Abstract
Abnormal aldosterone physiology has been implicated in the pathogenesis of cardio-metabolic diseases. Single aldosterone measurements capture only a limited range of aldosterone physiology. New methods of characterizing aldosterone physiology may provide a more comprehensive understanding of its relationship with cardio-metabolic disease. We evaluated whether novel indices of aldosterone responses to dietary sodium modulation, the Sodium-modulated Aldosterone Suppression-Stimulation Index (SASSI for serum and SAUSSI for urine), could predict cardio-metabolic risk factors. We performed cross-sectional analyses on 539 subjects studied on liberal (LIB) and restricted (RES) sodium diets with serum and urinary aldosterone measurements. SASSI and SAUSSI were calculated as the ratio of aldosterone on LIB (maximally suppressed aldosterone) to aldosterone on RES (stimulated aldosterone) diets, and associated with risk factors using adjusted regression models. Cardio-metabolic risk factors associated with either impaired suppression of aldosterone on LIB diet, or impaired stimulation on RES diet, or both; in all of these individual cases, these risk factors associated with higher SASSI or SAUSSI. In the context of abnormalities that comprise the metabolic syndrome (MetS), there was a strong positive association between the number of MetS components (0–4) and both SASSI and SAUSSI (P<0.0001) that was independent of known aldosterone secretagogues (angiotensin II, corticotropin, potassium). SASSI and SAUSSI exhibited a high sensitivity in detecting normal individuals with zero MetS components (86% for SASSI and 83% for SAUSSI). Assessing the physiologic range of aldosterone responses may provide greater insights into adrenal pathophysiology. Dysregulated aldosterone physiology may contribute to, and/or result from, early cardio-metabolic abnormalities.
Keywords: Aldosterone, Metabolic Sydrome, Renin, Adrenal, Physiology
INTRODUCTION
The renin angiotensin aldosterone system (RAAS) is a dynamic hormonal system. The manner in which the RAAS responds to physiologic provocations, such as dietary sodium modulation, characterizes RAAS physiology and abnormalities in RAAS regulation may be associated with cardio-metabolic diseases. This is highlighted in the literature that links aldosterone, using single cross-sectional measurements, with clinical outcomes and adverse cardio-metabolic profiles1–10. Improving the understanding of aldosterone dysregulation may provide insights into new avenues for the treatment and prevention of pathologic conditions associated with altered adrenal physiology.
Aldosterone dysregulation has been associated with cardio-metabolic risk factors; and individual components of the metabolic syndrome (MetS) associate with higher aldosterone concentrations in human studies 6–13. These studies used single aldosterone measures as the predictor, rather than evaluating the dynamic physiology that regulates aldosterone responses and actions and has been previously correlated with cardio-metabolic pathophysiology14–18. For example, high sodium dietary interventions maximally suppress adrenal aldosterone secretion; the inability to suppress aldosterone in this setting has been associated with insulin resistance, dyslipidemia, obesity, and diabetes7, 14, 19. In contrast, induction of a very restricted sodium balance, or the infusion of exogenous angiotensin II (AngII), stimulate adrenal aldosterone secretion; a blunted stimulation of aldosterone in these settings has also been associated with similar cardio-metabolic abnormalities14–16, 18, 20, 21. Therefore, we speculated that the integration of aldosterone suppression and stimulation would provide an improved representation of aldosterone physiology in disease states that could offer new insights into the pathogenesis and treatment of cardio-metabolic derangements.
We developed a novel index to reflect physiologic aldosterone responses to dietary sodium manipulation. This index integrates aldosterone physiology via a ratio of aldosterone levels on a liberal sodium (LIB) diet to levels on a restricted (RES) sodium diet. In this manner, this integrated index captures physiologic abnormalities in aldosterone suppression, aldosterone stimulation, and also when both of these responses are mildly or severely abnormal. For serum measures, we define the index as the Sodium-modulated Aldosterone Suppression-to-Stimulation Index (SASSI), and for urinary aldosterone measures we define the index as the Sodium-modulated Aldosterone Urinary Suppression-to-Stimulation Index (SAUSSI). We hypothesized that abnormal aldosterone responses to dietary salt interventions (high SASSI or high SAUSSI) would associate with individual cardio-metabolic risk factors and with aggregate constellations of cardio-metabolic risk, such as the MetS. These findings could better define the development of abnormal aldosterone physiology with progressive cardio-metabolic abnormalities, and provide mechanistic insights for future intervention studies.
