Abstract
Over the past decade, a growing recognition of the importance of neutralizing antibodies in host defense combined with the success of B-cell depletion therapies in treating autoimmune disorders has led to an increased focus on better understanding the pathways underpinning B-cell antibody production. In general, B cells require cognate interaction with T helper cells in the germinal center of lymphoid follicles to generate protective antibodies. However, recent evidence shows that B cells receive additional help from invariant natural killer T cells, dendritic cells, and various granulocytes, including neutrophils, eosinophils, and basophils. These innate immune cells enhance T-cell-dependent antibody responses by delivering B-cell helper signals both in the germinal center and at postgerminal center lymphoid sites such as the bone marrow. In addition to enhancing and complementing the B-cell helper activity of canonical T cells, invariant natural killer T cells, dendritic cells, and granulocytes can deliver T cell-independent B-cell helper signals at the mucosal interface and in the marginal zone of the spleen to initiate rapid innate-like antibody responses. Here, we discuss recent advances in the role of adaptive and innate B-cell helper signals in antibody diversification and production.
Keywords: Cytokines, Dendritic cells (DCs), Granulocytes, Immunoglobulins, Lymphocytes
Introduction
The mammalian immune system comprises of innate and adaptive branches that mount integrated protective responses against intruding microbes. The innate immune system includes dendritic cells (DCs), macrophages, granulocytes, and natural killer (NK) cells that mediate fast but nonspecific responses after recognizing generic microbial structures through invariant germline gene-encoded receptors often referred to as pattern recognition receptors, including Toll-like receptors (TLRs) (reviewed in [1]). In contrast, the adaptive immune system includes T and B cells that mediate specific but temporally delayed responses after recognizing discrete antigenic epitopes through highly diverse somatically recombined receptors (reviewed in [2]). The crosstalk between the innate and adaptive immune systems is exemplified by responses involving marginal zone (MZ) B cells or invariant NKT (iNKT) cells. Indeed, these lymphocyte subsets mount very early, innate-like adaptive responses after recognizing microbial carbohydrate and glycolipid antigens via both germline-encoded and somatically recombined receptors [3-5].
B cells confer immune protection by producing antibody molecules, also known as immunoglobulins (Igs), which can recognize antigen through either low- or high-affinity binding modes. Bone marrow B-cell precursors generate Ig recognition diversity by undergoing V(D)J gene recombination, an antigen-independent process that utilizes recombination activating gene (RAG) endonucleases to juxtapose noncontiguous variable (V), diversity (D) and joining (J) gene fragments into functional V(D)J genes encoding the antigen-binding V region of Ig molecules (reviewed in [6]). After further maturation events, multiple subsets of mature B cells co-expressing IgM and IgD emerge from the bone marrow and colonize different compartments of secondary lymphoid organs to initiate the antigen-dependent phase of B-cell development. In general, conventional follicular B cells, which are also called B-2 cells, predominantly participate in T-cell-dependent (TD) antibody responses to highly specific determinants usually associated with microbial proteins (reviewed in [7]). TD responses unfold in the germinal center of lymphoid follicles and generate high-affinity antibodies through a TD pathway that involves activation of B cells by follicular helper T (TFH) cells. This germinal center-associated T-cell subset expresses the inducible T-cell costimulator (ICOS) receptor, the chemokine receptor CXCR5, the programmed cell death-1 (PD-1) inhibitory receptor and the transcription factor Bcl6 [8-15]. TFH cells provide help to B cells via CD40 ligand (CD40L) and cytokines such as IL-21, IL-4, and IL-10 [16-19]. However, recent findings indicate that follicular antibody responses further involve additional T-cell subsets, including follicular regulatory T (TFR) cells and iNKT cells [4,5,20-22].
Unlike follicular B cells, certain subsets of extrafollicular B cells such as B-1 cells, splenic MZ B cells (also referred to as IgM memory B cells in humans) and bone marrow perisinusoidal B cells predominantly give rise to rapid T-cell-independent (TI) antibody responses to highly conserved carbohydrate and glycolipid determinants associated with microbes [3,23-30]. TI antibody responses usually unfold at the mucosal interface or in the splenic MZ and generate polyspecific and low-affinity antibodies through a TI pathway involving the interaction of B cells with DCs, macrophages, and granulocytes [3,30-34]. These innate immune cells deliver antibody-inducing signals via CD40L-like cytokines known as B-cell-activating factor of the TNF family (BAFF, also known as BLyS) and a proliferation-inducing ligand (APRIL) [3,30,35-39]. However, it must be noted that TD and TI responses are not rigidly compartmentalized within the B-2 and MZ/B-1 cell subsets. For instance, MZ B cells also participate in TD antibody production owing to their ability to shuttle to the follicle and present antigen to T cells [40,41]. Conversely, B-2 cells can initiate TI antibody responses in the intestine [42]. Here, we discuss recent advances in our understanding of the mechanisms by which adaptive and innate immune cells provide help to B cells.
B-cell helper signals from TH cells
Protein antigens initiate protective antibody responses in the follicles of secondary lymphoid organs, a microenvironment that favors the interaction of B and T cells with each other as well as with antigen presenting DCs and antigen exposing follicular dendritic cells (FDCs) (reviewed in [7]). After interacting with antigen through the B-cell receptor (BCR), which includes IgM and IgD (Fig. 1), naive B cells migrate to the boundary between the follicle and the outer T-cell zone [43]. At this location, B cells form dynamic conjugates with TFH cells, which deliver cognate B-cell help through a mechanism involving the tumor necrosis factor (TNF) family member CD40L and cytokines such as interferon-γ (IFN-γ, a cytokine also expressed by TH1 cells) and interleukin-4 (IL-4, a cytokine also expressed by TH2 cells) [13,14,43,44]. B cells thereafter differentiate along one of the two pathways. The follicular pathway generates Bcl6-positive germinal center B cells that further differentiate into long-lived memory B cells and plasma cells producing high-affinity antibodies, whereas the extrafollicular pathway generates Bcl6-negative blasts that further differentiate into short-lived plasma cells secreting low-affinity antibodies [14,45].
