Abstract
Neonatal hypoxia-ischemia (HI) is a devastating condition resulting in neuronal cell death and often culminates in neurological deficits. Granulocyte-colony stimulating factor (G-CSF) has been shown to have neuroprotective activity via inhibition of apoptosis and inflammation in various stroke models. Stem cell factor (SCF) regulates hematopoietic stem cells in the bone marrow and has been reported to have neuroprotective properties in an experimental ischemic stroke model. In this study we aim to determine the protective effects of G-CSF in combination with SCF treatment after experimental HI.
Methods
Seven-day old Sprague-Dawley rats were subjected to unilateral carotid artery ligation followed by 2.5 hours of hypoxia. Animals were randomly assigned to five groups: Sham (n=8), Vehicle (n=8), HI with G-CSF treatment (n=9), HI with SCF treatment (n=9) and HI with G-CSF+SCF treatment (coadministration group; n=10). G-CSF (50 µg/kg), SCF (50 µg/kg) and G-CSF+SCF (50 µg/kg) were administered intraperitoneally 1 hour post HI followed by daily injection for 4 consecutive days (five total injections). Animals were euthanized 14 days after HI for neurological testing. Additionally assessment of brain, heart, liver, spleen and kidney atrophy was performed.
Results
Both G-CSF and G-CSF+SCF treatments improved body growth and decreased brain atrophy at 14days post HI. No significant differences were found in the peripheral organ weights between groups. Finally, the G-CSF+SCF coadministration group showed significant improvement in neurological function.
Conclusion
Our data suggest that administration of G-CSF in combination with SCF not only prevented brain atrophy but also significantly improved neurological function.
Keywords: Granulocyte-colony stimulating factor (G-CSF), Stem cell factor (SCF), Hypoxia-ischemia (HI), Neurological outcome
Introduction
Hypoxic-ischemic (HI) brain injury remains a leading cause of mortality and morbidity in infants and affects 2–4 of 1000 full-term births and nearly 60% of premature births [1, 2]. Among survivors, 20–40% develops significant neurological impairments such as cerebral palsy, mental retardation, and epilepsy associated with life-long medical, social, emotional, and economic difficulties [3, 4]. However, effective treatment avenues are still lacking; thus necessitating alternative strategies to either replace or amplify current therapeutic protocols. Granulocyte-colony stimulating factor (G-CSF) and stem cell factor (SCF) are hematopoietic growth factors which play a role in hematopoiesis [5]. G-CSF is produced by a number of different cells (endothelium, macrophages and some immune cells) to stimulate the bone marrow to release stem cells and granulocytes. Its receptor is present on precursor cells in the bone marrow which when activated initiates proliferation and differentiation into mature granulocytes. Recently it has also been shown that it also has receptors expressed on neurons in the brain and spinal cord which, when activated by G-CSF, induces neurogenesis, increases neuroplasticity and reduces apoptosis [6, 7]. G-CSF stimulates survival, proliferation and development of neuronal stem cells and was in Phase I/II clinical trials for ischemic stroke treatment [6, 8, 9]. Multiple studies have shown that G-CSF is capable of conferring neuroprotection in a variety of in vivo brain injury models [10–13]. It was reported that rats treated with G-CSF after middle cerebral artery occlusion (MCAO) have smaller infarcts and better functional outcome compared with controls [14]. Furthermore it has also been shown that G-CSF prevented brain tissue loss and improved long-term neurological outcome in the neonatal HI model [15]. SCF binds to the tyrosine kinase receptor (c-kit) which is expressed on both primitive and mature hematopoietic progenitor cells. Once bound to its receptor it mediates cell proliferation, differentiation and migration in hematopoiesis [16]. In vivo SCF acts synergistically with G-CSF to mobilize and promote proliferation and survival of pluripotent progenitor cells [17, 18], and promote neuronal production from bone marrow [19].
Recently, accumulating evidence has shown that both G-CSF and SCF have therapeutic effects on the central nervous system [5]. SCF mutant mice showed deficits in special learning and memory [20] while G-CSF has been shown to have neuroprotective effect on brain ischemia. The administration of G-CSF alone [21–23] or in combination with SCF [23] during the acute phase of brain ischemia, or the subcutaneous injection of G-CSF+SCF during the subacute phase of brain ischemia [9] resulted in an reduction of infarction size. However, no study to date has examined whether administration of G-CSF with SCF induced neuroprotection translates into improvements in neurological outcome at the chronic phase of brain ischemia and whether there is an additive benefit against brain and peripheral organ atrophy with the coadministration treatment in neonatal HI.