RESEARCH DESIGN AND METHODS
Study Population and Protocol
Study Population
A cross-sectional analysis of participants studied in the International Hypertensive Pathotype (HyperPATH) Protocol, a dataset consisting of individuals who underwent rigorous profiling of the RAAS under controlled conditions, was conducted. Five centers contributed to this dataset: Brigham and Women’s Hospital (Boston, MA, USA) , University of Utah Medical Center (Salt Lake City, UT,USA) , Hospital Broussais (Paris, France) , University of Rome (Rome Italy), and Vanderbilt University (Nashville, TN, USA). For this analysis, we included individuals who successfully completed all study procedures, and had complete data for all 4 components of the metabolic syndrome according to the World Health Organization (WHO) criteria22 (hypertension, insulin resistance [fasting glucose and insulin], BMI, and hyperlipidemia [high density lipoprotein and triglyceride levels]). Although the MetS is heterogeneous and not inclusive of all risk factors (for example, age, race, and gender are not a part of the MetS definition), we used the MetS as a model of a pre-defined, and well known, compounded cardio-metabolic risk state. The HyperPATH cohort characterized hypertension as a seated diastolic blood pressure of ≥ 100 mmHg off antihypertensive medications, ≥ 90 mmHg taking ≥ 1 medication, or treatment with ≥ 2 medications. Type 2 diabetes mellitus (T2DM) was defined per American Diabetes Association criteria 23: fasting blood glucose ≥ 126 mg/dl, random blood glucose ≥ 140 mg/dl, HgA1c ≥ 6.5%, 2-hr OGTT blood glucose ≥ 200 mg/dl or a prior physician confirmed diabetes diagnosis 22. A participant was considered to have the MetS if they had T2DM (see above criteria), impaired glucose tolerance (2-hr oral glucose tolerance test (OGTT) glucose >140 mg/dl 24, impaired fasting glucose (≥ 100 mg/dl ADA citation), or insulin resistance (upper tertile of HOMA-IR in the HyperPATH: HOMA-IR ≥ 3) plus two or more of the characteristics listed below:
Hypertension (see above definition)
Hyperlipidemia: triglycerides measurement greater than or equal to 150 mg/dl and/or high density lipoprotein (HDL) <35 mg/dl in men and <39 mg/dl in women.
Obesity: BMI ≥30 kg/m2
Although other results from the HyperPATH cohort have been reported previously14, 16–18, the present analyses are original. All inclusion and exclusion criteria for the HyperPATH protocol are described elsewhere20. In brief, all participants received a screening examination with a medical history, physical examination, EKG, and laboratory evaluation. Participants with known or suspected secondary hypertension, coronary artery disease, stroke, overt renal insufficiency (serum creatinine >1.5mg/dl), psychiatric illness, current oral contraceptive use, current tobacco/illicit drug use or moderate alcohol use were excluded. Participants with abnormal electrolyte or thyroid/liver function tests or electrocardiographic evidence of heart block, ischemia, or prior coronary events at the screening exam were excluded. All participants were between the ages of 18 and 65 years. Race was obtained via participant self-report. The protocol was approved by the institutional review boards (IRB) of each site and informed consent was obtained prior to participant enrollment.
Study Protocol
Details of this protocol have been described previously20. In brief, to control for the influence of medications on components of the RAAS, all angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or mineralocorticoid receptor antagonists were discontinued for 3 months prior to study, and beta blockers, calcium-channel blockers, and diuretics were discontinued for at least 2 weeks prior to the study. If necessary, participants were briefly given amlodipine for blood pressure (BP) control; however, this was discontinued 2 weeks prior to the start of the study procedures.
Participants completed two diets for 5–7 days each: liberal sodium (LIB) (200mmol/day) and restricted sodium (RES) (10mmol/day) with each diet also containing 100 mmol/day potassium and 20 mmol/day calcium. Upon completion of each diet phase, participants were admitted overnight to the Clinical Research Center (CRC). Sodium balance was confirmed by 24-hour urine collection. For this analysis, only subjects with verified urinary sodium of ≥150 mmol sodium/d on LIB diet, and ≤40 mmol sodium/d on RES diet, were included. Baseline measurements for insulin, glucose, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), triglycerides, plasma renin activity (PRA), serum aldosterone, and blood pressure were obtained in the morning after overnight supine rest using standardized and validated methods as previously described 16, 25. After baseline blood draws were collected on RES diet, an infusion of angiotensin II (ANGII) (Bachem AG, Bubendorf, Switzerland) (3ng/kg/min for 60 min) was administered as previously described 25, and measurements for serum aldosterone and PRA were repeated at the end of the infusion. Insulin resistance was measured using the homeostatic model assessment (HOMA-IR) as previously described 26.
On the morning of the 5th day of the LIB diet, a portion of the non-diabetic participants reported to the ambulatory clinical research center and received a 75 g oral glucose tolerance test (OGTT). The OGTT was conducted as previously described 27, 28.
Development of the SASSI and SAUSSI
We used aldosterone and PRA measurements from our study protocols to develop integrated indices reflecting dynamic RAAS physiology that we could then use in the assessment of cardio-metabolic risk factors. Among the many methods to measure the RAAS (Table S1), we used only those that were obtained during the aforementioned control of diet, posture, and interfering medications. The ratio of single supine serum aldosterone measurements on LIB and RES diets was used to calculate the SASSI (ratio of dietary sodium-suppressed aldosterone to dietary sodium-stimulated aldosterone) (Table 1). The ratio of 24h urinary aldosterone excretion on both LIB and RES diets was used to calculate the SAUSSI (ratio of dietary sodium suppressed to dietary sodium stimulated urinary aldosterone excretion). The ratio of the supine serum aldosterone on LIB diet to the serum aldosterone following an infusion of ANGII on RES diet was termed the SASSI-II, and used as an index of the maximally dietary sodium-suppressed aldosterone to the AngII-stimulated aldosterone (Table 1). The SASSI-II was developed to provide more information than the SASSI or SAUSSI might alone, since ANGII stimulation on RES diet provides a measure of adrenal aldosterone stimulation that is independent of other endogenous RAAS components (ANGII and PRA). Thus, while the physiologic responses of PRA, ANGII, and aldosterone are expected to be parallel and correlated in a dietary sodium-modulated index such as the SASSI, the SASSI-II is expected to distinguish the contributions of aldosterone versus other RAAS components.
Table 1. New Indices to Represent Aldosterone Physiology.
