Abstract
OBJECTIVES:
To evaluate i) the relative importance of R0 resection, tumor size and peripancreatic lymph node (LN) status are significant determinants of survival benefit following upfront surgery for pancreatic adenocarcinoma (PaCa), ii) whether R0 resection confers survival benefit in all patients or a patient subset with certain favorable prognostic factors.
METHODS:
Retrospective analysis of patients (2001–2010) who underwent planned potentially curative surgical resection without neoadjuvant therapy for PaCa.
RESULTS:
Among 154 patients, median survival following R0 (n=105) and R1 resections was 26.8 and 17.7 months, respectively (P=0.010). Tumor size and LN status were significant determinants of survival following R0 resection. There were no differences in survival based on tumor size and LN in patients with R1 resection. Median survival was 17.7 months following R1 resection and was 70.9 months (P<0.001) and 22.2 months (P=0.44) in patients with tumor ≤25 mm in size and ≤1 involved LN and in the remaining patients in the cohort respectively following R0 resection.
CONCLUSIONS:
R0 resection is associated with dramatic survival benefit over R1 resection in a subset of patients with tumor size ≤25 mm and ≤1 involved LN. These findings underscore the importance of R0 resection and careful patient selection for upfront surgery in patients with PaCa.
INTRODUCTION
Pancreatic adenocarcinoma (PaCa) is the fourth most common cause of cancer death in the United States. More than 43,000 cases are diagnosed annually in the United States, and vast majority of them die from their disease within 1 year.1 Surgical resection is the standard of care for the management of patients with PaCa that seems potentially resectable on imaging. Patients who undergo an R0 resection have significantly better survival than those with R1/R2 resection.2, 3, 4 Achieving R0 resection has therefore become the goal of surgery in patients with PaCa, and increasingly extensive surgical procedures are being performed to that achieve that goal. However, it is not known whether there is a survival benefit in all patients in whom R0 resection is achieved. If that was the case, it would justify making every effort to achieve the goal of R0 resection, including increasingly extensive surgical resections. And if not, it would be useful to know the patient or tumor factors that predict significant survival benefit following R0 resection of the pancreatic tumor to help select patients in whom upfront surgery with curative intent is considered as preferred first-line treatment.
In this manuscript, we studied the relative significance of R0 resection, tumor size, peripancreatic lymph node (LN) status and patient age on survival following upfront surgical resection of PaCa. We then evaluated whether these characteristics can potentially help identify the subset of patients who benefit most from upfront surgical resection.
METHODS
Patient selection
This is a retrospective analysis and included patients treated for PaCa at Saint Louis University hospital or Missouri Baptist Medical Center from 2001 to 2010. A total of 921 patients were treated for PaCa; 451patients were found to have an unresectable tumor or metastatic disease on preoperative imaging and 64 patients were found to have metastatic disease during laparotomy. One hundred and seventy-seven patients were lost to follow-up and were also excluded. Among the 229 patients (24.8%) who underwent planned curative surgical resection, the following were excluded1 patients who died of causes unrelated to pancreatic cancer (n=16),2 those with multiple cancers (n=15),3 if the pancreatic tumor was a cystadenocarcinoma (n=26),4 if they received preoperative chemo±radiotherapy (n=15), and5 if they underwent a R2 resection (n=3). One hundred and fifty-four patients were finally included for analysis. Medical records were reviewed for clinical and surgical information.
The study was approved by the Institutional Review Board of the Saint Louis University School of Medicine and Missouri Baptist Medical Center.
Surgery and pathological analysis
Of the 154 patients, 128 (83.1%) underwent Whipple procedure and 26 (16.8%) underwent distal/total pancreatectomy. During surgery, frozen sections of the resection margins were taken for histological examination. In the operating room, the surgeon marked the resection margins at the level of bile duct, pancreatic duct, superior mesenteric artery, and portal vein margin. Resection margins were considered positive if the carcinoma was present at the final pancreatic neck, uncinate process, bile duct, duodenal, or retroperitoneal soft tissue margin. Resection specimens were analyzed for location, size, differentiation, resection margins, perineural invasion, venous or lymphatic vessel involvement, and status of LNs. An R0 resection was categorized when the tumor was excised in one piece without violating the tumor plane or when negative margins were achieved only after sequential re-excision of involved margins (n=9). An R1 resection involves a microscopically positive margin(s) anywhere, and an R2 resection involves a macroscopically positive margin(s) with visible tumor, as per surgeon's operative notes. Patients with involvement of portal vein or superior mesenteric vein underwent venous reconstruction as deemed appropriate. Tumors were categorized according to the American Joint Committee on Cancer/International Union Against Cancer. Post-operatively, patients were managed as clinically indicated as per the National Comprehensive Cancer Network guidelines.
The two centers, St Louis University Hospital and Missouri Baptist Medical Center, accounted for 45 and 109 cases, respectively, included in the present analysis. There were three experienced pancreatic surgeons involved in the care of these patients and there were no overall differences in outcomes based on surgeon.