We hypothesized that G-CSF in combination with SCF will not only have neuroprotective activity by reducing brain atrophy but will also show significant improvement in the neurological outcome after HI in neonatal rats. G-CSF, SCF and G-CSF+SCF were administered 1 hour post HI followed by daily injection for 4 consecutive days, and brain atrophy, organ weights and neurological outcome was measured at 14 days post HI.
Materials and Methods
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma University. All animal handling was executed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Sprague Dawley rat mothers, with litters of 10–12 pups, were purchased from Harlan Labs (Livermore, CA). A total of 44 unsexed rat pups were used. They were divided into the following groups: sham (n=8), HI + Vehicle (n=8), HI + G-CSF (n=9), HI + SCF (n=9) and HI + G-CSF + SCF (n=10).
Hypoxia-Ischemia model/Operative Procedure
The standard Rice-Vannucci neonatal Hypoxia-Ischemia model was used as previously described [24]. Briefly seven days after birth (P7), neonatal rat pups were placed into a temperature-controlled chamber for the induction of general anesthesia which was achieved with 3% isoflurane gas in air for induction, and 1.5% isoflurane in air for maintenance of anesthesia. Throughout the surgical and postoperative period, temperature was controlled with heating blankets and incubators. After induction of anesthesia the neck of the rats was prepared and draped using standard sterile techniques. Next a small midline neck incision on the anterior neck was made with a No. 11 blade surgical knife (approximately 3–5 mm in length). Using gentle blunt dissection, the right common carotid artery was isolated and gently separated from surrounding structures. The carotid artery was then ligated with 5-O surgical suture. Bleeding was controlled with gentle pressure and electrocautery as needed. The surgery was performed aseptically and its duration did not exceed more than 15 minutes. After the surgical procedure was completed, the rats were allowed to recover for 1 hour. Thereafter, they were placed in a 500-ml airtight jar partially submerged in a 37 °C water bath to maintain a constant thermal environment. A gas mixture of 8% Oxygen and 92% Nitrogen was delivered into the jars through inlet portals. The rat pups were exposed to this gas mixture for 2 hours and 30 minutes. Thereafter, animals were returned to their mothers.
Treatment Method
Rat pups were allowed to rest for 1 hour on a warm blanket before initiating therapy. Mouse recombinant SCF (50µ g/kg) (PeproTech, Rocky, USA), human recombinant G-CSF (50µ g/kg) (Amgen, Thousand Oaks, USA), G-CSF and SCF together or PBS were administered intraperitoneally daily for 4 days after the first injection at 1 hour after HI (a total of 5 injections). The doses for G-CSF and SCF chosen were previously shown to be affective in other ischemia models [15, 25].
Evaluation of Brain damage and systemic organ weight
The HI animal model shows brain damage on the ipsilateral side [26, 27], commonly assessed by hemispheric brain weight loss, which is highly correlated to histological loss of brain tissue [28, 29]. Brain tissue (sham: n = 8; vehicle: n = 8; G-CSF: n = 9; SCF: n = 9; G-CSF+SCF: n=10) was removed, and the hemispheres were separated by a midline incision and weighed on a high-precision balance (sensitivity ±0.001 g). Data are expressed as the ratio of ipsilateral (right) to contralateral (left) hemispheric weights. The heart, spleen, liver and kidney were also isolated and weighed. Data for systemic organs are expressed as the ratio of organ weight to body weight.
Neurobehavioral Tests
Modified Garcia and foot fault test were performed in order to assess the long- term sensorimotor dysfunction in rats 14 days post HI as previously described [30, 31]. Briefly, the modified Garcia test is a 21-point sensorimotor assessment system consisting of 7 tests with scores of 0 to 3 for each individual test (with 0 being worst score and 3 best; maximum score=21). These 7 tests evaluated spontaneous activity (1), reaction following side stroking (2) and vibrissae touch (3), limb symmetry (4), climbing (5), lateral turning (6), and forelimb walking (7).
The foot fault test is a test of motor coordination. The apparatus consists of a grid floor. The grid is suspended or raised above a surface. Each animal is placed at one end of the grid and monitored for 2min from the side as they walk across the grid. The number of forelimb and hind limb placement errors as the animal traverses the grid is scored. An error is counted whenever a limb misses a bar and extends downward through the plane of the bars.
Statistical Analysis
All data were expressed as mean +/− SEM. Statistical differences between groups were analyzed with one-way ANOVA followed by Tukey multiple-comparison post hoc analysis or Student-Newman-Keuls test on ranks. A P value of p<0.05 was considered statistically significant.