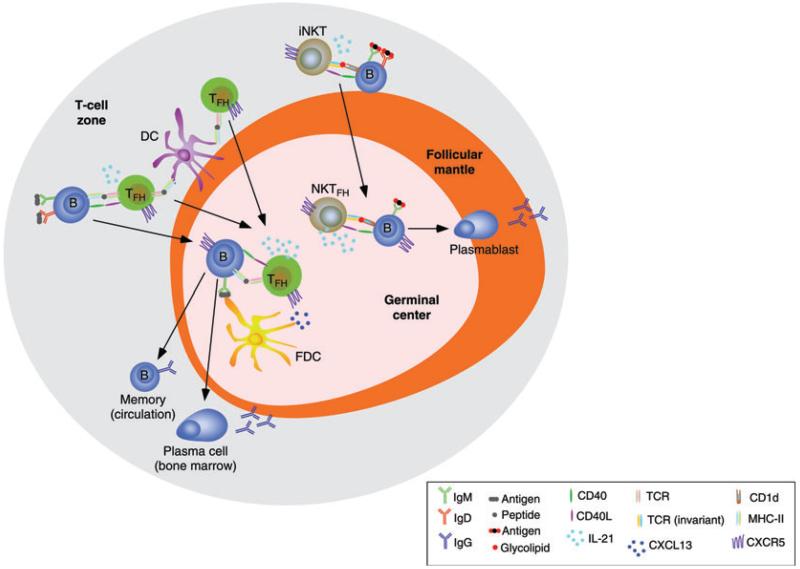
Figure 1.
TFH cells and NKTFH cells provide help to germinal center B cells. Cognate interaction of TFH cells with DCs and B cells induces migration of TFH cells and B cells to the germinal center of the follicle under the influence of CXCL13, a CXCR5 ligand released by from FDCs. Cognate interaction of iNKT cells with B cells induces a similar effect, leading to the differentiation of iNKT cells into NKTFH cells. Interaction of B cells with TFH cells involves internalization and processing of a protein antigen via the BCR (IgM or IgD), followed by presentation of a peptide–MHC class II complex to the TCR on TFH cells. Interaction of B cells with iNKT cells involves presentation of a CD1d-bound soluble glycolipid such as α-galactosylceramide to an invariant TCR on iNKT cells. In this interaction, B cells also recognize an α-galactosylceramide-containing antigen via the BCR. The ensuing activation of a Bcl6-dependent maturation program causes upregulation of IL-21 expression in both TFH cells and NKTFH cells. By expressing CD40L, IL-21, and other B-cell-stimulating cytokines, TFH cells sustain a germinal center reaction that involves induction of CSR from IgM to IgG as well as elicitation of SHM in B cells, followed by generation of high-affinity and long-lived memory B cells and plasma cells. Memory B cells enter the circulation, whereas IgG-secreting plasma cells are home to the bone marrow. Unlike TFH cells, NKTFH cells trigger a germinal center reaction characterized by induction of IgG CSR with little or no affinity maturation and generation of memory B cells. Instead, NKTFH cells elicit the formation of short-lived IgG-secreting plasmablasts.
After receiving activating signals from TFH cells at the border of the follicle with the T-cell zone, B cells upregulate the expression of the DNA-editing enzyme activation-induced cytidine deaminase (AID) and initiate somatic hypermutation (SHM) and class switch recombination (CSR), two Ig gene diversifying processes highly dependent on AID [46-49]. SHM introduces point mutations within V(D)J genes, thereby providing the structural correlate for selection of high-affinity Ig mutants by antigen (reviewed in [50]). By replacing constant (C) μ, and Cδ genes, which encode IgM and IgD, respectively, with Cγ, C, or C genes, which encode IgG, IgA, or IgE, respectively, CSR provides antibodies with novel effector functions without changing antigen specificity (reviewed in [51]). In humans, a noncanonical form of CSR from Cμ to Cδ has also been documented in lymphoid structures associated with the upper respiratory tract and generates B cells specialized in IgD production [52].
In addition to initiating CSR, the initial cognate interaction between B cells and TFH cells leads to the differentiation of B cells into highly proliferating germinal center B cells known as centroblasts [53,54]. These cells further upregulate AID expression and complete the processes of CSR and SHM [53-55]. After exiting the cell cycle, centroblasts become centrocytes that screen antigens on the surface of FDCs using their newly hypermutated surface Ig receptors [56,57]. By binding antigen through high-affinity Igs, centrocytes become capable of processing and presenting antigen to TFH cells [56,57]. These cells initiate their journey in the follicle after an initial cognate interaction with DCs in the T-cell zone [58]. Early TFH cells migrate to the T-B cell border to interact with B cells and then move to the follicle after further upregulating the expression of CXCR5 ([16,59], and reviewed in [60]), a chemokine receptor that is also expressed by germinal center B cells and that senses CXCL13 produced by FDCs [9,61]. In the presence of additional follicular signals, including ICOS ligand-dependent signals provided by B cells, TFH cell progenitors enter a Bcl6-dependent genetic program to become full-blown germinal center TFH cells [10].
B-cell help from TFH cells via CD40L, ICOS, and cytokines such as IL-21, IL-4, and IL-10 results in the survival and selection of high-affinity centrocytes, which stimulates the perpetuation of the germinal center reaction by inducing recycling of centrocytes into centroblasts, and provides signals for the differentiation of centrocytes into long-lived memory B cells and plasma cells expressing Igs with high affinity for antigen [15,17,57,62,63]. While TFH cells are essential for the germinal center reaction, their number needs to be tightly controlled to avoid the emergence of low affinity and autoreactive B-cell clones. This control involves a recently identified T-cell subset named TFR cells [20,21]. Although phenotypically similar to TFH cells, TFR cells originate from different precursors, express characteristics of regulatory T (Treg) cells such as the transcription factor Foxp3, and exert a suppressive activity on germinal center B cells and TFH cells [20,21]. By controlling the number of TFH cells, TFR cells limit the outgrowth of nonantigen-specific germinal center B cells and optimize antibody affinity maturation. Additional control signals are provided to TFH cells by plasma cells emerging from the germinal center reaction [64].