Follow-up and final diagnosis
Final diagnosis was based on surgical pathology. Follow-up information is rigorously collected for quality assurance in our clinical practice. It comprises periodic phone calls to patients, in addition to monitoring correspondence from the referring physicians and primary-care physicians, operative notes, surgical pathology, and imaging reports. The date of death recorded was checked from the medical records or social security death index. Corroboration with living patients was conducted via follow-up for our prospectively maintained database. The mean follow-up was 24.2±18.9 months (range 1.48–99.3) and was 36.8±20.3 months for patients who are still alive at the time of the study.
Statistical analysis
Survival was calculated in months from the day of surgery until death or in cases where patients remained alive until November 2011. Kaplan–Meier cumulative survival estimates were used to calculate the mean and median survival with 95% confidence interval (CI). Mantel–Cox log-rank test was used to compare differences in survival between the groups. Cox-proportional hazards regression was performed to test the effects of potential prognostic factors. Covariates included age, tumor size, and peripancreatic LN involvement. Covariates were tested in a univariate manner. Variables that were significant on univariate analysis at P<0.10 were included in multivariate analysis by forward stepwise manner. All analyses were two tailed and statistical significance was accepted as P<0.05. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS, Chicago, IL).
RESULTS
Patient characteristics
The characteristics of the patients included in the study are summarized in Table 1a. The mean age of the 154 patients (81 males) included in the study was 65.3±10.7 years (range 34–86). One hundred and five patients underwent R0 resection (68.1%) and 49 had R1 resection (31.8%). At the time of initial presentation, 109 (70.8%) presented with jaundice and 94 (61.0%) with abdominal pain. Eighty patients had history of weight loss, including 50 patients with ≥10 lb weight loss in preceding 3 months. Twenty-eight patients (18.2%) presented with acute pancreatitis. The overall median survival of the study subjects was 24.1 months (range 1.1–99.3).
Table 1a. Patient characteristics.
| R0 resection N=105 (%) | R1 resection N=49 (%) | Total N=154 (%) | |
|---|---|---|---|
| Age (years) | 65.8±10.4 (35–86) | 64.3±11.4 (34–84) | 65.3±10.7 (34–86) |
| Gender | |||
| Male | 54 (35.1) | 27 (17.5) | 81 (52.6) |
| Female | 51 (33.1) | 22 (14.3) | 73 (47.4) |
| Race | |||
| Caucasian | 95 (61.7) | 45 (29.2) | 140 (90.9) |
| African-American | 10 (6.5) | 4 (2.6) | 14 (9.1) |
| Associated symptoms | |||
| Jaundice | 77 (50.0) | 32 (20.8) | 109 (70.8) |
| Weight loss | |||
| ≥10 lbs | 28 (18.2) | 25 (14.3) | 50 (32.5) |
| <10 lbs | 19 (12.3) | 11 (7.1) | 30 (19.5) |
| No weight loss | 58 (37.7) | 16 (10.4) | 74 (48.1) |
| Acute pancreatitis | 21 (13.6) | 7 (4.5) | 28 (18.2) |
| Abdominal pain | 66 (42.9) | 28 (18.2) | 94 (61.0) |
| Incidental mass | 4 (2.6) | 4 (2.6) | 8 (5.2) |
| Follow-up; mean±s.d. (months) range | 25.6±20.3 (2–100) | 21.1±15.0 (5–69) | 24.2±18.9 (2–100) |
Table 1b summarizes the tumor characteristics in study patients based on examination of surgical pathology specimens. The median tumor size (range) in patients who underwent R0 resection was 30 mm (8–80 mm) and those with R1 resection was 33 mm (17–100 mm). Tumor was located in the head/uncinate process in 130 patients, in the body in 14 patients, and in the tail in 10 patients. Regional LN involvement by tumor and perineural invasion was present in 103 (66.8%) and 131 (85.0%), respectively.
Table 1b. Tumor characteristics based on surgical pathology findings.