Results
Treatment with G-CSF and SCF improved physical development after HI
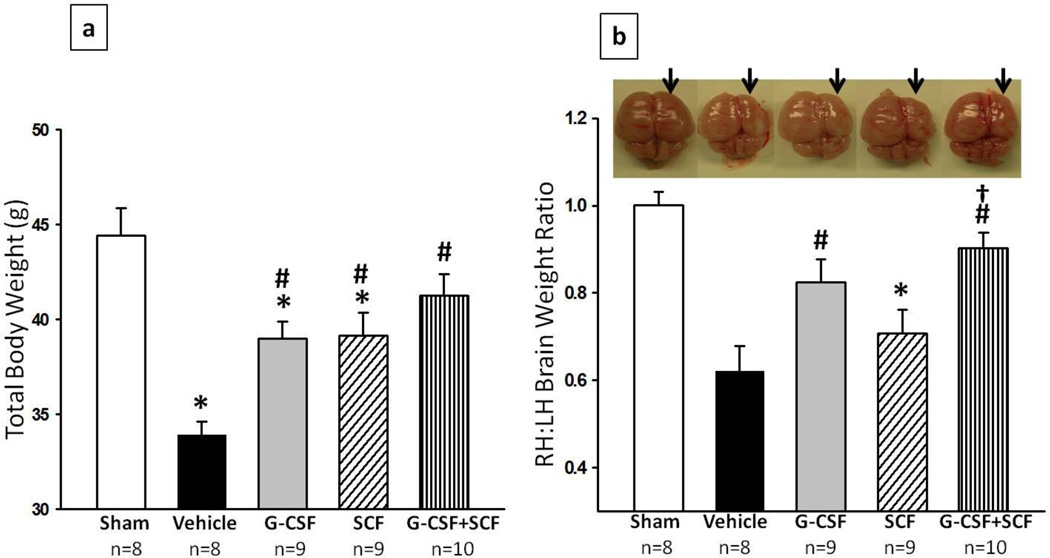
Figure 1a shows the difference in physical development between rats injected with vehicle (PBS), G-CSF, SCF or G-CSF+SCF at the completion of the 5-day treatment period. At 14 days post HI, vehicle rats (n=8) had gained significantly less weight compared to sham (n=8), numbers in parenthesis represent mean raw score ± standard error (p<0.0533.919 ± 0.693 vs 44.413 ± 1.454 g) which was attenuated by G-CSF, SCF (n=9) and G-CSF+ SCF (n=10) treatment (p<0.05, compared to vehicle). Furthermore, animals treated with both G-CSF and SCF showed higher weight gain then animals treated with G-CSF or SCF only (p>0.05, compared to Sham; 41.267 ± 1.123 sham vs 38.974 ± 2.69 G-CSF vs 39.13 ± 1.211 SCF g).
Fig. 1. G-CSF and SCF improved body weight (a) and reduced brain atrophy (b) 14days post HI.
Postnatal day-7 rats were subjected to HI. Intraperitoneal (IP) treatment with GCSF, SCF or G-CSF+SCF began at 1hour post HI and continued daily for 4days. (a) G-CSF, SCF and G-CSF+SCF treatment groups demonstrated an improved body weight (g) 14days post HI, with the combination treatment group showing best results. (b) Significant loss of right-to-left hemispheric (RH:LH) weight ratio is evident in vehicle rats and significantly improved with G-CSF and G-CSF+SCF treatment at 14days post HI (representative pictures shown; Data represent +/− SEM; *p<0.05 versus sham, #p<0.05 versus vehicle, †p<0.05 versus SCF ).