Memory B cells generated during the germinal center reaction enter the circulation and form extrafollicular aggregates in lymphoid organs [65,66]. Some of these memory B cells rapidly differentiate into extrafollicular IgG-secreting plasmablasts in response to recall antigens whereas others re-initiate the germinal center reaction [65]. Although cognate interaction of memory B cells with TFH cells is likely to be required for anamnestic antibody responses, some evidence points to the existence of an alternative pathway involving antigen-independent polyclonal stimulation of memory B cells by microbial TLR ligands [67]. Unlike memory B cells, plasma cells generated during a germinal center response home to the bone marrow and populate survival niches that contain eosinophils and promote tonic release of high-affinity antibodies [68-70].
B-cell helper signals from iNKT cells
As mentioned earlier, the regulation of follicular B cells responses is not restricted to TFH cells, but involves additional T-cell subsets, including iNKT cells. These cells express an invariant Vα14+ T-cell receptor (TCR) that recognizes glycolipid antigens presented by the nonpolymorphic MHC-I-like molecule CD1d [71,72]. After recognizing the glycolipid α-galactosylceramide on CD1d-expressing paracortical DCs or subcapsular macrophages, iNKT cells can deliver noncognate help to B cells by inducing formation of efficient antigen presenting DCs and macrophages via CD40L and interferons [71,72]. Subsequent expansion of antigen-experienced TFH cells leads to a germinal center reaction that induces moderate IgG production, affinity maturation via SHM, and immune memory [73].
More recent studies have shown that iNKT cells further help B cells in a cognate manner (Fig. 1). Indeed, a subpopulation of iNKT cells upregulates CXCR5 after interacting with glycolipids presented by B cells expressing CD1d [5]. Subsequent entry into the follicle stimulates these iNKT cells to activate the Bcl6 program and differentiate into NKTFH cells that express CD40L, IL-21, and other typical TFH cell-associated molecules, including ICOS and PD-1 [4,5]. The ensuing germinal center reaction induces strong primary IgG production but little affinity maturation and no immune memory [4,5]. A similar CD1d-dependent iNKT cell–B-cell interaction can occur in the extrafollicular area, but predominantly induces IgM and only some IgG production [74]. Similar to TI pathways, these iNKT cell-dependent pathways enable B cells to mount a rapid wave of IgG and IgM antibodies against pathogens.
B-cell helper signals from DCs and epithelial cells
In mucosa-associated lymphoid follicles such as Peyer’s patches, B cells are less dependent on cognate help from TFH cells to generate protective antibodies, perhaps because B cells can receive alternative helper signals from FDCs [75,76]. These cells release BAFF, APRIL, and retinoic acid, a metabolite of vitamin A, upon “priming” by TLR signals from commensal bacteria [76]. Intestinal FDCs also release large amounts of active TGF-β, a cytokine critically involved in IgA CSR, and utilize their dendrites to organize antigens in “periodic” arrays to trigger BCR and TLR molecules on follicular B cells more efficiently [76]. By releasing TGF-β, BAFF, and APRIL, and by antigenically stimulating antigen receptors on B cells, intestinal FDCs dramatically enhance the IgA-inducing function of TFH cells. Intestinal TFH cells utilize CD40L, IL-21, and TGF-β to enhance the production of noninflammatory IgA and concomitantly inhibit the production of pro-inflammatory IgG [77]. Peyer’s patches may also support some IgA production through a TI mechanism [78]. In addition to IgA-inducing FDCs, Peyer’s patches include TipDCs, a TNF-inducible nitric oxide synthase (iNOS)-producing DC subset that usually occupies the intestinal lamina propria [79]. These TipDCs elicit IgA production by increasing the expression of the TGF-β receptor on B cells via nitric oxide, thereby rendering B cells more responsive to IgA-inducing signals provided by TGF-β [79]. Of note, recent findings show that IgA-secreting plasma cells acquire TipDC-like phenotypic features in the intestinal microenvironment, including expression of the antimicrobial mediators, TNF and iNOS [80]. Thus, some of the functions previously ascribed to intestinal TipDCs also involve IgA-secreting plasma cells.
Follicular B cells from Peyer’s patches and mesenteric lymph nodes further undergo IgA CSR and production in response to TI signals from plasmacytoid DCs (pDCs), which release large amounts of BAFF and APRIL upon being “primed” by type I interferon from intestinal stromal cells [81]. Together with Peyer’s patches and mesenteric lymph nodes, isolated lymphoid follicles represent another intestinal site for IgA induction. Isolated lymphoid follicles contain lymphoid tissue-inducer cells that release the TNF family member lymphotoxin-β upon exposure to TLR signals from commensals [42]. The interaction of lymphotoxin-β with its cognate receptor stimulates local stromal cells to release TNF and DC-attracting chemokines such as CCL19 and CCL21 [42]. By inducing DC production of matrix metalloproteases 9 and 13, TNF stimulates DCs to process active TGF-β from a latent precursor protein [42]. In the presence of TLR signals, DCs further release BAFF and APRIL, which activate a TI pathway for IgA production by cooperating with TGF-β [42].