The table lists novel indices that were devised in this study to represent physiologic aldosterone responses and their interpretation.
| Index of Aldosterone Physiology |
Abbreviation | Interpretation |
|---|---|---|
|
Serum aldosterone on LIB diet ------------------------------------ Serum aldosterone on RES diet |
SASSI |
|
| Serum aldosterone on LIB diet ------------------------------------- ANGII stimulated serum aldosterone on RES diet |
SASSI-II |
|
| Urinary aldosterone on LIB diet -------------------------------------- Urinary aldosterone on RES diet |
SAUSSI |
|
Although plasma ANGII measurements were not available on the vast majority of participants, single supine PRA measurements were available on LIB and RES diets for all subjects. The ratio of these measurements (PRA on LIB diet:PRA on RES diet) was used to evaluate the physiologic range of PRA to dietary sodium manipulation. In addition, PRA was measured after the infusion of ANGII on RES diet, and used in an index to parallel the SASSI-II (PRA on LIB: PRA on RES after ANGII).
Statistical Analyses
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, N.C., U.S.A.). Population characteristics for individuals with and without MetS were compared using a Student’s t-test. A chi-square analysis was used for comparison of categorical variables. Data are represented as means ± standard deviation. The main analyses for this study included: 1) univariate regression analyses to analyze the relationship between individual cardio-metablic risk factors and measures of aldosterone; 2) The relationship between these aldosterone measurements and increasing components of the MetS (0–4) and the odds of having MetS (yes/no) using linear and logistic regression adjusted for age, gender, and race29–31. Sensitivity and specificity analyses were conducted to evaluate whether physiologic aldosterone measurements could distinguish healthy individuals (zero MetS components) from those with any one MetS component (0 versus 1) or those with any combination of MetS components (0 versus 1, 2, 3, or 4). Since specific aldosterone measurements associated with cardio-metabolic risk have not been established, we chose cut-points for the sensitivity and specificity analyses based on the highest tertile value for each aldosterone measurement32. All statistical tests were 2-sided. Significance is indicated for p<0.05.
RESULTS
The Study Population
Twenty-six percent of the study population had MetS, and as expected, these individuals were older in age, and had higher BMI, fasting blood glucose, and blood pressure (BP) (Table 2). Consistent with prior reports6, 9, 10, aldosterone concentrations and PRA on LIB diet were significantly higher in individuals with MetS compared to those without MetS. In contrast, a non-significant trend towards lower serum and urinary aldosterone levels was observed during RES diet conditions.
Table 2. Baseline characteristics of the study population based on MetS status.
Where applicable, variables are depicted when subjects were maintained on LIB and RES diets. Values are represented as means±standard deviation.
| Characteristics | MetS (−) | MetS(+) | p value | |
|---|---|---|---|---|
| N | 398 | 141 | ||
| Age (years) | 45.9±10.7 | 48.8±8.7 | 0.004 | |
| Female Gender (%) | 199 (50%) | 48 (34%) | 0.001 | |
| Race | ||||
| Caucasian (%) | 336(84%) | 109(78%) | 0.08 | |
| African American (%) | 44(11%) | 26 (18%) | 0.08 | |
| Other (%) | 18 (5%) | 6 (4%) | ||
| Body Mass Index (kg/m2) | ||||
| LIB diet | 26.5±3.7 | 30.7±4.0 | <0.0001 | |
| RES diet | 25.8±3.7 | 29.9±4.1 | <0.0001 | |
| Fasting Blood Glucose (mg/dl) | ||||
| LIB diet | 88.8±16.8 | 103.9±21.3 | <0.0001 | |
| RES diet | 96.0±32.6 | 104.9±22.6 | <0.0001 | |
| Mean Arterial Pressure (mmHg) | ||||
| LIB diet | 97.2±16.0 | 106.7±13.7 | <0.0001 | |
| RES diet | 88.8±13.2 | 97.6±11.7 | <0.0001 | |
| Serum Aldosterone Baseline (ng/dl) | ||||
| LIB diet | 4.3±2.9 | 5.3±3.9 | 0.0007 | |
| RES diet | 18.0±12.3 | 17.5±11.0 | 0.7 | |
|
Urinary Aldosterone Excretion Rate (mcg/24h) |
||||
| LIB diet | 10.1±7.9 | 13.8±29.4 | <0.0001 | |
| RES diet | 45.9±29.8 | 41.9±26.9 | 0.2 | |
| Plasma Renin Activity (ng/mL/h) | ||||
| LIB diet | 0.43±0.43 | 0.63±1.2 | <0.01 | |
| RES diet | 2.47±2.0 | 2.79±3.0 | 0.20 | |
| Urinary Sodium (mmol/24hrs) | ||||
| Lib diet | 240.8±66.2 | 250.1±74.5 | 0.20 | |
| RES diet | 12.7±8.9 | 13.8±8.3 | 0.20 | |
| Urinary Potassium (mEq/24hrs) | ||||
| Lib diet | 72.5±24.3 | 76.6±24.1 | 0.1 | |
| RES diet | 73.3±19.6 | 71.8±20.0 | 0.4 | |
The SASSI and SAUSSI as Predictors of Individual and Aggregate Cardio-Metabolic Risk
We evaluated the correlation between aldosterone measurements and individual cardio-metabolic risk factors. Serum aldosterone on LIB diet was positively correlated with BP, BMI, and lipid parameters, suggesting that the lack of aldosterone suppressibility is a predictor of these risk factors (Table 3a). In contrast, serum aldosterone on RES diet was inversely associated with age and BMI, suggesting an inability to appropriately stimulate aldosterone as a predictor for these risk factors. When these aldosterone measures were integrated as the SASSI, this single index displayed strong positive associations with age, male gender, BP, and BMI; thereby combining the predictive power of single aldosterone measurements on LIB and RES diets. Similar relationships were seen with urine aldosterone measurements and the integrated SAUSSI (Table 3b).