| R0 resection N=105 (%) | R1 resection N=49 (%) | Total N=154 (%) | |
|---|---|---|---|
| Tumor size (mm) | |||
| Mean±s.d. | 32.0±13.6 | 36.1±13.2 | 33.6±13.8 |
| Median | 30 | 33 | 31 |
| Range | 8–80 | 17–70 | 8–80 |
| Tumor location | |||
| Head/uncinate | 89 (57.8) | 41 (26.6) | 130 (84.4) |
| Body | 10 (6.5) | 4 (2.6) | 14 (9.1) |
| Tail | 6 (3.9) | 4 (2.6) | 10 (6.5) |
| Surgery | |||
| Whipple | 88 (57.1) | 40 (26.0) | 128 (83.1) |
| Distal pancreatectomy | 16 (10.4) | 9 (5.8) | 25 (16.2) |
| Total pancreatectomy | 1 (0.6) | — | 1 (0.6) |
| Cytological differentiation | |||
| Well differentiated | 22 (14.3) | 5 (3.2) | 27 (17.5) |
| Moderately differentiated | 57 (37.0) | 32 (20.8) | 89 (57.8) |
| Poorly differentiated | 26 (16.9) | 12 (7.8) | 38 (24.7) |
| Regional lymph node involvement | |||
| None | 32 (20.7) | 19 (12.3) | 51 (33.1) |
| 1 | 21 (13.6) | 4 (2.5) | 25 (16.2) |
| 2 | 15 (9.7) | 11 (7.1) | 26 (16.8) |
| >2 | 37 (24.0) | 15 (9.7) | 52 (33.8) |
| Perineural invasion | 84 (54.5) | 47 (30.5) | 131 (85.1) |
| Tumor stage | |||
| IA | 5 (3.2) | — | 5 (3.2) |
| IB | 8 (5.2) | 6 (3.9) | 14 (9.1) |
| IIA | 19 (12.3) | 8 (5.2) | 27 (17.5) |
| IIB | 71 (46.1) | 28 (18.2) | 99 (64.3) |
| III | 2 (1.3) | 7 (4.5) | 9 (5.8) |
Determinants of survival after R0 resection
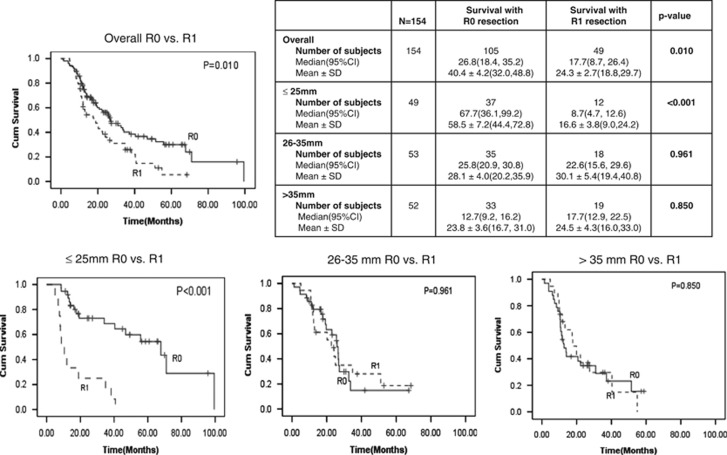
Figure 1 shows the Kaplan–Meier cumulative survival estimates based on tumor size. The overall median (95% CI) and mean survival after R0 resection was 26.8 (18.4, 35.2) and 40.4±4.2 months and after R1 resection was 17.7 (8.7, 26.4) and 24.3±2.7 months, respectively (P=0.010). The median and mean survival was 19.5 and 21.4 months in patients in whom R0 resection was achieved following an initial R1 resection margin. We then performed survival analysis based on tumor size to determine whether tumor size influenced survival after R0 resection and if so what tumor sizes were associated with significant survival benefit (provided asSupplementary data). On the basis of these analyses, we divided patients into three categories based on tumor sizes: patients with tumor size ≤25 mm, with 26–35 mm, and >35 mm. In patients with tumors ≤25 mm in size, the median (95% CI) and mean survival was 67.7 (36.1, 99.2) and 58.5±7.2, respectively, after R0 resection and was 8.7 (4.7, 12.6) and 16.6±3.8, respectively, after R1 resection (P<0.001). The median and mean survivals after R0 resection in patients with tumors 26–35 mm or >35 mm in size were not significantly better than those with R1 resection.
Figure 1.
Tumor size as a determinant of survival after R0 resection of pancreatic adenocarcinoma. CI, confidence interval.
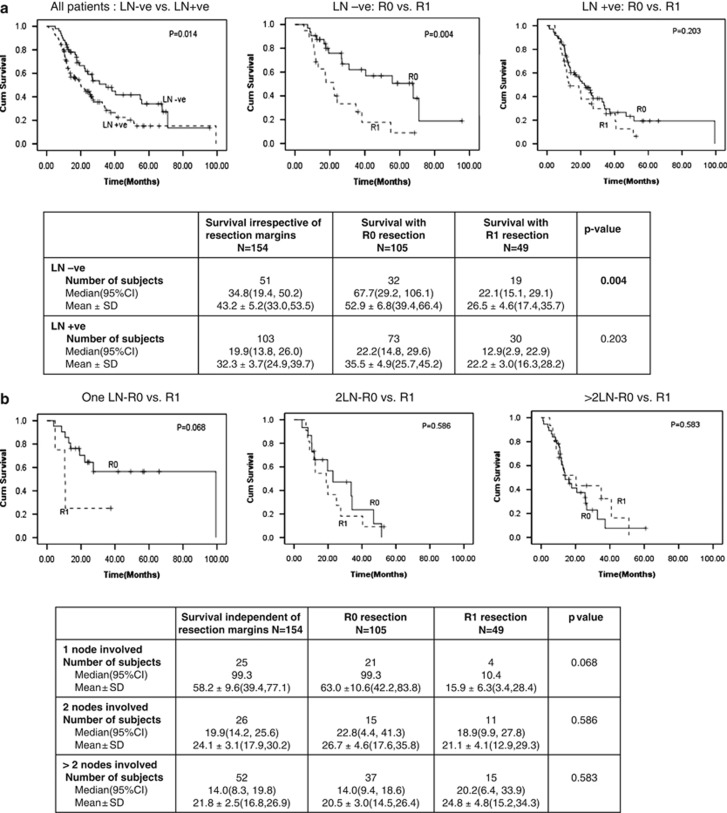
Figure 2a shows analysis of the influence of LN involvement by tumor on survival in study patients. The median number of LN(s) examined and reported by pathologists in surgical resection specimen in our cohort was 15 (1–45). Patients without LN involvement by tumor had significantly longer median survival than those with metastatic tumor in LN (34.8 months (19.4, 50.2) vs. 19.9 months (13.8, 26.0); P=0.014). R0 resection was associated with significantly better survival than R1 resection only in patients without LN involvement and not so in those with it. Figure 2b shows the influence of the number of peripancreatic nodes involved by tumor on survival benefit following R0 resection. Survival benefit was noted with tumor involvement of none (P<0.004) or single LN (P=0.068) but not when two (P=0.586) or greater than two LN were involved by tumor (P=0.583).