In addition to isolated lymphoid follicles, the intestinal lamina propria contains a diffuse lymphoid tissue comprised of scattered B cells that can undergo IgA class switching and production, although less efficiently and at a lower frequency than follicular B cells (reviewed in [82,83]). This IgA production is supported by multiple subsets of lamina propria DCs that can activate B cells in a TI manner. When exposed to microbial TLR signals, lamina propria TipDCs release nitric oxide, which in turn enhances the production of BAFF and APRIL [79]. Another lamina propria DC subset with IgA-licensing function is represented by DCs constitutively expressing the flagellin receptor TLR5 [84]. These DCs express little or no TLR4 and induce TI IgA class switching and production by releasing retinoic acid and IL-6 upon sensing flagellin from commensal bacteria [84]. Also, epithelial cells deliver IgA-inducing signals to lamina propria B cells by releasing BAFF and APRIL after recognizing bacteria via multiple TLRs [38,85]. This epithelial pathway contributes to the generation of IgA2 antibodies (that are resistant to bacterial degradation) by human IgM-expressing B cells or to the generation of IgA1 class-switched B cells that derive from Peyer’s patches [38].
Epithelial cells further amplify the IgA-inducing function of local DCs by releasing thymic stromal lymphopoietin (TSLP), an IL-7-like cytokine that enhances BAFF and APRIL production by TLR-stimulated DCs [38,85]. In addition to releasing B-cell helper factors, DCs may present intact TI antigens to B cells [34]. Indeed, a subset of mucosal DCs sample bacteria from the intestinal lumen by extending dendrites through epithelial cell junctions or across transcellular pores formed by specialized epithelial cells called M cells [86-88]. An additional subset of mucosal DCs captures small molecular weight antigens across passages formed by goblet cells [89]. All these mucosal DCs may recycle unprocessed TI antigens to the cell surface to present them to B cells [90]. Considering that BAFF and APRIL also provide survival signals to plasma cells [91], the combined B-cell helper function of epithelial cells and DCs may provide an alternative pathway for the continuous production of IgA antibodies against mucosal commensal bacteria.
B-cell helper signals from neutrophils
TI Ig responses also occur in the MZ of the spleen, a B-cell area positioned at the interface between the circulation and the immune system (reviewed in [92,93]). B cells lodged in the MZ are in a state of active readiness that enables them to mount very early Ig responses to blood-borne TI antigens from pathogenic or commensal bacteria (reviewed in [92,93]). Remarkably, blood-borne antigens stimulate the homing of DCs, as well as neutrophils, to the MZ of the spleen [3]. While the role of DCs in the activation of MZ B cells is well documented [3], the role of neutrophils remains less understood, but clearly these cells have the ability to release large amounts of innate B-cell-stimulating factors, such as BAFF and APRIL, particularly after stimulation by cytokines or microbial ligands [37,94]. Consistent with this observation, recent findings show that neutrophils occupy peri-MZ areas of the spleen in the absence of infection, recruited via a noninflammatory pathway that starts during fetal life and accelerates after birth, a time that coincides with the colonization of mucosal surfaces by bacteria [30]. The splenic microenvironment stimulates conventional neutrophils to become B-cell helper neutrophils (NBH cells) through a process that involves the delivery of neutrophil reprogramming signals from splenic sinusoidal endothelial cells and possibly other cell types, including macrophages (Fig. 2). These signals include the anti-inflammatory cytokine, IL-10 [30].
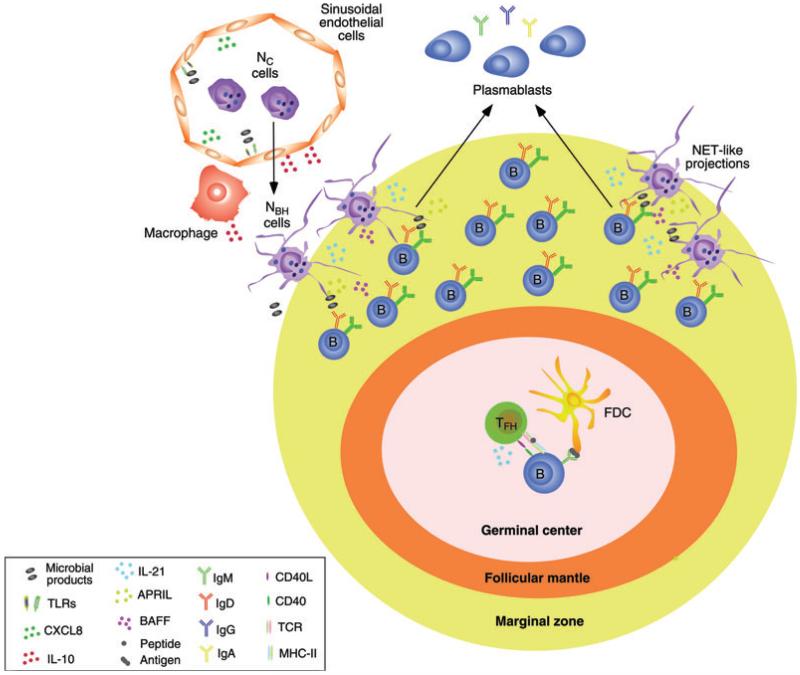
Figure 2.
Neutrophils provide help to splenic MZ B cells. Splenic sinusoidal endothelial cells sense microbial products, including commensal antigens from mucosal surfaces, via TLRs. The ensuing release of chemokines such as CXCL8 (IL-8) enhances the migration of conventional circulating neutrophils (NC cells) to perifollicular areas of the spleen. Further release of cytokines such as IL-10 by perifollicular sinusoidal endothelial cells and macrophages stimulates the reprogramming of NC cells into B-cell-helper neutrophils (NBH cells). NBH cells trigger CSR from IgM to IgG or IgA as well as moderate levels of SHM (at least in humans) and differentiation of plasmablasts by activating a subset of MZ B cells through BAFF, APRIL, and IL-21. Antigen trapped on the surface of NET-like structures emanating from NBH cells might further increase the activation of MZ B cells by binding to the BCR (IgM or IgD) and TLRs. The interaction of NBH cells with MZ B cells facilitates the formation of an innate repertoire of IgM, IgG, and IgA antibodies to provide a fast second line of defense against systemic invasion by microbes, including bacteria breaching the mucosal barrier.