Table 3. Univariate Relationships Between Serum (A) and Urinary (B) Aldosterone Measurements and Individual Cardio-Metabolic Parameters.
Effect estimates (β) and P-values are presented for each variable.
| A) | ||||||
|---|---|---|---|---|---|---|
| Cardio- Metabolic Parameters |
LIB Diet Serum Aldosterone |
RES Diet Serum Aldosterone |
SASSI | |||
| β | p value | β | p value | β | p value | |
| Age | 0.01 | 0.3 | −0.43 | <0.0001* | 0.007 | <0.0001* |
| Gender (male) | −0.35 | 0.2 | −4.34 | 0.6 | 0.03 | <0.0001* |
| Race (African American) |
−0.56 | 0.7 | −1.71 | 0.6 | −0.02 | 0.8 |
| SBP | 0.04 | <0.0001* | −0.02 | 0.4 | 0.004 | <0.0001* |
| BMI | 0.07 | 0.05* | −0.36 | 0.004* | 0.01 | 0.001* |
| HDL | −0.02 | 0.003* | −0.07 | 0.06 | 0.00004 | 0.9 |
| Triglycerides | 0.004 | 0.02* | −0.002 | 0.7 | 0.0003 | 0.08 |
| HOMA-IR | 0.14 | 0.06 | −0.06 | 0.8 | 0.01 | 0.2 |
| B) | ||||||
|---|---|---|---|---|---|---|
| Cardio- Metabolic Parameters |
LIB Diet Urine Aldosterone |
RES Diet Urine Aldosterone |
SAUSSI | |||
| β | p value | β | p value | β | p value | |
| Age | 0.04 | 0.2 | −1.001 | <0.0001* | 0.007 | <0.0001* |
| Gender (male) | −0.05 | 0.9 | 1.99 | 0.4 | 0.01 | 0.7 |
| Race (African American) | −1.26 | 0.04* | −10.11 | 0.03* | 0.18 | 0.7 |
| SBP | 0.05 | <0.0001* | −0.35 | <0.0001* | 0.004 | <0.0001* |
| BMI | 0.11 | 0.1 | −1.03 | 0.0008* | 0.003 | 0.4 |
| HDL | −0.03 | 0.08 | 0.18 | 0.05* | −0.001 | 0.2 |
| Triglycerides | 0.007 | 0.03* | −0.07 | <0.0001* | 0.0002 | 0.09 |
| HOMA-IR | −0.0004 | 0.9 | −0.67 | 0.2 | −0.01 | 0.05* |
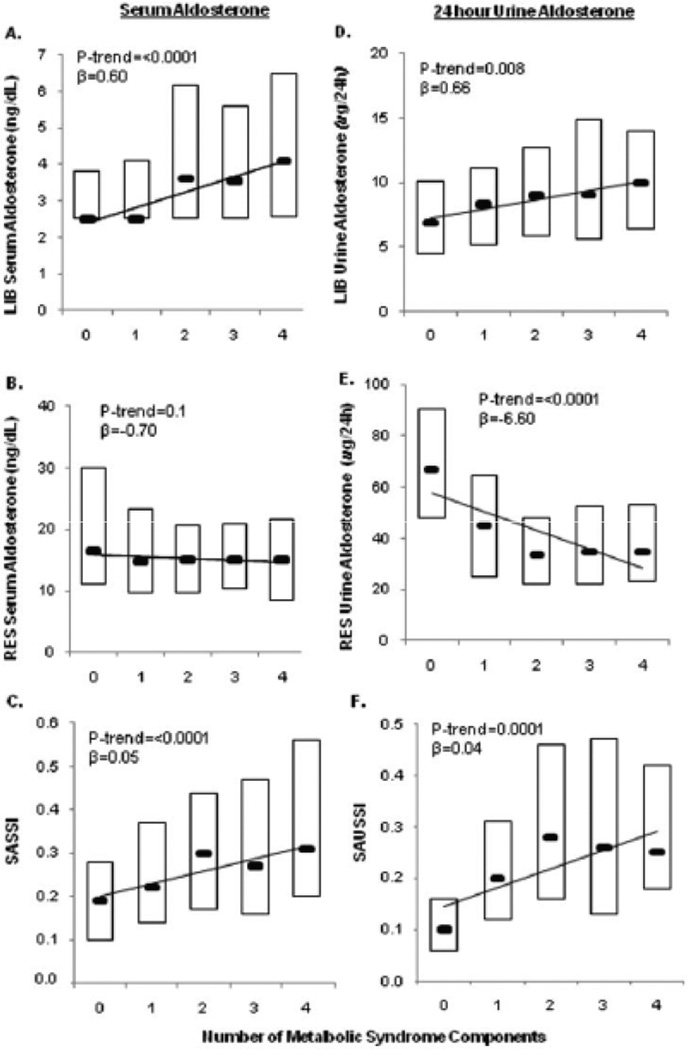
In addition to providing an additive integration of associations of aldosterone measures on either diet alone, the SASSI and SAUSSI also seemed to distinguish individuals with MetS. Although the MetS definition does not include age, gender, and race (potential cardio-metabolic risk factors that associate with aldosterone measurements in Table 3a and 3b), subjects with MetS had a higher SASSI and a non-significant trend towards higher SAUSSI values when compared to those without MetS (SASSI: 0.41 ± 0.36 vs. 0.33 ± 0.32, P=0.01; SAUSSI: 0.42 ± 0.59 vs. 0.34 ± 0.48, P=0.15), indicating an association between MetS and higher aldosterone suppression-to-stimulation ratios. We evaluated the impact of how successive components of the MetS would affect physiologic aldosterone responses. In comparison to healthy subjects who lack any component of the MetS (zero components), those with increasing numbers of components exhibited a failure to suppress serum and urinary aldosterone on LIB diet, and a failure to appropriately stimulate aldosterone on RES diet (Figure 1 A–B, 1D–E). In reflection of this dampening of the physiologic range of aldosterone with progressive MetS components, both the SASSI and SAUSSI were observed to be higher, and strongly associated, with a greater number of MetS components (Figure 1C and 1F).