Combinational treatment of G-CSF with SCF reduced brain atrophy and tends to improve peripheral organ weight
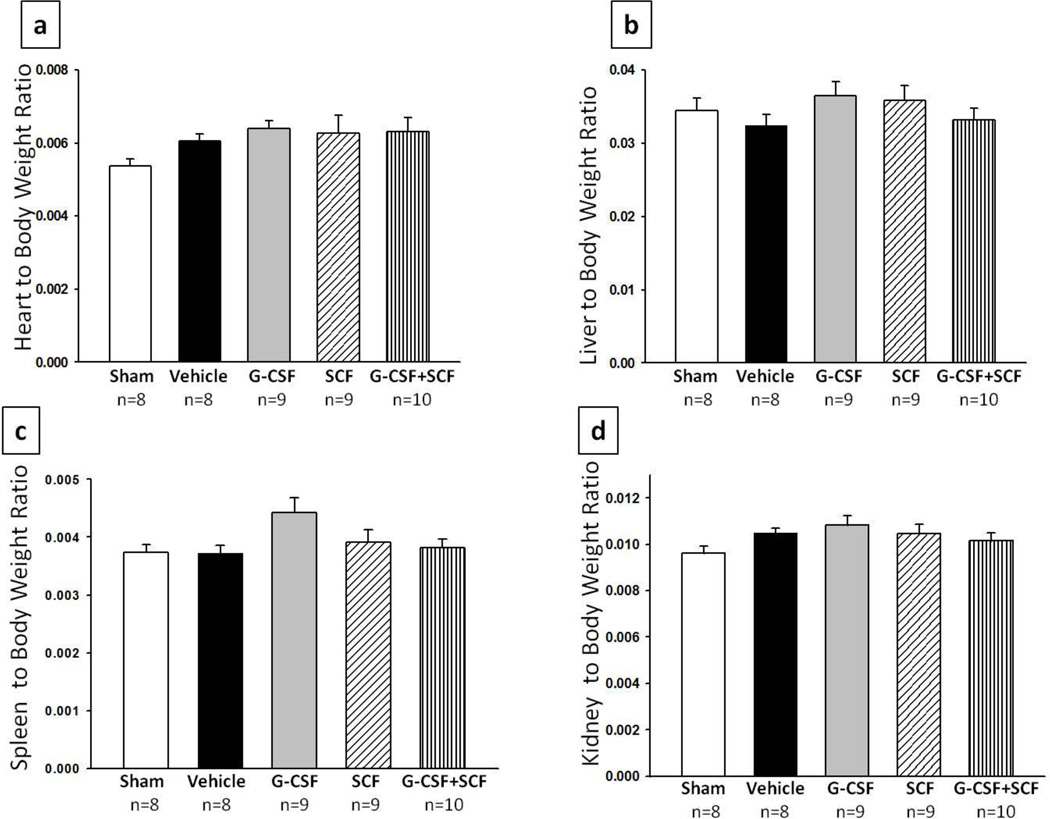
Hi injury caused severe brain atrophy of the lesioned hemisphere as seen in figure 1b (arrows represent the injured side). Rats injected with G-CSF resulted in significantly lesser brain tissue atrophy when analyzed at 14 days post HI compared to vehicle (p<0.05, 0.825 ± 0.0515 vs 0.621 ± 0.0575 right to left brain weight ratio). A tendency was seen in rats injected with SCF only, to reduce brain tissue atrophy in comparison to vehicle, nevertheless no statistical significant difference was found between the groups. However, rats injected with both G-CSF and SCF showed significant less brain tissue atrophy when compared to vehicle and SCF only treatment group (p<0.05; 0.902 ± 0.0357 vs 0.621 ± 0.0575 vs 0.708 ± 0.0547 right to left brain weight ratio). Although G-CSF, SCF and G-CSF+SCF treatment had a tendency to improve spleen, liver, kidney and heart weights, no statistical significant difference was detected between the groups. As seen from figure 2, vehicle rats tend to have smaller liver compared to sham and G-CSF treatment showed to be most effective in increasing liver size, but once again there is no significant difference between the groups.
Fig. 2. Treatment groups showed no effect on organ weight at 14days post HI.
Treatment did not appear to improve heart to body (a)liver to body (b)spleen to body (c) or kidney to body (d) weight ratios when measured 14days post HI. Although G-CSF+SCF treatment appeared to show some improvement in the above listed organs, statistical significance was not reached.
G-CSF and SCF improved neurological outcome at 2 weeks post HI
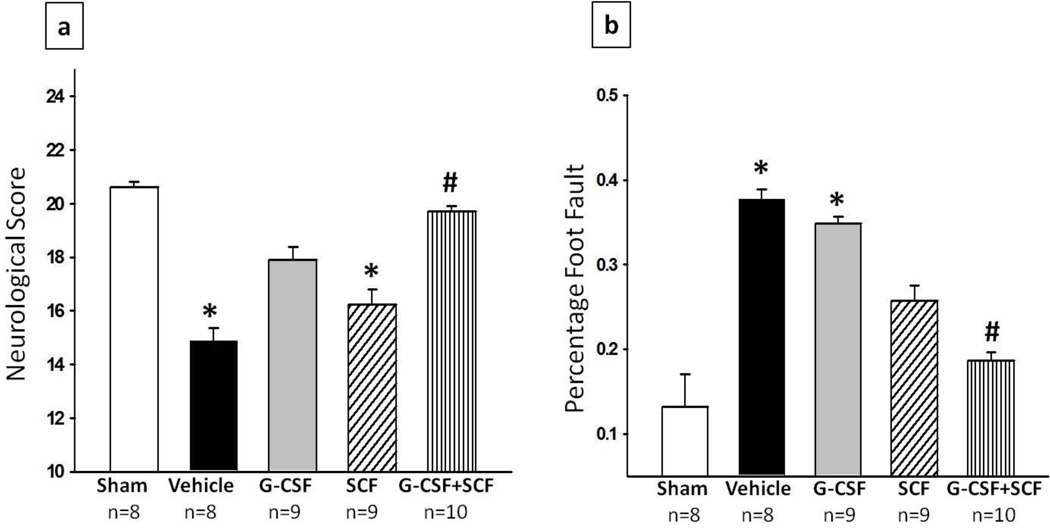
In order to test the effects of G-CSF, SCF and G-CSF + SCF treatment on the neurobehavioral impairments induced by HI, neurological outcome was assessed using modified Garcia test and foot fault test at 14 days after HI. In both behavioural tests, animals in the vehicle group performed significantly worse than sham operated rats (p<0.05). In the modified Garcia test, rats administered with G-CSF or SCF showed a tendency to improve neurological outcome however no significance was reached. Nonetheless, the combinational treatment of G-CSF +SCF showed to significantly improve neurological outcome compared to vehicle treated rats (p<0.05, 19.7 ± 0.213 vs 14.875 ± 0.479). In addition, the combinational treatment group also significantly improved the sensorimotor coordination as assessed by foot fault test (p<0.05, compared to vehicle; 0.186 ± 0.0106 vs 0.378 ± 0.0117). The animals in the G-CSF or SCF groups did not show any significant improvements (fig. 3)
Fig. 3. G-CSF and SCF improved functional outcome at 14days post HI.