Figure 2.
Peripancreatic lymph node involvement and survival. (a) Peripancreatic lymph node involvement as a determinant of survival after R0 resection. (b) Does the number of malignant peripancreatic nodes involved by tumor influence survival after R0 resection. CI, confidence interval; LN, lymph node.
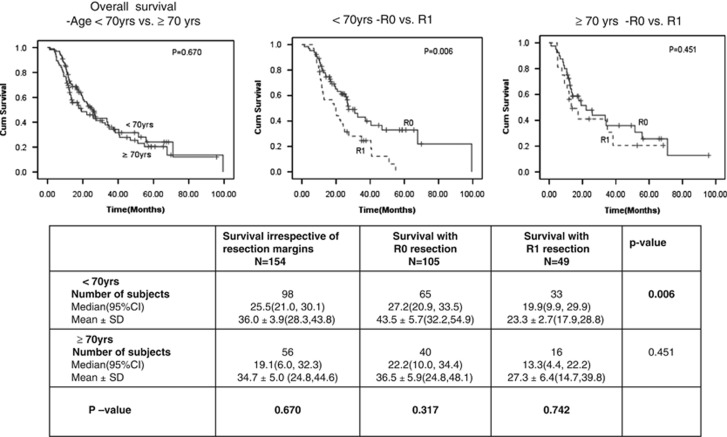
Figure 3 shows the influence of patient's age on survival after R0 resection. Comparison of survival benefit with R0 vs. R1 resection using patient age cutoff 70 years suggested significant survival benefit in patients <70 years of age (median survival of 27.2 months in R0 vs. 19.9 in R1; P=0.006). Among patients ≥70 years of age, there was a trend to survival benefit with R0 resection (22.2 months vs. 13.3 months with R1), but it did not reach statistical significance (P=0.451). The survival differences in patients <70 and ≥70 years after R0 or R1 resection were not statistically significant.
Figure 3.
Age as a determinant of survival after R0 resection. CI, confidence interval.
Prognostic factors for survival after R0 resection
Table 2 shows the univariate and multivariate analysis for tumor size, and LN involvement and patient age as prognostic indicators of survival in patients who underwent R0 resection. R1 resection was used as reference to test for survival benefit in patients with R0 resection. The tumor size of ≤25 mm and ≤1 LN involved by tumor were associated with significant survival benefit. The age of ≥70 years was not statistically significant on univariate analysis. On multivariate analysis, after entering the variables in forward stepwise manner, patients with R0 resection and tumor size >35 mm had worse survival than patients with R1 resection (hazard ratio=1.08; 95% CI, 0.6–1.8). In patients with tumor size 26–35 mm, though the survival was slightly better than reference group, statistical significance was not reached.
Table 2. Regression analysis to test survival benefit after R0 resection.
|
Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||
| <70 | 1.00 (Reference) | |||
| ≥70 | 1.09 (0.7, 1.6) | 0.670 | ||
| Tumor size (mm) | <0.001 | 0.003 | ||
| R1 | 1.00 (Reference) | 1.00 (Reference) | ||
| R0 and >35 | 1.03 (0.6, 1.7) | 0.887 | 1.08 (0.6, 1.8) | 0.753 |
| R0 and 26–35 | 0.73 (0.4, 1.2) | 0.272 | 0.70 (0.4, 1.2) | 0.201 |
| R0 and ≤25 | 0.27 (0.1, 0.5) | <0.001 | 0.33 (0.1, 0.6) | 0.001 |
| LN involvement | ||||
| ≤1 LN | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥2 LN | 2.21 (1.4, 3.3) | <0.001 | 1.78 (1.1, 2.7) | 0.009 |
Abbreviations: CI, confidence interval; HR, hazard ratio; LN, lymph node.These results in bold letters are statistically significant (P<0.05).