Figure 1. The association of between the number of successive components of the MetS and aldosterone measures.
Serum aldosterone concentrations are on LIB diet (A) and RES diet (B) are paralleled with urinary aldosterone excretion rates on LIB diet (D) and RES diet (E). Serum measures are expressed as the SASSI with respect to MetS components (C) and urine measures are expressed as the SAUSSI (F). Data are presented as box plots where boxes represent the 25th-75th percentiles and black horizontal dashes represent the median value.
Predicting Aggregate Cardio-Metabolic Risk Using SASSI and SAUSSI
We examined the sensitivity and specificity for identifying healthy individuals (zero MetS components) using SASSI or SAUSSI, when compared to individuals with any one single MetS component. Both the SASSI and SAUSSI displayed a higher sensitivity for distinguishing individuals with zero cardio-metabolic risk factors when compared to single serum or urinary measures of aldosterone on either diet (86% for SASSI, 83% for SAUSSI) (Table S2). In contrast, the ability to distinguish healthy individuals from any of the remaining subjects with 1, 2, 3, or 4 MetS components was not remarkably different among aldosterone measures (Table S2).
Is Abnormal Aldosterone Physiology a Consequence of a Primary Adrenal Defect or Secondary to Other Known Regulators of Aldosterone?
We explored whether the observed relationships between aldosterone responses and components of the MetS were due to a primary dysfunction of the adrenal glands, or driven by other factors known to influence adrenal aldosterone physiology, such as ANGII activity, serum potassium, or corticotropin (ACTH).
ANGII Activity
We used measures of PRA as a surrogate for ANGII since it is known to be highly correlated with ANGII concentrations. In logistic regression models adjusted for age, gender, and race, an inability to suppress aldosterone or PRA on LIB diet predicted the odds of having MetS (Table 4 – top row). Progressive impairments in physiologic aldosterone responses to dietary sodium manipulations (higher SASSI) were associated with a higher odds of having MetS, but impaired PRA responses to dietary sodium manipulation (PRA on LIB:PRA on RES) were not (Table 4 – middle row). Like the SASSI, higher SASSI-II values were also associated with the odds of having MetS, suggesting that a blunted range of adrenal aldosterone responsiveness (and not endogenous PRA or ANGII) was correlated with MetS (Table 4 – bottom row).
Table 4. Physiologic RAAS Measurements and the Odds for Metabolic Syndrome.
Every aldosterone measurement, regardless of interventional provocation, significantly predicted the odds of having MetS (yes/no). In contrast, this was not true of all PRA measurements. Values represent odds ratios and 95% confidence intervals.
| Physiologic Aldosterone Measurements |
Aldosterone Measure |
PRA Measure |
Physiologic PRA Measurements |
|
|---|---|---|---|---|
| Serum aldosterone on LIB diet | 1.10 [1.04–1.16]** |
2.10 [1.40–3.20]*** |
Supine PRA on LIB diet | |
|
LIB diet aldosterone --------------------- RES diet aldosterone |
(SASSI) | 2.20 [1.2–3.9]** |
1.50 [0.94–2.40] |
PRA on LIB diet ------------------------------- PRA on RES diet |
|
LIB diet aldosterone -------------------- RES diet ANGII-stimulated aldosterone |
(SASSI-II) | 8.33 [1.16–60.1]* |
1.40 [0.87–2.20] |
PRA on LIB diet ------------------------------- PRA after ANGII stimulation on RES diet |
P<0.05,
P<0.01,
****P<0.001.
Serum Potassium
Serum potassium, a major regulator of aldosterone, was no different in those with versus without MetS (LIB diet: 4.16 ± 0.32 vs 4.16 ± 0.34 mmol/L p=0.90; RES diet: 4.18 ± 0.33 vs 4.25 ± 0.36 mmol/L, p=0.08). There was no association between increasing components of the MetS and serum potassium on either dietary condition (p=0.50 for LIB diet and p=0.10 for RES diet).
ACTH
We used serum cortisol measures as a surrogate for ACTH, which was not directly measured. Serum cortisol levels did not differ between individuals with versus without the MetS on either diet (LIB diet: 10.8 ± 4.0 vs 11.3 ± 4.5 pg/mL, p=0.30; RES diet: 11.5 ± 3.8 vs 12.1 ± 4.5 pg/mL, p=0.20), and were not associated with increasing components of the MetS on either LIB (p=0.30) or RES (p=0.20) diets.