The combination treatment group (G-CSF + SCF) showed significant improvement in neurological function according to (a) Modified Garcia test, where sensori-motor function was evaluated. Vehicle and SCF groups scored significantly worse on the 21 score scale system when compared to sham while the G-CSF+SCF treatment group showed significant improvement compared to vehicle and (b) Foot fault test, which shows the percentage of foot faults of the contralateral forelimb. The results show that ischemia-induced foot slips were significantly reduced by treatment with SCF and G-CSF+SCF (Data represent +/− SEM; *p<0.05 versus sham, #p<0.05 versus vehicle).
Discussion
This study demonstrates the efficacy of G-CSF+SCF combination therapy for prevention of brain atrophy and improvement of neurological function in established rat model of neonatal HI injury. Our data show that G-CSF+SCF improved body weight, reduce brain tissue loss and improved the neurological outcome following HI in the neonatal rat pups. These findings provide important information for potential development of new therapeutic strategies against HI-induced brain injury in neonates.
Hypoxia-ischemia is a devastating condition that causes severe brain damage to the newborn infant. Up to date effective treatment avenues are still lacking hence the need for future research in this field. The current study focuses on two major bone marrow related growth factors, granulocyte-colony stimulating factor (G-CSF) and stem cell factor (SCF) which might have a promising therapeutic effect in the onset of stroke. Both G-CSF and SCF are known to stimulate the survival, proliferation and differentiation of bone marrow cells, such as the hematopoietic cells [32–37]. While G-CSF is important for stimulating the production and recruitment of cells from the bone marrow into the circulation, SCF might be responsible for the proliferation of vascular endothelial cells, many of which are derived from the bone marrow [25]. Furthermore, co-administration of G-CSF with SCF may further increase G-CSF protective effect by increasing tyrosine kinase-related downstream effects and increasing prosurvival signals [38]. PhaseI/II clinical trials have shown that SCF treatment alone resulted in little effect on peripheral blood or bone marrow cell numbers. However, when combined with G-CSF it showed a 2–3 fold increase in cells that express CD34 antigen (cells expressing this antigen are hematopoietic cells, progenitor cells, endothelial cells, etc. critical for prosurvival)[18, 39]. Previous studies also reported that when G-CSF was combined with SCF it further increased CD34+ circulating cells versus treatment with G-CSF alone [32] hence further enhancing G-CSF protective effect by increasing prosurvival signals [38].
G-CSF is one of the most studied growth factors in the setting of stroke. Previous studies have reported that G-CSF can pass the blood brain barrier [40] and has shown to be neuroprotective in a rat model of focal cerebral ischemia [21, 41, 42]. Clinically, increasing evidences have shown the therapeutic potential of G-CSF and SCF in patients with ischemic stroke [8, 18, 43]. SCF has been found to stimulate early pluripotent and committed stem cells to form colony-forming units and act synergistically with other growth factors such as GCSF to increase the production of hematopoietic cells [44, 45]. Previous findings have also reported G-CSF’s ability to reduce lesion volumes and improve neurological outcome at 48 hours after cerebral ischemia in rodents [21, 46].
Based on the above findings it is of particular interest to us to study the effects of the combinational treatment of G-CSF with SCF on neurological outcome, brain atrophy and peripheral organ protection. We tested this hypothesis by administering G-CSF, SCF and a combination of G-CSF+SCF at 1 hour post HI and consecutively for 4 days. To our knowledge the long-term neuroprotective effect of the combinational treatment has not been studied to date.