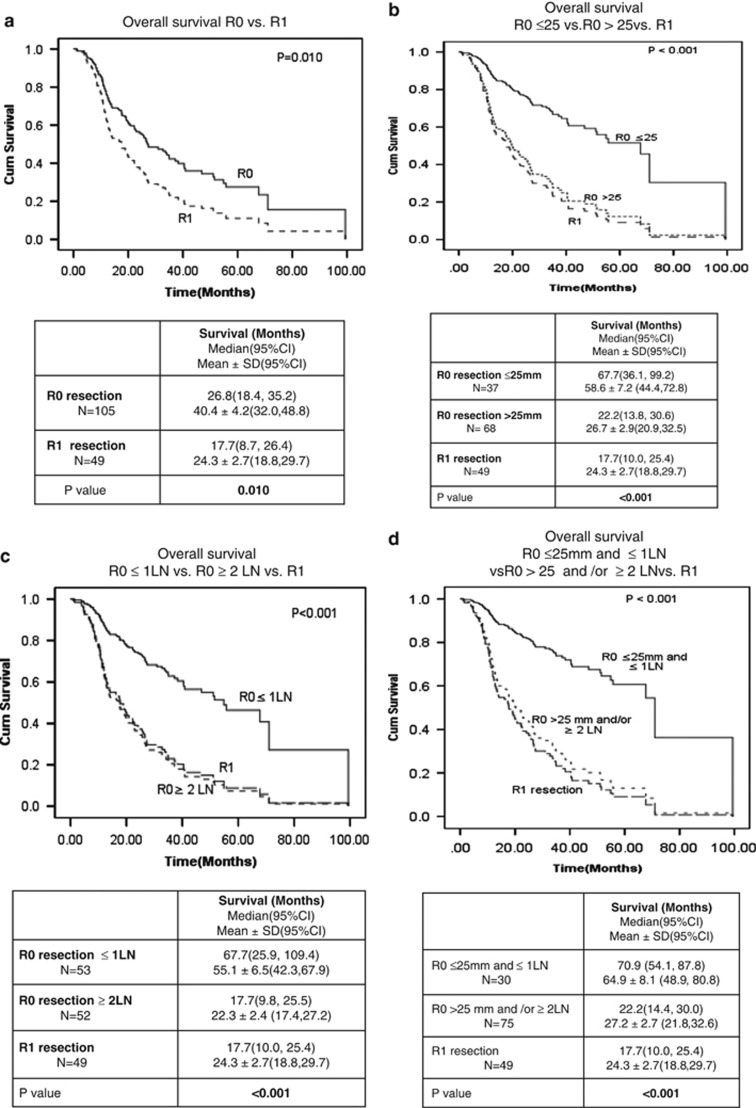
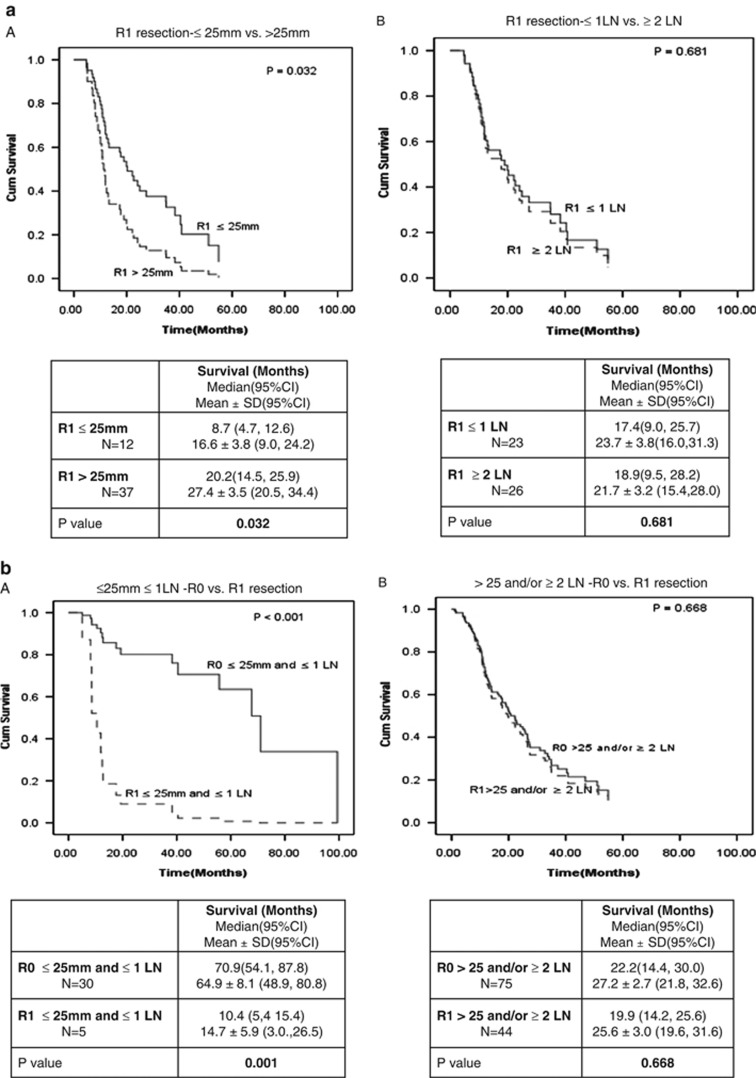
We then sought to determine the influence on survival benefit if one or both of these criteria were favorable or unfavorable. Figure 4 shows survival analysis in all patients with R0 resection vs. patients with R1 resection (Figure 4a), in patients with R0 resection and tumors size ≤25 mm vs. R0 resection with tumor size >25 mm and patients with R1 resection (Figure 4b), in patients with R0 resection with ≤1 involved LN(s) vs. patient with R0 resection and ≥2 involved LN(s) vs. patients with R1 resection (Figure 4c), and in patients with R0 resection and tumor size ≤25 mm and ≤1 involved LN vs. patients with R0 resection and tumor size >25 mm or ≥2 involved LN(s) or both vs. patients with R1 resection (Figure 4d). As noted in the accompanying tables, the median survival was 26.8 months in all patients with R0 resection, 67.7 months in those with tumor size ≤25 mm (irrespective of LN status), 67.7 months in patients with ≤1 involved LN (irrespective of tumor size), and 70.9 months in patients with tumor size ≤25 mm with ≤1 involved LN. There was no significant survival benefit following R0 resection if either the tumor was >25 mm in size or ≥2 involved LN(s) were present (P=0.64).
Figure 4.
Relative importance of tumor size and peripancreatic lymph node involvement on post-operative survival following R0 resection. (a) Overall survival R0 vs. R1. (b) Overall survival R0 ≤25 mm vs. R0 >25 mm vs. R1. (c) Overall survival R0≤1 LN vs. R0≥2 LN vs. R1. (d) Overall survival R0≤25 mm and ≤1 LN vs. rest of R0 vs. R1. CI, confidence interval.
Relative importance of tumor size, LN involvement, and resection margin status on survival
Is survival benefit with upfront surgery in patients with PaCa largely a function of the patient factors including tumor size and LN status or it is contingent upon achieving a R0 resection surgically? We evaluated survival benefit due to tumor size and LN status in patients with R1 resection in our cohort. Figure 5a shows Kaplan–Meier survival analysis in study patients who had R1 resection based on tumor size less than or greater than 25 mm and LN involvement by tumor (≤1 LN or ≥2 LN). Unlike in patients with R0 resection, there were no significant differences in survival based on LN involvement and relatively modest (though statistically significant) difference in survival based on tumor size in patients with R1 resection emphasizing the importance of R0 resection.
Figure 5.
