) and no statistically significant concentration dependence could be concluded. Figure 4B suggested that the effect of PTX on uRBCs was not dose-dependent (p=0.67) in the concentration range between 20 to 100 μg/ml.
After 2 h incubation with PTX, the uRBC dynamic deformability seemed to be slightly enhanced (from 42.79 to 52.03 μm/s). The effect remained for incubation up to 6 h (Figure 4C). Simultaneous deformability measurement was obtained for iRBCs and no statistical significant effect was observed up to 6 h PTX incubation (Figure 4D).
The combined effect of PTX and ART is shown in Figure 4E. Compared with ART only experiments, PTX does not seem to provide additional influences to the dynamic deformability of both iRBC and uRBC when applied in combination with ART.
Discussion
It is widely accepted that ring-stage P. falciparum parasites are the only asexual intra-erythrocytic parasite forms able to travel through human circulation without being recognized by the human host. Antimalarial drugs affecting ring-stage iRBC membrane mechanical properties would help the host with early identification of parasitized red blood cells, and consequently their effective clearance by the human spleen.
In the present study we explored the effect of two different drugs (artesunate and pentoxifylline) on the microcirculatory velocity of RBCs after malaria infection. Both drugs are used alone or in combination for the treatment of p. falciparum malaria: artesunate (ART) is known to facilitate splenic parasite clearance 38, and pentoxifylline (PTX) has been suggested as an effective adjunctive therapy with ART 39, but their relevant effects on the RBC mechanical properties are not yet well understood.
Our results demonstrate that ART treatment significantly stiffens ring-stage iRBCs based on both quasi-static and dynamic single cell deformability assays. Additionally, our microfluidic set up mimicking RBC microcirculatory behavior revealed that ART has a significant impact on the velocity separation resolution between iRBCs and uRBCs. Both observations suggest the role of ART in influencing the dynamic circulation of iRBCs and uRBCs. Since human spleen is well known as a “mechanical filter” that removes old and abnormal RBCs16, and splenic retention of artificially hardened uRBCs has been demonstrated by Buffet et al. using an ex vivo spleen8, 32, we speculate ART induced alternation in RBC membrane stiffness and dynamic microcirculatory behavior would have a significant effect on splenic RBC filtration24.
Pentoxifylline, on the other hand, was initially developed to improve blood flow in the microvasculature 39. It was suggested as a possible ancillary therapy for cerebral malaria but clinical trials showed conflicting results 26, 40. In this study, we attempted to evaluate how PTX and PTX-ART combinational therapy impact on the microcirculatory behavior of RBCs infected with P.falciparum malaria. The microfluidic based dynamic deformability assay with ring-stage iRBCs treated with PTX suggested a marginal albeit significant enhancement on uRBC deformability (p<0.05). This result is consistent with several other deformability studies using different measurement platform 36, 41. However, we did not observe any significant effect of PTX on iRBC deformability. Experiments with ART-PTX combinational therapy did not reveal any beneficial effect of adding PTX.