DISCUSSION
Our findings show strong associations between abnormal aldosterone physiology with individual cardio-metabolic risk factors, and suggest that progressive clustering of risk factors, as seen in the MetS, are also associated with pathophysiologic aldosterone regulation. These findings build upon, and integrate, prior reports which suggest that an inability to appropriately stimulate14, 17, 18, 33 or suppress7, 8, 19 aldosterone in response to physiologic stimuli is associated with cardio-metabolic disease. Our study is distinguished from previous studies that evaluated the role of aldosterone in disease in that it analyzed a very large sample size of subjects, and employed novel indices to represent dynamic aldosterone physiology. Whereas static aldosterone measurements in a specific environmental milieu (LIB diet or RES diet) may predict some cardio-metabolic risk, our findings show that an index of aldosterone physiology that reflects the entire dynamic of sodium-induced aldosterone regulation provides a more complete integration of cardio-metabolic risk associations (Table 3). Our novel indices of aldosterone regulation not only associated with individual cardio-metabolic risk variables, but were also sensitive at distinguishing healthy individuals from those with mild cardio-metabolic risk, and predicted the odds of having MetS and the severity of MetS. Furthermore, our analyses suggest that the abnormal aldosterone physiology seen with the progressive accrual of cardio-metabolic risk factors is independent of demographic variables and known aldosterone secretagogues. In totality, these findings provide new insights into the role of aldosterone regulation in disease: cardio-metabolic derangements may be caused by, or result in, progressive dysregulation of physiologic aldosterone suppression and/or stimulation.
Our findings extend and clarify those of others before us. Prior cross-sectional studies, that often lacked control of environmental confounders of aldosterone, found that higher aldosterone levels were associated with an increased prevalence of MetS9 10 6. Conversely, a large case-control study of approximately 1,800 individuals found no difference in glucose and lipid values between individuals with and without primary hyperaldosteronism34. With our strict study design, we confirm that higher aldosterone levels on a fixed diet of liberal sodium intake associate with multiple cardio-metabolic risk factors. We extend these findings to show that the inability to appropriately stimulate aldosterone with sodium restriction also associates with similar risk factors, but in addition correlates with other risk factors (such as older age), which are not associated with the lack of aldosterone suppression on liberal sodium intake. These associations support our initial hypothesis that an integrated assessment of the dynamic range of aldosterone (suppression and stimulation) may better characterize the pathophysiologic role aldosterone plays in cardio-metabolic diseases. Indeed, our integrated indices of aldosterone physiology associated with all of the cardio-metabolic variables that correlated with aldosterone levels on either LIB diet and/or RES diet. Although it is not clear why some risk factors associate with abnormal aldosterone suppression while others associate with abnormal aldosterone stimulation, our findings indicate that knowledge of the full range of adrenal aldosterone regulation may be important in understanding the pathogenesis of aldosterone-associated cardio-metabolic diseases.
We used the MetS as an example of a heterogeneous clustering of cardio-metabolic risk factors. Although MetS does not include important aldosterone-associated variables such as age and race, our findings still suggest that the presence of any single MetS component is associated with a significant alteration in aldosterone physiology such that it is distinguished from that of a healthy individual. In contrast, with the successive accrual of MetS components, the dampening of the physiologic aldosterone range appears to plateau (Figures 1C and 1F). Based on these observations, we speculate that the association between cardio-metabolic risk factors and aldosterone physiology is most notable in the early development of cardio-metabolic disease. With the progressive accrual of cardio-metabolic risk factors, the dynamic range of aldosterone may approach a fixed asymptote that diminishes its ability to distinguish additional risk. Measurements of the SASSI or SAUSSI are not simple, not generalizable, and are unlikely to be adopted on a large scale. Our study does not support the use of these indices as diagnostics for disease or risk; however, it does provide novel insights into the dynamic and subtle alterations in adrenal physiology that occur with cardio-metabolic disease. This knowledge may help guide future therapies and prevention measures to lower cardio-metabolic risk.
What mechanisms may account for the dampening of the physiologic aldosterone range we observed with progressive cardio-metabolic risk factors? One could postulate that the progressive accumulation of MetS components results in abnormalities in adrenal aldosterone regulation, or that alternatively, a predisposition to abnormal adrenal physiology increases the likelihood of developing MetS components. We explored potential mechanisms by evaluating known regulators of aldosterone, such as PRA-ANGII axis, ACTH, and serum potassium; however, observed no meaningful correlations to suggest that these factors were responsible for the resultant aldosterone responses. Therefore, we speculate that the development of abnormal aldosterone physiology with cardio-metabolic disease is due to either a primary adrenal gland dysfunction, or due to other unknown or unmeasured regulators of aldosterone (such as adipose tissue) that warrant further characterization35–37.
Our study has several strengths. Our sample size was large (>500 subjects) and included more than 1,000 inpatient study visits (2 per subject) dedicated to characterizing the RAAS under tightly controlled environmental conditions. Hormonal measurements were conducted after participants ingested a diet that controlled electrolytes known to affect RAAS components in humans38, 39, and dietary compliance was confirmed with urinary assessments. Subjects were withdrawn from all interfering medications to increase the confidence in RAAS measures. Further, participants were admitted to an in-patient Clinical Research Center and studied after overnight supine rest. Aldosterone was characterized using serum and urinary measures, and we observed parallel findings with both assessments, highlighting the robust nature of these relationships. Notable limitations of our study include its cross-sectional nature. We cannot confirm a causal relationship between inappropriate aldosterone physiology and cardio-metabolic risk factors or MetS. Our analyses were not designed to evaluate the diagnostic utility of our indices, which are impractical; rather the focus was to provide insights into adrenal pathophysiology that may underlie cardio-metabolic disease states. Interventional studies to evaluate the effect of RAAS antagonists in individuals with abnormal SASSI/SAUSSI are needed to better assess causality.