Importance of margin status on survival. (a) Influence of tumor size and lymph node (LN) status on survival in patients with R1 resection. (b) Overall survival benefit in study subjects in patients ≤25 mm ≤1 LN and >25 and/or ≥2 LN. CI, confidence interval.
Figure 5b illustrates the cumulative post-operative survival following R0 and R1 resections in a subset of patients with tumor size ≤25 mm and ≤1 involved LN (Figure 5a), and the remaining patients in our cohort (Figure 5b). R0 resection was associated with dramatic and statistically significant difference in survival only in patients with tumor size ≤25 mm and ≤1 involved LN, but not in the remaining patients in our cohort. These findings illustrate the significance of achieving and confirming R0 resection in patients undergoing upfront surgical resection for PaCa.
DISCUSSION
Margin-negative resection (R0), tumor size, and LN status are known to be significant determinants of post-operative survival following upfront surgical resection in patients with PaCa.2, 3, 4 The relative importance of these factors in predicting survival benefit with surgery, however, has not been clearly established. In the present cohort, we found survival benefit following R0 resection (vs. R1 resection) only in patients with tumor size ≤25 mm and ≤1 involved LN. Median survival was 17.7 months following R1 resection and was 70.9 months (P<0.001) and 22.2 months (P=0.44) in patients with tumor ≤25 mm in size and ≤1 involved LN and in the remaining patients in the cohort respectively following R0 resection.
Tumor size and involvement of peripancreatic LN(s) has been reported by several authors to be significant prognostic factor survival following surgical resection.5, 6, 7, 8, 9, 10, 11, 12, 13 We similarly noted tumor size and LN status to be significant prognostic factors for post-operative survival if patients were pooled together regardless of the margin status. In addition, we found statistically significant survival benefit following R0 resection only in patients with tumors ≤25 mm in size. We also found that LN status to be a significant factor for survival only within the subset of patients who had a R0 resection but not in patients with R1 resection. Murakami et al.14 had suggested that the patients with one involved LN had prognosis similar to those without any LN involvement. In our cohort, we also noted that tumor involvement of a single LN did not preclude survival benefit associated with R0 resection. However, R0 resection was not associated with survival benefit in patients with ≥2 involved LN(s).