In general, neutrophils are the first immune cells that migrate to sites of infection and inflammation to eliminate microbes and necrotic cells and initiate adaptive immune responses (reviewed in [95]). Remarkably, NBH cells occupy peri-MZ areas of the spleen under homeostatic conditions, that is, in the absence of infection or inflammation [30]. The colonization of the spleen by NBH cells correlates with postnatal deposition of microbial products that likely originate from mucosal surfaces, including lipopolysaccharide [30]. Compared with circulating neutrophils, NBH cells are more activated as they express increased amounts of B-cell-stimulating molecules such as BAFF, APRIL, CD40L, and IL-21, as well as increased levels of immunostimulatory cytokines such as IL-12 and TNF [30]. However, this activation is counterbalanced by an increased expression of immune regulatory molecules, including protease inhibitors and T-cell suppressor factors such as arginase and iNOS [30].
Consistent with this phenotype, NBH cells induce IgM secretion, as well as IgG and IgA CSR, by stimulating MZ B cells via BAFF, APRIL, IL-21, and possibly CD40L, at least in humans [30]. On the other hand, NBH cells express T-cell-suppressive factors such as arginase and iNOS and suppress T-cell proliferation in a contact-independent manner [30]. By exerting this dual B-cell helper/T-cell suppressor function, NBH cells may maximize extrafollicular B-cell responses to TI antigens while minimizing follicular B-cell responses to TD antigens and inflammation. Accordingly, NBH cells are excluded from splenic follicles under homeostatic conditions, but then infiltrate follicles under inflammatory conditions, perhaps to activate T cells (Fig. 2; [30]). Remarkably, NBH cells can induce SHM through a mechanism that could involve exposure of microbial TI antigens such as TLR ligands to MZ B cells [30]. This possibility is consistent with studies suggesting that MZ B cells activate the SHM machinery through a TI pathway activated by TLR ligation [27,96-100]. Additional evidence indicates that MZ B cells also undergo SHM through a typical TD pathway, which may reflect the ability of MZ B cells to deposit antigen in the follicle and activate T cells [41,101]. In mice, MZ B cells express unmutated Ig genes under steady-state conditions, but other B-cell subsets have been shown to induce SHM via a TI pathway involving TLR signaling [100,102,103].
The mechanism by which NBH cells activate MZ B cells likely involves mucosal colonization by bacteria [30]. Discrete amounts of microbial products such as lipopolysaccharide undergo peri-MZ deposition soon after birth [30]. The resulting activation of TLR4 on sinusoidal endothelial cells would then cause the release of neutrophil-attracting chemokines, such as CXCL8, as well as perifollicular accumulation and activation of NBH cells, some of which form postapoptotic DNA-containing cellular projections similar to neutrophil traps (NETs) [30]. In addition to exposing trapped TI antigens to MZ B cells, NBH cell-derived NETs might “inject” apoptotic CpG DNA into the peri-MZ microenvironment, thereby delivering adjuvant-like TLR signals to MZ B cells, as has been postulated for the autoimmune disease SLE [104,105]. The interaction of CpG DNA with TLR9 could then trigger survival, activation, SHM, as well as CSR, signals in MZ B cells [67,98,106,107].
In general, the crosstalk of MZ B cells with NBH cells may be instrumental to enhance the generation of a second line of innate (or natural) antibody defense against systemic invasion by commensal antigens and microbes that breach first line defenses at the mucosal barrier. An insufficiency of NBH cells may contribute to the pathogenesis of systemic infections by mucosal bacteria in patients with neutropenia. Conversely, harnessing NBH cells may enhance vaccine-induced Ig responses to poorly immunogenic TI antigens and mucosal pathogens in healthy individuals.
B-cell helper signals from eosinophils, basophils, and mast cells
Plasma cells emerging from the germinal center reaction home to the bone marrow, a highly vascularized lymphoid compartment containing a specialized niche that promotes long-term plasma cell survival, as well as continuous plasma cell release of high-affinity antibodies into the circulation (reviewed in [108]). Although it is known to be different from the bone marrow niche sustaining early B-cell precursors, the bone marrow niche supporting plasma cells has remained poorly defined. Recent evidence shows that this niche contains eosinophils (Fig. 3), a granulocyte subset that produces APRIL and is in close contact with stromal cells that release CXCL12, a chemokine that binds to a CXCR4 receptor highly expressed by plasma cells [70]. Engagement of CXCR4 on plasma cells by CXCL12 from stromal cells stimulates plasma cells to navigate toward and colonize eosinophil-containing niches [70]. Of interest, eosinophils also express CXCR4, which would explain their ability to colocalize with stromal cells and plasma cells in the bone marrow [70]. By releasing large amounts of APRIL and the cytokine IL-6, bone marrow eosinophils facilitate the long-term survival of plasma cells [70]. This effect may be further enhanced by megakaryocytes, a platelet-generating hematopoietic cell that also releases APRIL [109].