Currently the underlying molecular mechanism of ART-induced RBC stiffening is undetermined. Clinical studies revealed that the drug action is mainly attributed by the endoperoxide-bridge. Deoxyartemisinin, an Artemisinin analog lacking the endoperoxide bridge, is clinically proven devoid of antimalarial activity 42, indicating the important role of peroxide in Artemisinin's and its derivative ART's drug action. Artesunate induced oxidative stress, a likely consequence due to its endoperoxide structure, was also suggested by Krungkrai et al to be the key for the drug's antimalarial activity 43. It is therefore possible that the endoperoxide-bridge could also be responsible for ART-induced RBC stiffening. This is confronted by our microfluidic experiments with iRBCs treated with deoxyartemisinin, which revealed that deoxyartemisinin has no effect on the deformability of both uRBCs and iRBCs. (S2)
It is noted that due to the complex nature of RBC deformability, different measurement tools could lead to different experimental observations. For example, prior deformability measurements by rotational ektacytometry reported that up to 4 h ART exposure would not affect uRBC deformability 14, but our microfluidic and micropipette experiments showed significant uRBC stiffening after 2 h ART treatment. Additionally, the ektacytometry measurement suggests that ≥ 2 h ART exposure would result 20% reduction in the deformability separation between healthy and ring samples; our micropipette data showed that the deformability separation between uRBCs and iRBCs dropped from 0.36 to 0.18 (i.e. 50% drop) only at 2 h time point, and then restored back to 0.40 and 0.51 respectively after 4 h and 6 h ART treatment. Similarly, the microfluidic system measured 14% drop (from 0.30 to 0.36) in the deformability separation only at 2 h incubation time point and the separation would then increase to 0.54 and 0.55 after 4 h and 6 h ART treatment respectively. The exact cause accounting for the discrepancies among different measurement tools remains unclear. It is likely that the difference could come from the different formulations of deformability, such as “elongation index” in ektacytometry, “membrane shear modulus” in micropipette aspiration, and “transit velocity” in microfluidics. Compared to the microfluidic and micropipette single cell measurements, ektacytometry measures bulk RBC deformability, which has an additional parameter of cell-to-cell interaction that could influence the overall result. Different shear rates were used in different systems, leading to different results due to the stress-strain (as well as stress-strain rate) relationship44.
Comparing the single cell deformability results measured by microfluidic and micropipette assays the stiffening trend differs slightly, even though both systems reported decreased RBC deformability after ART treatment (Fig 2A & Fig 3). The microfluidic experiments showed more significant reduction in iRBC transit velocity (50%) as compared to the drop in uRBC transit velocity (10%). On the other hand micropipette aspiration displayed less differentiation in uRBC (14%) and iRBC (27%) stiffening. It is possible that the microfluidic measurement reflects the overall microcirculatory behavior of RBCs with several determining factors including RBC geometry, intracellular fluidic viscosity, as well as RBC membrane viscoelasticity44, whereas for the micropipette aspiration, the contribution of cell geometry and viscosity is insignificant45, 46. Experiments measuring the size of RBCs containing 5% parasitemia (Beckman Coulter) revealed no significant change in RBC size after 6h ART treatment (S3), suggesting that the difference between quansi-static and dynamic RBC deformability results may due to intracellular viscosity rather than cell size. Heme irons are believed to be actively involved for ART's selectivity 47. Redox chemistry of RBCs is highly complicated, involving abundant hemoglobin in RBCs. Since parasites in iRBCs actively metabolize hemoglobin, and release high quantities of free heme irons, this difference may lead to differential redox-response toward ART, and possibly differentially alter the dynamic versus quasi-static deformability of iRBCs over uRBCs.
Our study attempted to understand the impact on drug-related changes on the ring-stage mechanical properties of P. falciparum iRBCs, by recreating the microcirculatory blood flow in vitro using a microfluidic device, and measuring their quasi-static membrane behavior using a micropipette device. By putting iRBCs through repeated physical and mechanical barriers, compared with the quasi-static data, we were able to quantify the drug related dynamic deformability modification for iRBCs and uRBCs.
In conclusion, this work demonstrates that in vitro dynamic and quasi-static deformability measurements can be used to study subtle cell deformability changes resulting from various environmental factors such as in vitro drug treatment. ART drug treatment on malaria infected RBCs shows significant alternations in RBC's membrane stiffness and microcirculatory behavior. Since human spleen is a “mechanical filter”, reduced RBC stiffness may enhance splenic clearance of less deformable parasite iRBCs from the circulation 24, 32. On the other hand, the drug stimulus may also aggravate the loss of uRBCs as significant decrease in uRBC deformability was also observed in vitro. These hypotheses, while insightful and important, are certainly yet to be further corroborated by future in vivo and clinical studies. From the bioengineering perspectives, these measurements could provide a well-controlled in-vitro experimental platform to test novel anti-malarial compounds, or clarify the drug's mode of action in relation to splenic clearance, which is generally difficult to do in vivo due to the lack of viable animal models and ethical considerations.