One of the main signs of HI related outcome is growth retardation which can be used as an indicator for general well being [47]. Previous studies have shown that neonatal hypoxiaischemia retards the development of somatic growth starting from 1 day after the insult [48, 49].The body weight data in the present study showed that injured animals significantly lost weight at 14 days post HI while G-CSF or SCF treatment groups significantly reversed that effect. A similar result was seen in previous publication from our lab where G-CSF administered for 5days or 10days significantly increased body weights when compared to vehicle treated animals [15. However, the combinational treatment group even further improved weight gain (Fig.1a). This suggests that G-CSF + SCF treatment significantly improved physical development during the critical period following brain injury.
Aside from promoting weight gain, it is well known that HI causes brain injury which leads to high risks of future behavioural deficits [48]. Hence, we tested the effect of G-CSF + SCF on injured rats and results showed that the treatment significantly reduced brain atrophy (Fig. 1b). Animals in the combinational treatment group showed to prevent the loss of brain tissue. The anti-apoptotic and anti-inflammatory properties of those two growth factors might be responsible for attenuating long -term brain damage; however the exact mechanism is still unknown. HI has been shown to affect not only the brain but other main peripheral organs. The interruption of blood flow to the brain causes a redistribution in cardiac output resulting in more blood pumped to the brain at the expense of blood flow to kidney, liver, spleen etc. resulting in organ injury [50]. Body weight is accompanied by decreased weight in peripheral organs such as the heat, liver, spleen and kidney [51, 52]. As stated above one possible explanation would be the lack of adequate oxygen supply and lack of nutrients which result from preferential blood flow to the brain. Due to those effects seen in this study we further went to measure the organ to body weight ratio (fig. 2), however, no statistical significant results were found between the groups. This might be because the neonatal rat can compensate for those losses. Although there was no statistically significant difference found, the combinational treatment group appeared to show some improvement in organ weights however the G-CSF only treated groups tend to have larger organs when compared to sham. This latter finding may be due to organ hypertrophy due to treatment with growth factor. Spleen enlargements in particular have been reported previously in naive and injured animals due to G-CSF and SCF administration [18, 53]. These findings are in agreement with previous studies form our lab, where it has been shown that G-CSF did not show any significant difference on peripheral organ weights [15].
The hippocampus and the sensorimotor cortex are critical for regulation of sensorimotor function and are highly affected by HI [54]. As already known damage to those regions causes severe deterioration in functional performance. According to modified Garcia and foot fault test G-CSF and SCF treatment groups alone did not show any significant improvement in neurological score, however the combinational treatment group did show significant improvements. According to the Garcia test, HI injured rats performed poorly, which was reversed by the combinational treatment group. Similar effect was seen in foot fault where the percentage of foot faults to contralateral forelimb was high in HI injured rats and reversed in treated animals. Our results are consistent with previous work done from our lab, where G-CSF given for 5days or 10 days promoted physical development, reduced brain atrophy and improved neurological outcome according to T-maze, foot fault, Garcia and rota rod tests at 5 weeks post HI [15]. From the present study and previous studies we can conclude that G-CSF shows significant neurological improvement as early as 2 weeks and as late as 5 weeks, and G-CSF+SCF showed even further improvement when assessed at 2 weeks post HI. A few more studies have been done in our lab on different models that have shown G-CSF to have similar neuroprotective outcome [55, 56].
From this study we can conclude that G-CSF+SCF can improve body weight, reduce brain tissue atrophy and improve neurological outcome following HI in the neonatal rat pup. The exact mechanism through which G-CSF+SCF act to achieve this neuroprotective effect are not well understood. It is speculated that HI may activate intrinsic protective mechanisms to combat neonatal brain injury, which is consistent with prior observations [11]. G-CSF may decrease proapoptotic factors meanwhile increase antiapoptotic factors by activation of the JAK/STAT3 [57], PI3K/Akt [6] and MAPK/ERK pathways which have been largely confirmed in brain ischemia models in vivo 22]. Combining G-CSF with SCF may further increase the protective effects of G-CSF by augmenting tyrosine-kinase related effects and further increase prosurvival signals [38].
Overall, the above findings from this study are clinically relevant and provide foundation for exploring clinical translation. Both G-CSF and SCF are attractive candidates for therapeutic treatment. They both showed neuroprotective properties, specifically attenuating long- term brain atrophy, and also have minimal side effects such as bone or musculoskeletal pain, anemia, thrombocytopenia and injection site reactions [58, 59]. Nevertheless, the study anticipates the need for potentially a longer neurobehavioral assessment time period as well as a more comprehensive evaluation of safety before moving this treatment into clinical setting.