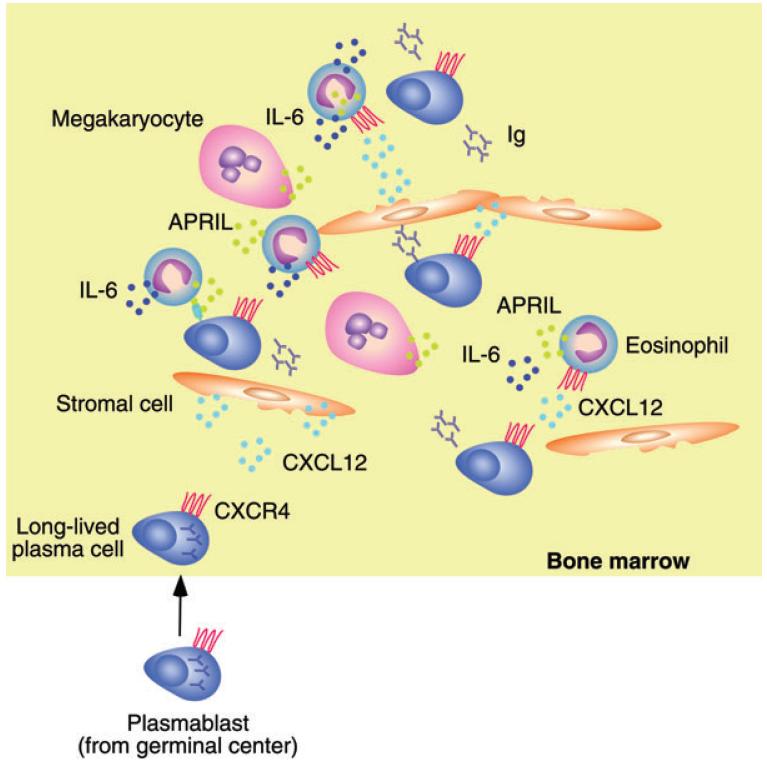
Figure 3.
Eosinophils provide help to bone marrow plasma cells. Plasma cells emerging from the germinal center reaction upregulate the expression of the chemokine receptor CXCR4 and home to the bone marrow in response to CXCL12, a chemokine released by bone marrow stromal cells. By expressing CXCR4, eosinophils home to the same bone marrow niche occupied by plasma cells. APRIL and IL-6 release by eosinophils enhances the survival of Ig-secreting plasma cells. A similar effect is induced by megakaryocytes through the release of APRIL.
Similar to eosinophils, mast cells have long been known for their participation in pathological allergic reactions characterized by dysregulated production of the inflammatory antibody isotype IgE (reviewed in [110]). However, a number of studies have also implicated mast cells in the development of adaptive immune responses, including antibody production by B cells [111-116]. By releasing the regulatory cytokines, IL-10 and TGF-β, mast cells also contribute to the modulation and possibly formation of Treg cells expressing the transcription factor Foxp3 [117]. In the intestine, Treg cells express CD40L, IL-10, and TGF-β and thereby promote homeostatic IgA responses by B cells while inhibiting inflammatory IFN-γ and IL-17 responses by TH1 and TH17 cells, respectively [118-120]. Furthermore, intestinal Treg cells differentiate into TFH cells, which are critical for the induction of germinal centers, as well as IgA CSR and production in intestinal Peyer’s patches [119]. Some Treg cells also infiltrate germinal centers to negatively regulate TFH cells and this process would lead to higher affinity B-cell responses [20,21]. Finally, mast cells also directly activate B cells to induce IgA production via CD40L, IL-6, and IL-10 [121]. This activation may contribute to TI IgA responses in the intestinal lamina propria.
Basophils, an innate cell type closely related to mast cells, also deliver helper signals to B cells via both direct and indirect mechanisms (Fig. 4). Firstly, under certain circumstances, basophils can migrate to draining lymph nodes where they release IL-4 to induce the formation of TH2 cells, an IL-4-producing T-cell subset critically involved in the induction of protective IgG1 and IgE responses against various allergens and pathogens, including helminths [122-125]. Secondly, after secondary immunization, basophils recognize antigen through prebound antigen-specific IgE generated during a primary immune response [126]. Antigen recognition via IgE causes upregulation of CD40L and release of IL-4 and IL-6, which provide antibody-inducing signals to B cells not only directly, but also indirectly via enhancement of IL-4, IL-6, IL-10, and IL-13 production by TH2 cells [112,126]. Presumably, the antigen-IgE interaction does not trigger pathological release of preformed highly inflammatory compounds, such as histamine, from basophils owing to the low affinity of IgE for antigen. It must be also noted that IgE can also bind DCs, which raises the possibility that DCs could account for at least part of the Th2-inducing activity ascribed to basophils [127].
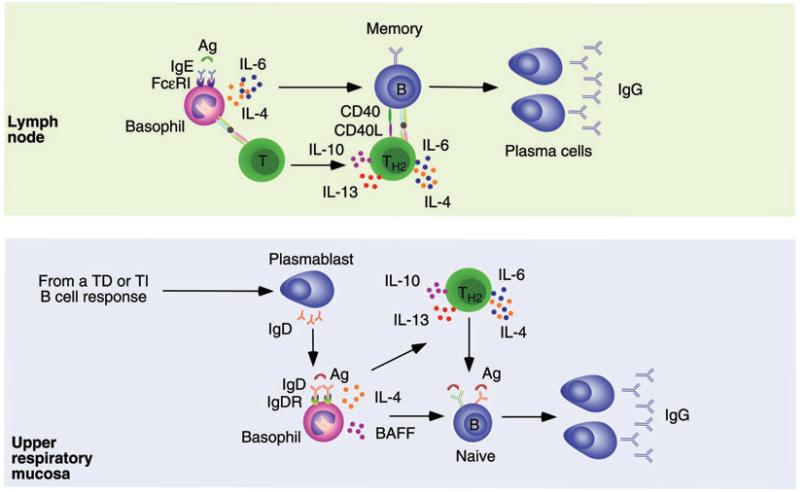
Figure 4.
Basophils provide help to lymph node and mucosal B cells. Upper panel. Basophils bind to IgE generated during a primary immune response through a high-affinity receptor named FcRI. In a secondary immune response, binding of recall antigen to IgE via either conventional or unconventional mechanisms triggers basophil release of IL-4 and IL-6, which enhances the conversion of naïve T cells into TH2 cells secreting IL-4, IL-6, IL-10, and IL-13. Together with CD40L, these cytokines enhance the activation and expansion of IgG-expressing memory B cells in the context of a cognate interaction that leads to the generation of IgG-secreting plasma cells. Basophils may further enhance the activation of memory B cells by delivering helper signals independently of T cells. Bottom panel. B cells from the human upper respiratory mucosa undergo noncanonical CSR from IgM to IgD by following either TD or TI pathways. This process leads to the formation of mucosal and circulating plasmablasts that secrete IgD antibodies reactive against respiratory bacteria. Mucosal IgD would enhance local immunity and homeostasis after translocating across epithelial cells, whereas circulating IgD binds to basophils via an unknown receptor (IgDR). In addition to delivering rapid innate responses and alerting the immune system as to the presence of invading bacteria, basophils exposed to IgD-reactive antigens may enhance immune responses in both systemic and mucosal lymphoid organs. These basophils enhance IgG (but also IgA) CSR and antibody production by activating B cells via BAFF, IL-4, and other B-cell-activating factors, including CD40L and APRIL (not shown). Similar to IgE-stimulated basophils, IgD-stimulated basophils might further enhance the local antibody response by promoting the formation of TH2 cells via IL-4.