Supplementary Material
Artemisinin is an important drug for malaria treatment, but its drug mechanism remains unclear. Past studies largely focused on the biochemistry of artemisinin to explain drug's mechanism. In this paper, ART (artemisinin derivative)-induced change in RBC membrane stiffness is investigated by passing RBCs through a microfluidic device that mimics and simulates splenic filtration and other in vivo RBC deformations during microcirculation. After ART treatment, the membrane stiffness of both healthy and infected RBCs increases moderately (~10%), corresponding to a significant decrease in RBC transit velocities. Our result suggests that drug-induced RBC stiffening could significantly alter blood circulation and enhances the efficiency and specificity of splenic parasite clearance, elucidating a previously unexplored, mechanical aspect of artemisinin drug mechanism.
Acknowledgments
The authors would like to thank Dr. Subra Suresh for helpful discussions. Device fabrications were carried out at MIT Microsystems Technology Laboratories. This work is mainly supported by the National Research Foundation (Singapore) through Singapore-MIT Alliance for Research and Technology (SMART) Center (BioSyM and ID IRG), and the U. S. National Institutes of Health (Grant R01 HL094270-01A1). One of the authors (A.U.) gratefully acknowledges support by the Alexander von Humboldt-Foundation in form of a Feodor Lynen Research Fellowship.
Footnotes
Disclosure
S. Huang, M. Diez-Silva, M. Dao, S. Suresh, J. Han, along with others, have filed two US provisional patents based on a portion of the contents of this paper.
References
- 1.2010. O. World Health.
- 2.Trampuz A, Jereb M, Muzlovic I, Prabhu R. Critical Care. 2003;7:315–323. doi: 10.1186/cc2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier AG, Cooke BM, Cowman AF, Tilley L. Nat. Rev. Microbiol. 2009;7:341–354. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 4.Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- 5.Buffet PA, Safeukui I, Deplaine G, Brousse V, Prendki V, Thellier M, Turner GD, Mercereau-Puijalon O. Blood. 2011;117:381–392. doi: 10.1182/blood-2010-04-202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seed TM, Kreier JP. In: Malaria, Volume 2. Pathology, vector studies, and culture. Kreier JP, editor. 1980. pp. 1–46. [Google Scholar]
- 7.Dondorp AM, Pongponratn E, White NJ. Acta Tropica. 2004;89:309–317. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Buffet PA, Milon G, Brousse V, Correas J-M, Dousset B, Couvelard A, Kianmanesh R, Farges O, Sauvanet A, Paye F, Ungeheuer M-N, Ottone C, Khun H, Fiette L, Guigon G, Huerre M, Mercereau-Puijalon O, David PH. Blood. 2006;107:3745–3752. doi: 10.1182/blood-2005-10-4094. [DOI] [PubMed] [Google Scholar]
- 9.Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Seufferlein T. Acta Biomater. 2005;1:15–30. doi: 10.1016/j.actbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Safeukui I, Correas J-M, Brousse V, Hirt D, Deplaine G, Mulé S, Lesurtel M, Goasguen N, Sauvanet A, Couvelard A, Kerneis S, Khun H, Vigan-Womas I, Ottone C, Molina TJ, Tréluyer J-M, Mercereau-Puijalon O, Milon G, David PH, Buffet PA. Blood. 2008;112:2520–2528. doi: 10.1182/blood-2008-03-146779. [DOI] [PubMed] [Google Scholar]