PERSPECTIVES
In summary, we conclude that abnormal aldosterone physiology, when represented by integrated indices that reflect the extremes of dietary sodium modulations, strongly predicts individual and compounded cardio-metabolic risk factors. The earliest manifestations of cardio-metabolic derangements may exhibit abnormalities in aldosterone suppression and/or stimulation that are detected by integrated indices such as the SASSI and SAUSSI. Future studies to investigate the mechanisms underlying the blunting of the dynamic range of aldosterone may identify new therapeutic approaches to prevent or treat cardio-metabolic risk.
Supplementary Material
NOVELTY and SIGNIFICANCE.
1) What is New? We developed and tested novel indices (SASSI and SAUSSI) to reflect the dynamic physiologic range of aldosterone in response to dietary sodium modulation.
2) What is Relevant? Integrated indices of aldosterone suppression-to-stimulation associate strongly with individual cardio-metabolic risk factors, predict the odds and severity of metabolic syndrome, and discriminate healthy individuals from those with even mild cardio-metabolic risk.
Summary: Abnormal aldosterone physiology, when represented by integrated indices that reflect the extremes of dietary sodium modulations, strongly predicts cardio-metabolic risk factors, and provides novel insights into the pathophysiology that may underlie aldosterone-mediated disease states.
ACKNOWLEDGMENTS
We thank the fellows, trainees, and nursing staff who have contributed to the studies involving the HyperPath cohort.
SOURCES OF FUNDING: The project was supported in part by the following grants: U54LM008748 from the National Library of Medicine, UL1RR025758, Harvard Clinical and Translational Science Center, from the National Center for Research Resources and M01-RR02635, Brigham & Women's Hospital, General Clinical Research Center, from the National Center for Research Resources. As well as NIH grants HL47651, HL59424, F32 NR013318 (PCU), K24 HL103845 (GKA), K23 HL111771-01 (AV), Specialized Center of Research (SCOR) in Molecular Genetics of Hypertension P50HL055000.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST/DISCLOSURES: none
REFERENCES
- 1.Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep. 13:163–172. doi: 10.1007/s11906-011-0182-2. [DOI] [PubMed] [Google Scholar]
- 2.Krug AW, Ehrhart-Bornstein M. Aldosterone and metabolic syndrome: Is increased aldosterone in metabolic syndrome patients an additional risk factor? Hypertension. 2008;51:1252–1258. doi: 10.1161/HYPERTENSIONAHA.107.109439. [DOI] [PubMed] [Google Scholar]
- 3.Tirosh A, Garg R, Adler GK. Mineralocorticoid receptor antagonists and the metabolic syndrome. Curr Hypertens Rep. 12:252–257. doi: 10.1007/s11906-010-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: Role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis. 52:401–409. doi: 10.1016/j.pcad.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W. Association of plasma aldosterone with cardiovascular mortality in patients with low estimated gfr: The ludwigshafen risk and cardiovascular health (luric) study. Am J Kidney Dis. 2011;57:403–414. doi: 10.1053/j.ajkd.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, Fox CS, Meigs JB, Levy D, Larson MG, Selhub J, D'Agostino RB, Sr, Wang TJ, Vasan RS. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: The framingham offspring study. Circulation. 2007;116:984–992. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 7.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab. 95:1986–1990. doi: 10.1210/jc.2009-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, Shamlaye C, Burnier M. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 10.Hannemann A, Wallaschofski H, Ludemann J, Volzke H, Markus MR, Rettig R, Lendeckel U, Reincke M, Felix SB, Empen K, Nauck M, Dorr M. Plasma aldosterone levels and aldosterone-to-renin ratios are associated with endothelial dysfunction in young to middle-aged subjects. Atherosclerosis. 219:875–879. doi: 10.1016/j.atherosclerosis.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension. 1995;25:30–36. doi: 10.1161/01.hyp.25.1.30. [DOI] [PubMed] [Google Scholar]
- 12.Rossi GP, Belfiore A, Bernini G, et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–2571. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 13.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–362. doi: 10.1002/j.1550-8528.1999.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferri C, Bellini C, Desideri G, Valenti M, De Mattia G, Santucci A, Hollenberg NK, Williams GH. Relationship between insulin resistance and nonmodulating hypertension: Linkage of metabolic abnormalities and cardiovascular risk. Diabetes. 1999;48:1623–1630. doi: 10.2337/diabetes.48.8.1623. [DOI] [PubMed] [Google Scholar]
- 15.Gaboury CL, Hollenberg NK, Hopkins PN, Williams R, Williams GH. Metabolic derangements in nonmodulating hypertension. Am J Hypertens. 1995;8:870–875. doi: 10.1016/0895-7061(95)00160-Q. [DOI] [PubMed] [Google Scholar]