Several studies have previously investigated prognostic factors for patient survival following surgical resection of PaCa. Kuhlman et al.15 reported that resection margin status, LN status, tumor differentiation, and tumor size were independent variables for survival following surgery. Winter et al.16 similarly found tumor size ≥30 mm, positive LN(s), positive resection margins, and poor differentiation of tumor as markers of poor prognosis following surgery. Ability to preoperatively identify patients with higher likelihood of having a R0 resection and better post-operative survival would allow better selection of patients for surgery. Boa et al.17 evaluated computed tomography (CT) and endoscopic ultrasound (EUS) findings that could predict R0 resection and better post-operative survival. They found that EUS stage ≤1A, absence of vascular infiltration on CT and EUS stages 1B or 2A, or EUS stage 2B but the tumor size ≤26 mm favorably predicted R0 resection and recommended surgery as primary treatment in these patients. Patient in the low-risk group based on these criteria had significantly better survival (20.3 months) compared with those in the high-risk group (12.1 months). We noted survival trends similar to these studies in our cohort. In addition, we observed that R0 resection by itself did not confer significant survival benefit in all patients. Rather, it was a subset of these patients with favorable factors that derived significant survival benefit with R0 resection. We identified tumor size ≤25 mm and ≤1 involved LN as two of these favorable factors. The median survival in all study patients was 24.1 months and in those with R0 resection was 26.8 months, in patients with R0 resection and tumor size ≤25 mm and ≤1 involved LN, the median survival was 70.9 months. Survival in the remaining patients with R0 resection was identical to patients with R1 resection. Survival benefit from R0 resection was completely abrogated if either of these criteria were unfavorable (i.e., tumor was >25 mm or ≥2 involved LN).
Though R0 resection is oncologically desirable and has been shown to be associated with better survival in several studies, the importance of R0 resection (vs. R1 resection) has been debated recently. In separate cohorts of PaCa patients treated with neoadjuvant therapy before surgery, Raut et al.18 and Breslin et al.19 found no significant survival differences between R0 and R1. Even in studies where R0 resection was clearly associated with better survival, the differences in median survival between patients with R0 and R1 resection were relatively modest. In the present cohort, we found that in the subset of patients with tumor size ≤25 mm and ≤1 involved LN, who seemed to benefit most with R0 resection, the median survival with R0 resection was dramatically better than in patients with similarly sized tumors with R1 resection. Unlike in patients with R0 resection, LN status were not significant prognostic indicators in patients with R1 resection. These data emphasize the importance of R0 resection, though the benefit of R0 resection was not uniform and largely confined to a subset of patients with favorable tumor size and LN status.
Tumor size and LN status in our study were based on surgical pathology. There is insufficient data on how accurately the tumor size and LN involvement can be assessed preoperatively. The only published study on correlation of tumor size measurements by CT and EUS with actual tumor size (Arvold et al.) suggested a poor correlation, but the study has several weaknesses. A major limitation is that the endosonographers and radiologists currently do not make any special effort to precisely measure the size of the tumor as it is not used in decision making for resectable tumors. As a result, the poor correlation suggested by Arvold et al.20 does not necessarily reflect the limitation of the CT and EUS technologies. Analysis of our database suggested that even though EUS measurements are not accurate for large tumors, it is quite reliable for tumors ≤35 mm in size (data not presented). Even though, enlarged LN(s) noted by imaging with CT/magnetic resonance imaging can be reactive and without tumor infiltration, endoscopic ultrasound-guided fine needle aspiration has high positive predictive value for preoperative determination of malignant LN infiltration.21, 22 Diagnosing malignant infiltration in ≥2 peripancreatic LNs by endoscopic ultrasound-guided fine needle aspiration can potentially help identify patients who are unlikely to benefit from upfront surgery. If the analyses of patient databases from other institutions confirm the findings of this study, endosonographers will be encouraged to precisely measure tumor size and biopsy all abnormal-appearing peripancreatic LNs until at least two nodes are positive during EUS examination of patients with pancreatic cancer. This information can then potentially be used for devising treatment plan for the patients with pancreatic cancer. Future studies will need to determine feasibility and accuracy of preoperative determination of tumor size and malignant LN infiltration by various imaging techniques, including endoscopic ultrasound-guided fine needle aspiration before these criteria can be recommended for use in routine patient management.
If upfront surgery with R0 resection does not confer survival benefit vs. R1 resection, two questions then arise: (1) is there any survival benefit with surgery compared with similar-staged patients who are not operated upon and (2) what could be alternate treatment options for patients with resectable tumors on imaging but with tumor size >25 mm or ≥2 involved LN(s). Our data do not exclude a survival benefit due to surgical resection in patients with tumors >35 mm in size and ≥2 involved LN(s) compared with similarly staged patients who do not undergo surgery. Neoadjuvant therapy is increasingly being used for downstaging borderline resectable tumors.23 Patients with potentially resectable tumors with size >25 mm or ≥2 involved LN(s) potentially could be classified as borderline resectable and considered for neoadjuvant treatment, similar to other locally advanced (T3Nx or TxN1) gut cancers such as esophageal and rectal cancer. The benefit of neoadjuvant therapy in this patient subset will need to be evaluated in future studies.