- 11.Mills JP, Diez-Silva M, Quinn DJ, Dao M, Lang MJ, Tan KSW, Lim CT, Milon G, David PH, Mercereau-Puijalon O, Bonnefoy S, Suresh S. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9213–9217. doi: 10.1073/pnas.0703433104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chotivanich K, Udomsangpetch R, McGready R, Proux S, Newton P, Pukrittayakamee S, Looareesuwan S, White NJ. Journal of Infectious Diseases. 2002;185:1538–1541. doi: 10.1086/340213. [DOI] [PubMed] [Google Scholar]
- 13.Angus BJ, Chotivanich K, Udomsangpetch R, White NJ. Blood. 1997;90:2037–2040. [PubMed] [Google Scholar]
- 14.Chotivanich K, Udomsangpetch R, Dondorp A, Williams T, Angus B, Simpson JA, Pukrittayakamee S, Looareesuwan S, Newbold CI, White NJ. Journal of Infectious Diseases. 2000;182:629–633. doi: 10.1086/315718. [DOI] [PubMed] [Google Scholar]
- 15.Newton PN, Chotivanich K, Chierakul W, Ruangveerayuth R, Teerapong P, Silamut K, Looareesuwan S, White NJ. Blood. 2001;98:450–457. doi: 10.1182/blood.v98.2.450. [DOI] [PubMed] [Google Scholar]
- 16.Mebius RE, Kraal G. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 17.Diez-Silva M, Park Y, Huang S, Bow H, Mercereau-Puijalon O, Deplaine G, Lavazec C, Perrot S, Bonnefoy S, Feld M, Han J, Dao M, Suresh S. Scientific Reports. 2012 doi: 10.1038/srep00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessis M MN, Feo C. Blood Cells. 1980;6:315–327. [PubMed] [Google Scholar]
- 19.Mokken FC, Kedaria M, Henny CP, Hardeman MR, Gelb AW. Annals of Hematology. 1992;64:113–122. doi: 10.1007/BF01697397. [DOI] [PubMed] [Google Scholar]
- 20.Nash GB, Obrien E, Gordonsmith EC, Dormandy JA. Blood. 1989;74:855–861. [PubMed] [Google Scholar]
- 21.Chien S, Sung KL, Skalak R, Usami S, Toezeren A. Biophysical Journal. 1978;24:463–487. doi: 10.1016/S0006-3495(78)85395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lekka M, Laidler P, Gil D, Lekki J, Stachura Z, Hrynkiewicz AZ. European Biophysics Journal. 1999;28:312–316. doi: 10.1007/s002490050213. [DOI] [PubMed] [Google Scholar]
- 23.Brody JP, Han Y, Austin RH, Bitensky M. Biophysical Journal. 1995;68:2224–2232. doi: 10.1016/S0006-3495(95)80443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bow H, Pivkin IV, Diez-Silva M, Goldfless SJ, Dao M, Niles JC, Suresh S, Han J. Lab on a Chip. 2011;11:1065–1073. doi: 10.1039/c0lc00472c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graninger W, Thalhammer F, Locker G. Journal of Infectious Diseases. 1991;164:829. doi: 10.1093/infdis/164.4.829. [DOI] [PubMed] [Google Scholar]
- 26.Looareesuwan S, Wilairatana P, Vannaphan S, Wanaratana V, Wenisch C, Aikawa M, Brittenham G, Graninger W, Wernsdorfer WH. The American Journal of Tropical Medicine and Hygiene. 1998;58:348–353. doi: 10.4269/ajtmh.1998.58.348. [DOI] [PubMed] [Google Scholar]
- 27.Kremsner PG, Grundmann H, Neifer S, Sliwa K, Sahlmuller G, Hegenscheid B, Bienzle U. Journal of Infectious Diseases. 1991;164:605–608. doi: 10.1093/infdis/164.3.605. [DOI] [PubMed] [Google Scholar]
- 28.Trager W, Jensen JB. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 29.Lambros C, Vanderberg JP. The Journal of Parasitology. 1979;65:418–420. [PubMed] [Google Scholar]