- 16.Raji A, Williams GH, Jeunemaitre X, Hopkins PN, Hunt SC, Hollenberg NK, Seely EW. Insulin resistance in hypertensives: Effect of salt sensitivity, renin status and sodium intake. J Hypertens. 2001;19:99–105. doi: 10.1097/00004872-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya A, Sun B, Larson C, Forman JP, Williams JS. Vitamin d3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: An interventional study. J Clin Endocrinol Metab. 2012;97:2456–2465. doi: 10.1210/jc.2012-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Underwood PC, Chamarthi B, Williams JS, Vaidya A, Garg R, Adler GK, Grotzke MP, Staskus G, Wadwekar D, Hopkins PN, Ferri C, McCall A, McClain D, Williams GH. Nonmodulation as the mechanism for salt sensitivity of blood pressure in individuals with hypertension and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:3775–3782. doi: 10.1210/jc.2012-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price DA, De'Oliveira JM, Fisher ND, Williams GH, Hollenberg NK. The state and responsiveness of the renin-angiotensin-aldosterone system in patients with type ii diabetes mellitus. Am J Hypertens. 1999;12:348–355. [PubMed] [Google Scholar]
- 20.Vaidya A, Forman JP, Underwood PC, Hopkins PN, Williams GH, Pojoga LH, Williams JS. The influence of body-mass index and renin-angiotensin-aldosterone system activity on the relationship between 25-hydroxyvitamin d and adiponectin in caucasian men. Eur J Endocrinol. doi: 10.1530/EJE-11-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pojoga LH, Underwood PC, Goodarzi MO, et al. Variants of the caveolin-1 gene: A translational investigation linking insulin resistance and hypertension. J Clin Endocrinol Metab. 2011;96:E1288–E1292. doi: 10.1210/jc.2010-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a who consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Vimaleswaran KS, Radha V, Jayapriya MG, Ghosh S, Majumder PP, Rao MR, Mohan V. Evidence for an association with type 2 diabetes mellitus at the pparg locus in a south indian population. Metabolism. 2010;59:457–462. doi: 10.1016/j.metabol.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Giles WH. A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care. 2003;26:575–581. doi: 10.2337/diacare.26.3.575. [DOI] [PubMed] [Google Scholar]
- 25.Shoback DM, Williams GH, Hollenberg NK, Davies RO, Moore TJ, Dluhy RG. Endogenous angiotensin ii as a determinant of sodium-modulated changes in tissue responsiveness to angiotensin ii in normal man. J Clin Endocrinol Metab. 1983;57:764–770. doi: 10.1210/jcem-57-4-764. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Trout KK, Homko C, Tkacs NC. Methods of measuring insulin sensitivity. Biol Res Nurs. 2007;8:305–318. doi: 10.1177/1099800406298775. [DOI] [PubMed] [Google Scholar]
- 28.Ayers K, Byrne LM, Dematteo A, Brown NJ. Differential effects of nebivolol and metoprolol on insulin sensitivity and plasminogen activator inhibitor in the metabolic syndrome. Hypertension. 59:893–898. doi: 10.1161/HYPERTENSIONAHA.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okosun IS, Liao Y, Rotimi CN, Prewitt TE, Cooper RS. Abdominal adiposity and clustering of multiple metabolic syndrome in white, black and hispanic americans. Ann Epidemiol. 2000;10:263–270. doi: 10.1016/s1047-2797(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 30.Sartorio A, Agosti F, Adorni F, Pera F, Lafortuna CL. Effect of age degree and distribution of adiposity on the prevalence of the metabolic syndrome in a cohort of obese italian women. Diabetes Res Clin Pract. 2007;78:225–233. doi: 10.1016/j.diabres.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt MI, Duncan BB, Watson RL, Sharrett AR, Brancati FL, Heiss G. A metabolic syndrome in whites and african-americans. The atherosclerosis risk in communities baseline study. Diabetes Care. 1996;19:414–418. doi: 10.2337/diacare.19.5.414. [DOI] [PubMed] [Google Scholar]
- 32.Vaes B, Delgado V, Bax J, Degryse J, Westendorp RG, Gussekloo J. Diagnostic accuracy of plasma nt-probnp levels for excluding cardiac abnormalities in the very elderly. BMC Geriatr. 2010;10:85. doi: 10.1186/1471-2318-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoback DM, Williams GH, Moore TJ, Dluhy RG, Podolsky S, Hollenberg NK. Defect in the sodium-modulated tissue responsiveness to angiotensin ii in essential hypertension. J Clin Invest. 1983;72:2115–2124. doi: 10.1172/JCI111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matrozova J, Steichen O, Amar L, Zacharieva S, Jeunemaitre X, Plouin PF. Fasting plasma glucose and serum lipids in patients with primary aldosteronism: A controlled cross-sectional study. Hypertension. 2009;53:605–610. doi: 10.1161/HYPERTENSIONAHA.108.122002. [DOI] [PubMed] [Google Scholar]
- 35.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 36.Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A. 2003;100:14211–14216. doi: 10.1073/pnas.2336140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids. 1999;60:401–405. doi: 10.1016/s0952-3278(99)80020-9. [DOI] [PubMed] [Google Scholar]
- 38.Dluhy RG, Axelrod L, Underwood RH, Williams GH. Studies of the control of plasma aldosterone concentration in normal man. Ii. Effect of dietary potassium and acute potassium infusion. J Clin Invest. 1972;51:1950–1957. doi: 10.1172/JCI107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams GH, Cain JP, Dluhy RG, Underwood RH. Studies of the control of plasma aldosterone concentration in normal man. I. Response to posture, acute and chronic volume depletion, and sodium loading. J Clin Invest. 1972;51:1731–1742. doi: 10.1172/JCI106974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.