The present study has limitations inherent because of its retrospective design even though it was based on objective and easily verifiable data. We excluded patients with preoperative chemoradiation and only patients who had upfront surgery were included for analysis. Patients with pancreatic cystadenocarcinoma were excluded as they have earlier been shown to have significantly better survival.24 Our cohort is comparable to those from previously published patient series in terms of survival and other major trends, and we noted similar influence of tumor size and LN involvement on overall post-operative survival. We, however, performed a focused analysis of the subset of patients with R0 resection to determine whether all patients have survival benefit from R0 resection and if not then try to identify factors that could identify patients with PaCa most likely to benefit from upfront surgery. We did not review the surgical pathology to re-evaluate the surgical margins for the revised R1 definition.25 It is likely that such reclassification of margin status may lead to the survival differences between revised R0 and R1 resection becoming slightly more pronounced. However, this would not influence the conclusions of this paper that survival benefit with upfront surgery especially in subset of patients with tumor ≤25 mm in size and ≤1 involved peripancreatic LN is dependent on achieving an R0 resection. The dramatic survival benefit with R0 resection in patient with tumors ≤25 mm in size and ≤1 LN may even justify a higher operative risk in patients with comorbidities in this patient subset. Owing to retrospective nature of this study, the findings are not definitive but can easily be verified by other investigators by similar analysis using their existing surgical databases.
In conclusion, R0 resection is associated with dramatic survival benefit over R1 resection in subset of patients with tumor size ≤25 mm and ≤1 involved peripancreatic LNs. These findings underscore the importance of R0 resection and careful patient selection for upfront surgery in patients with PaCa.
Study Highlights
Guarantor of the article: Banke Agarwal, MD.
Specific author contributions: Data collection, interpretation, manuscript preparation, and statistical analysis: Pavan Tummala; data interpretation, critical revision, and approval of the final draft submitted: Todd Howard; conceiving the study, data interpretation, critical revision, and approval of the final draft submitted: Banke Agarwal.
Financial support: None.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and TranslationalGastroenterology website (http://www.nature.com/ctg)
This work was presented at American Pancreatic Association on 3 November 2011 (Chicago, IL, USA).
Supplementary Material
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics. . CA Cancer J Clin. 2010;2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Benassai G, Mastrorilli M, Quarto G, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73:212–218. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Konstantinidis IT, Deshpande V, Zheng H, et al. Does the mechanism of lymph node invasion affect survival in patients with pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2010;14:261–267. doi: 10.1007/s11605-009-1096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143:75–83. doi: 10.1001/archsurg.2007.17. [DOI] [PubMed] [Google Scholar]
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HJ, An JY, Heo JS, et al. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37–43. doi: 10.1097/01.mpa.0000194609.24606.4b. [DOI] [PubMed] [Google Scholar]
- Zacharias T, Jaeck D, Oussoultzoglou E, et al. Impact of lymph node involvement on long-term survival after R0 pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas. J Gastrointest Surg. 2007;11:350–356. doi: 10.1007/s11605-007-0113-3. [DOI] [PubMed] [Google Scholar]
- Massucco P, Ribero D, Sgotto E, et al. Prognostic significance of lymph node metastases in pancreatic head cancer treated with extended lymphadenectomy: not just a matter of numbers. Ann Surg Oncol. 2009;16:3323–3332. doi: 10.1245/s10434-009-0672-5. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Uemura K, Sudo T, et al. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg. 2010;211:196–204. doi: 10.1016/j.jamcollsurg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Kuhlmann KF, de Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–558. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Bao P, Potter D, Eisenberg DP, et al. Validation of a prediction rule to maximize curative (R0) resection of early-stage pancreatic adenocarcinoma. HPB (Oxford) 2009;11:606–611. doi: 10.1111/j.1477-2574.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- Arvold ND, Niemierko A, Mamon HJ, et al. Pancreatic cancer tumor size on CT Scan versus pathologic specimen: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys. 2011;80:1383–1390. doi: 10.1016/j.ijrobp.2010.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc. 1999;50:786–791. doi: 10.1016/s0016-5107(99)70159-8. [DOI] [PubMed] [Google Scholar]
- Săftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1–17. doi: 10.1002/jcu.20534. [DOI] [PubMed] [Google Scholar]
- Katz MH, Pisters PW, Lee JE, et al. Borderline resectable pancreatic cancer: what have we learned and where do we go from here. Ann Surg Oncol. 2011;18:608–610. doi: 10.1245/s10434-010-1460-y. [DOI] [PubMed] [Google Scholar]
- Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339–2349. doi: 10.1111/j.1572-0241.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.