- 30.Mairey E, Genovesio A, Donnadieu E, Bernard C, Jaubert F, Pinard E, Seylaz J, Olivo-Marin J-C, Nassif X, Dum茅nil G. The Journal of Experimental Medicine. 2006;203:1939–1950. doi: 10.1084/jem.20060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald IC, Ragan DM, Schmidt EE, Groom AC. Microvascular Research. 1987;33:118–134. doi: 10.1016/0026-2862(87)90011-2. [DOI] [PubMed] [Google Scholar]
- 32.Deplaine G, Safeukui I, Jeddi F, Lacoste F, Brousse V, Perrot S, Biligui S, Guillotte M, Guitton C, Dokmak S, Aussilhou B, Sauvanet A, Cazals Hatem D, Paye F, Thellier M, Mazier D, Milon G, Mohandas N, Mercereau-Puijalon O, David PH, Buffet PA. Blood. 117:e88–e95. doi: 10.1182/blood-2010-10-312801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buffet PA, Safeukui I, Milon G, Mercereau-Puijalon O, David PH. Current Opinion in Hematology. 2009;16:157–164. doi: 10.1097/MOH.0b013e32832a1d4b. 110.1097/MOH.1090b1013e32832a32831d32834b. [DOI] [PubMed] [Google Scholar]
- 34.Hien TT, White NJ, White The Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- 35.Bethell DB, Teja-Isavadharm P, Phuong CXT, Thuy PTT, Mai TTT, Thuy TTN, Ha NTT, Phuong PT, Kyle D, Day NPJ, White NJ. Transactions of the Royal Society of Tropical Medicine and Hygiene. 91:195–198. doi: 10.1016/s0035-9203(97)90222-4. [DOI] [PubMed] [Google Scholar]
- 36.Ohshima N, Sato M. Angiology. 1981;32:752–763. doi: 10.1177/000331978103201103. [DOI] [PubMed] [Google Scholar]
- 37.Horvath B, Marton Z, Halmosi R, Alexy T, Szapary L, Vekasi J, Biro Z, Habon T, Kesmarky G, Toth K. Clinical Neuropharmacology. 2002;25:37–42. doi: 10.1097/00002826-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Sinclair D DS, Lalloo DG. Cochrane Database of Systematic Reviews. 2011 doi: 10.1002/14651858.CD005967.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Lell B, Kohler C, Wamola B, Olola C, Kivaya E, Kokwaro G, Wypij D, Mithwani S, Taylor T, Kremsner P, Newton C. Malaria Journal. 9:368. doi: 10.1186/1475-2875-9-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das BK, Mishra S, Padhi PK, Manish R, Tripathy R, Sahoo PK, Ravindran B. Tropical Medicine & International Health. 2003;8:680–684. doi: 10.1046/j.1365-3156.2003.01087.x. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu T, Sekizuka E, Oshio C, Tsukada K, Nagai T, Hokari R, Minamitani H. The automatic image analysis of red blood cell deformability and blood flow in microchannels with an image-intensified high-speed video camera system. 1998 [Google Scholar]
- 42.Meshnick SR, Tsang TW, Lin FB, Pan HZ, Chang CN, Kuypers F, Chiu D, Lubin B. Progress in clinical and biological research. 1989;313:95–104. [PubMed] [Google Scholar]
- 43.Krungkrai SR, Yuthavong Y. Transactions of the Royal Society of Tropical Medicine and Hygiene. 81:710–714. doi: 10.1016/0035-9203(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 44.Chien S. Annual Review of Physiology. 1987;49:177–192. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- 45.Hochmuth RM, Berk DA, Wiles HC. Annals of the New York Academy of Sciences. 1983;416:207–224. doi: 10.1111/j.1749-6632.1983.tb35190.x. [DOI] [PubMed] [Google Scholar]
- 46.Tözeren H, Chien S, Tözeren A. Biophysical Journal. 1984;45:1179–1184. doi: 10.1016/S0006-3495(84)84266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meshnick SR, Yang YZ, Lima V, Kuypers F, Kamchonwongpaisan S, Yuthavong Y. Antimicrobial Agents and Chemotherapy. 1993;37:1108–1114. doi: 10.1128/aac.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.