Acknowledgments
This study was supported by NIH grant R01NS060936 to J. Tang
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Vannucci RC, Vannucci SJ. A model of perinatal hypoxic-ischemic brain damage. Ann N Y Acad Sci. 1997;835:234–249. doi: 10.1111/j.1749-6632.1997.tb48634.x. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7(1):56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Bracewell M, Marlow N. Patterns of motor disability in very preterm children. Ment Retard Dev Disabil Res Rev. 2002;8(4):241–248. doi: 10.1002/mrdd.10049. [DOI] [PubMed] [Google Scholar]
- 4.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351(19):1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 5.Zhao LR, Singhal S, Duan WM, Mehta J, Kessler JA. Brain repair by hematopoietic growth factors in a rat model of stroke. Stroke. 2007;38(9):2584–2591. doi: 10.1161/STROKEAHA.106.476457. [DOI] [PubMed] [Google Scholar]
- 6.Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115(8):2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitzer C, Kruger C, Plaas C, Kirsch F, Dittgen T, Muller R, Laage R, Kastner S, Suess S, Spoelgen R, et al. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. 2008;131(Pt 12):3335–3347. doi: 10.1093/brain/awn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006;174(7):927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113(5):701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- 10.Solaroglu I, Tsubokawa T, Cahill J, Zhang JH. Anti-apoptotic effect of granulocytecolony stimulating factor after focal cerebral ischemia in the rat. Neuroscience. 2006;143(4):965–974. doi: 10.1016/j.neuroscience.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yata K, Matchett GA, Tsubokawa T, Tang J, Kanamaru K, Zhang JH. Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia-ischemia in rats. Brain Res. 2007;1145:227–238. doi: 10.1016/j.brainres.2007.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popa-Wagner A, Stocker K, Balseanu AT, Rogalewski A, Diederich K, Minnerup J, Margaritescu C, Schabitz WR. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke. 2010;41(5):1027–1031. doi: 10.1161/STROKEAHA.109.575621. [DOI] [PubMed] [Google Scholar]
- 13.Solaroglu I, Cahill J, Tsubokawa T, Beskonakli E, Zhang JH. Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol Res. 2009;31(2):167–172. doi: 10.1179/174313209X393582. [DOI] [PubMed] [Google Scholar]
- 14.Beck H, Voswinckel R, Wagner S, Ziegelhoeffer T, Heil M, Helisch A, Schaper W, Acker T, Hatzopoulos AK, Plate KH. Participation of bone marrow-derived cells in long-term repair processes after experimental stroke. J Cereb Blood Flow Metab. 2003;23(6):709–717. doi: 10.1097/01.WCB.0000065940.18332.8D. [DOI] [PubMed] [Google Scholar]
- 15.Fathali N, Lekic T, Zhang JH, Tang J. Long-term evaluation of granulocyte-colony stimulating factor on hypoxic-ischemic brain damage in infant rats. Intensive Care Med. 2010;36(9):1602–1608. doi: 10.1007/s00134-010-1913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DE, Lyman SD. Characterization of the gene-product of the Steel locus. Prog Growth Factor Res. 1991;3(4):235–242. doi: 10.1016/0955-2235(91)90002-l. [DOI] [PubMed] [Google Scholar]
- 17.Six I, Gasan G, Mura E, Bordet R. Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003;458(3):327–328. doi: 10.1016/s0014-2999(02)02785-1. [DOI] [PubMed] [Google Scholar]
- 18.McNiece IK, Briddell RA. Stem cell factor. J Leukoc Biol. 1995;58(1):14–22. doi: 10.1002/jlb.58.1.14. [DOI] [PubMed] [Google Scholar]
- 19.Corti S, Locatelli F, Strazzer S, Salani S, Del Bo R, Soligo D, Bossolasco P, Bresolin N, Scarlato G, Comi GP. Modulated generation of neuronal cells from bone marrow by expansion and mobilization of circulating stem cells with in vivo cytokine treatment. Exp Neurol. 2002;177(2):443–452. doi: 10.1006/exnr.2002.8004. [DOI] [PubMed] [Google Scholar]
- 20.Motro B, Wojtowicz JM, Bernstein A, van der Kooy D. Steel mutant mice are deficient in hippocampal learning but not long-term potentiation. Proc Natl Acad Sci U S A. 1996;93(5):1808–1813. doi: 10.1073/pnas.93.5.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, Sommer C, Schwab S. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34(3):745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 22.Komine-Kobayashi M, Zhang N, Liu M, Tanaka R, Hara H, Osaka A, Mochizuki H, Mizuno Y, Urabe T. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2006;26(3):402–413. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- 23.Zhao LR, Berra HH, Duan WM, Singhal S, Mehta J, Apkarian AV, Kessler JA. Beneficial effects of hematopoietic growth factor therapy in chronic ischemic stroke in rats. Stroke. 2007;38(10):2804–2811. doi: 10.1161/STROKEAHA.107.486217. [DOI] [PubMed] [Google Scholar]