Basophils may deliver similar B-cell helper signals by interacting with IgD (Fig. 4), an enigmatic antibody isotype released by IgD class-switched plasmablasts originating in the human upper respiratory mucosa [52,128]. In spite of being heavily hypermutated, these IgD antibodies are largely polyreactive and may afford mucosal protection by binding not only to commensals and pathogens but also to their virulence factors [52,83,129,130]. In addition to crossing the epithelial barrier to reach the surface of upper respiratory mucosal surfaces, IgD binds to circulating basophils, monocytes, and neutrophils, as well as mucosal mast cells, via an unknown receptor [52]. Crosslinking of prebound IgD induces basophil release of BAFF, IL-4, and IL-13, which in turn stimulate B cells to undergo IgM production, as well as CSR to IgG and IgA, in a TI manner [52]. CD40L and APRIL further help the activation of B cells by IgD-activated basophils [52]. Thus, basophils may utilize both prebound IgE and IgD as immune amplifiers of both systemic and mucosal B-cell responses.
Conclusions
TFH cells, TFR cells, NKTFH cells, and Treg cells play a pivotal role in TD antibody responses against microbial proteins. A finely tuned division of labor between these T-cell subsets regulates the activation, expansion, diversification, selection, and survival of B cells engaged in TD antibody responses at different lymphoid sites and at different times after immunization or infection. Thus, while TFH and NKTFH cells are clearly essential to support IgG responses in systemic lymphoid follicles, other T-cell subsets such as Treg cells are crucial to initiate IgA responses in mucosal lymphoid follicles. The stimulating signals provided by TFH cells and NKTFH cells to germinal center B cells are counterbalanced by inhibitory signals provided by TFR cells. These cells are critical to select germinal center B cells with optimal affinity for antigen and may also influence the decision of germinal center B cells to differentiate along either plasma cell or memory B-cell pathways. Plasma cells and memory B cells generated by the germinal center reaction require additional helper signals from eosinophils and possibly basophils to extend their lifespan in postgerminal center niches. Finally, the generation of short-lived plasmablasts during natural or postimmune B-cell responses to TI antigens such as microbial carbohydrates and glycolipids involves multiple subsets of myeloid and plasmacytoid DCs, FDCs, epithelial cells, neutrophils, basophils, and mast cells, particularly in the MZ of the spleen and at mucosal sites.
The identification of novel helping partners for B cells opens up novel avenues for therapeutic intervention. In addition to harnessing the power of DCs and TFH cells, vaccines may need to target NKTFH cells, TFR cells, granulocytes, and mast cells to optimize the quantity, quality, and lifespan of antibodies produced by systemic and mucosal B cells. Conversely, inhibiting helper signals from DCs, TFH cells, NKTFH cells, granulocytes, and mast cells may be useful to dampen the production of pathogenic antibodies by autoreactive B cells and plasma cells that appear in autoimmune disorders.
Abbreviations
- AID
activation-induced cytidine deaminase
- CSR
class switch recombination
- MZ
marginal zone
- NK
natural killer
- SHM
somatic hypermutation
- TI
T-cell-independent
- TD
T-cell-dependent
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 4.King IL, Fortier A, Tighe M, Dibble J, Watts GF, Veerapen N, Haberman AM, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 2012;13:44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 2012;13:35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 6.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109:S45–S55. doi: 10.1016/s0092-8674(02)00675-x. Suppl. [DOI] [PubMed] [Google Scholar]
- 7.MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 8.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med. 2011;208:1377–1388. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 20.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, et al. Foxp3 +follicular regulatory T cells control the germinal center response. Nat. Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonardo SM, De Santis JL, Malherbe LP, Gauld SB. Cutting edge: in the absence of regulatory T cells, a unique th cell population expands and leads to a loss of B cell anergy. J. Immunol. 2012;188:5223–5226. doi: 10.4049/jimmunol.1103731. [DOI] [PubMed] [Google Scholar]
- 23.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 24.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 25.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, Rose H, et al. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J. Exp. Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 32.Colino J, Shen Y, Snapper CM. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J. Exp. Med. 2002;195:1–13. doi: 10.1084/jem.20011432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 35.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 36.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, Cassatella MA. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J .Exp. Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 39.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, Shan M, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J. Exp. Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr., Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov, Itoh K, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumjohann D, Okada T, Ansel KM. Cutting edge: distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol. 2011;187:2089–2092. doi: 10.4049/jimmunol.1101393. [DOI] [PubMed] [Google Scholar]
- 45.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J. Immunol. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 46.Toellner KM, Gulbranson-Judge A, Taylor DR, Sze DM, MacLennan IC. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J. Exp. Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 48.Pape KA, Kouskoff V, Nemazee D, Tang HL, Cyster JG, Tze LE, Hippen KL, et al. Visualization of the genesis and fate of isotype-switched B cells during a primary immune response. J. Exp. Med. 2003;197:1677–1687. doi: 10.1084/jem.20012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolar GR, Mehta D, Pelayo R, Capra JD. A novel human B cell subpopulation representing the initial germinal center population to express AID. Blood. 2007;109:2545–2552. doi: 10.1182/blood-2006-07-037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papavasiliou FN, Schatz DG. Somatic hypermutation of immunoglobulin genes: merging mechanisms for genetic diversity. Cell. 2002;109:S35–S44. doi: 10.1016/s0092-8674(02)00706-7. Suppl. [DOI] [PubMed] [Google Scholar]
- 51.Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat. Rev. Immunol. 2004;4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 52.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu YJ, Barthelemy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7–1 and B7–2. Immunity. 1995;2:239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 54.Liu YJ, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, Mills FC, et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241–250. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 55.Crouch EE, Li Z, Takizawa M, Fichtner-Feigl S, Gourzi P, Montano C, Feigenbaum L, et al. Regulation of AID expression in the immune response. J. Exp. Med. 2007;204:1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 57.Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 58.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 61.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 62.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 63.Lohning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, Hamelmann E, et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J. Exp. Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. Plasma cells negatively regulate the follicular helper T cell program. Nat. Immunol. 2010;11:1110–1118. doi: 10.1038/ni.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, et al. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 66.Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J. Exp. Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 68.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 69.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J. Exp. Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 71.Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, Mathew B, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J. Clin. Invest. 2004;114:1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barral P, Polzella P, Bruckbauer A, van Rooijen N, Besra GS, Cerundolo V, Batista FD. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat. Immunol. 2010;11:303–312. doi: 10.1038/ni.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl. Acad. Sci. USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, Besra GS, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, et al. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Dullaers M, Li D, Xue Y, Ni L, Gayet I, Morita R, Ueno H, et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergqvist P, Stensson A, Lycke NY, Bemark M. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J. Immunol. 2010;184:3545–3553. doi: 10.4049/jimmunol.0901895. [DOI] [PubMed] [Google Scholar]
- 79.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 80.Fritz JH, Rojas OL, Simard N, McCarthy DD, Hapfelmeier S, Rubino S, Robertson SJ, et al. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature. 2012;481:199–203. doi: 10.1038/nature10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 83.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu. Rev. Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uematsu S, Fujimoto K, Ho Jang M, Yang B-G, Jung Y-J, Nishiyama M, Sato S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 85.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 86.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 87.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lelouard H, Fallet M, de Bovis B, Meresse S, Gorvel JP. Peyer’s patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology. 2012;142:592–601. e593. doi: 10.1053/j.gastro.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 89.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 91.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 93.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu. Rev. Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 94.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Invest. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 96.Weller S, Faili A, Garcia C, Braun MC, Le Deist FF, de Saint Basile GG, Hermine O, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. USA. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scheeren FA, Nagasawa M, Weijer K, Cupedo T, Kirberg J, Legrand N, Spits H. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J. Exp. Med. 2008;205:2033–2042. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aranburu A, Ceccarelli S, Giorda E, Lasorella R, Ballatore G, Carsetti R. TLR ligation triggers somatic hypermutation in transitional B cells inducing the generation of IgM memory B cells. J. Immunol. 2010;185:7293–7301. doi: 10.4049/jimmunol.1002722. [DOI] [PubMed] [Google Scholar]
- 99.Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, He B, et al. Human memory B cells originate from three distinct germinal center-dependent and - independent maturation pathways. Blood. 2011;118:2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seifert M, Kuppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J. Exp. Med. 2009;206:2659–2669. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toellner KM, Jenkinson WE, Taylor DR, Khan M, Sze DM, Sansom DM, Vinuesa CG, et al. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J. Exp. Med. 2002;195:383–389. doi: 10.1084/jem.20011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mao C, Jiang L, Melo-Jorge M, Puthenveetil M, Zhang X, Carroll MC, Imanishi-Kari T. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 104.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J. Immunol. 2004;173:4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 107.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J. Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 108.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 109.Winter O, Moser K, Mohr E, Zotos D, Kaminski H, Szyska M, Roth K, et al. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood. 2010;116:1867–1875. doi: 10.1182/blood-2009-12-259457. [DOI] [PubMed] [Google Scholar]
- 110.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galli SJ, Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984;226:710–713. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- 112.Gauchat JF, Henchoz S, Mazzei G, Aubry JP, Brunner T, Blasey H, Life P, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 113.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J. Clin. Invest. 1997;99:1492–1499. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 115.Ott VL, Cambier JC, Kappler J, Marrack P, Swanson BJ. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat. Immunol. 2003;4:974–981. doi: 10.1038/ni971. [DOI] [PubMed] [Google Scholar]
- 116.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat. Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 117.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, et al. CD4+CD25 +regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 120.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 121.Merluzzi S, Frossi B, Gri G, Parusso S, Tripodo C, Pucillo C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood. 2010;115:2810–2817. doi: 10.1182/blood-2009-10-250126. [DOI] [PubMed] [Google Scholar]
- 122.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4(+) T cells. Nat. Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 124.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, et al. MHC class II-dependent basophil-CD4(+) T cell interactions promote T(H)2 cytokine-dependent immunity. Nat. Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, Maus R, et al. Basophils enhance immunological memory responses. Nat. Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 127.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arpin C, de Bouteiller O, Razanajaona D, Fugier-Vivier I, Briere F, Banchereau J, Lebecque S, et al. The normal counterpart of IgD myeloma cells in germinal center displays extensively mutated IgVH gene, Cmu-Cdelta switch, and lambda light chain expression. J. Exp. Med. 1998;187:1169–1178. doi: 10.1084/jem.187.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu YJ, de Bouteiller O, Arpin C, Briere F, Galibert L, Ho S, Martinez-Valdez H, et al. Normal human IgD+IgM− germinal center B cells can express up to 80 mutations in the variable region of their IgD transcripts. Immunity. 1996;4:603–613. doi: 10.1016/s1074-7613(00)80486-0. [DOI] [PubMed] [Google Scholar]
- 130.Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, Jared M, et al. Mature B cells class switched to IgD are autoreactive in healthy individuals. J. Clin. Invest. 2007;117:1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]