- 24.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 25.Toth ZE, Leker RR, Shahar T, Pastorino S, Szalayova I, Asemenew B, Key S, Parmelee A, Mayer B, Nemeth K, et al. The combination of granulocyte colony-stimulating factor and stem cell factor significantly increases the number of bone marrow-derived endothelial cells in brains of mice following cerebral ischemia. Blood. 2008;111(12):5544–5552. doi: 10.1182/blood-2007-10-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer C, Vannucci RC, Towfighi J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr Res. 1990;27(4 Pt 1):332–336. doi: 10.1203/00006450-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y, Fratkins JD, LeBlanc MH. Treatment with tamoxifen reduces hypoxicischemic brain injury in neonatal rats. Eur J Pharmacol. 2004;484(1):65–74. doi: 10.1016/j.ejphar.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Bona E, Johansson BB, Hagberg H. Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatr Res. 1997;42(5):678–683. doi: 10.1203/00006450-199711000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Hagberg H, Gilland E, Diemer NH, Andine P. Hypoxia-ischemia in the neonatal rat brain: histopathology after post-treatment with NMDA and non-NMDA receptor antagonists. Biol Neonate. 1994;66(4):205–213. doi: 10.1159/000244109. [DOI] [PubMed] [Google Scholar]
- 30.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102(3):318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 32.Hess DA, Levac KD, Karanu FN, Rosu-Myles M, White MJ, Gallacher L, Murdoch B, Keeney M, Ottowski P, Foley R, et al. Functional analysis of human hematopoietic repopulating cells mobilized with granulocyte colony-stimulating factor alone versus granulocyte colony-stimulating factor in combination with stem cell factor. Blood. 2002;100(3):869–878. doi: 10.1182/blood.v100.3.869. [DOI] [PubMed] [Google Scholar]
- 33.Broudy VC, Kovach NL, Bennett LG, Lin N, Jacobsen FW, Kidd PG. Human umbilical vein endothelial cells display high-affinity c-kit receptors and produce a soluble form of the c-kit receptor. Blood. 1994;83(8):2145–2152. [PubMed] [Google Scholar]
- 34.Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, Fleming PA, Drake CJ, Ogawa M. Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004;186(2):134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 36.Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25(2):296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 37.Takamiya M, Okigaki M, Jin D, Takai S, Nozawa Y, Adachi Y, Urao N, Tateishi K, Nomura T, Zen K, et al. Granulocyte colony-stimulating factor-mobilized circulating c-Kit+/Flk-1+ progenitor cells regenerate endothelium and inhibit neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2006;26(4):751–757. doi: 10.1161/01.ATV.0000205607.98538.9a. [DOI] [PubMed] [Google Scholar]
- 38.Duarte RF, Frank DA. SCF and G-CSF lead to the synergistic induction of proliferation and gene expression through complementary signaling pathways. Blood. 2000;96(10):3422–3430. [PubMed] [Google Scholar]
- 39.Rosenstrauch D, Poglajen G, Zidar N, Gregoric ID. Stem celltherapy for ischemic heart failure. Tex Heart Inst J. 2005;32(3):339–347. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao LR, Navalitloha Y, Singhal S, Mehta J, Piao CS, Guo WP, Kessler JA, Groothuis DR. Hematopoietic growth factors pass through the blood-brain barrier in intact rats. Exp Neurol. 2007;204(2):569–573. doi: 10.1016/j.expneurol.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110(13):1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 42.Yanqing Z, Yu-Min L, Jian Q, Bao-Guo X, Chuan-Zhen L. Fibronectin and neuroprotective effect of granulocyte colony-stimulating factor in focal cerebral ischemia. Brain Res. 2006;1098(1):161–169. doi: 10.1016/j.brainres.2006.02.140. [DOI] [PubMed] [Google Scholar]
- 43.Sprigg N, Bath PM, Zhao L, Willmot MR, Gray LJ, Walker MF, Dennis MS, Russell N. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092) Stroke. 2006;37(12):2979–2983. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- 44.McNiece IK, Langley KE, Zsebo KM. Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp Hematol. 1991;19(3):226–231. [PubMed] [Google Scholar]
- 45.Galli MC, Giardina PJ, Migliaccio AR, Migliaccio G. The biology of stem cell factor, a new hematopoietic growth factor involved in stem cell regulation. Int J Clin Lab Res. 1993;23(2):70–77. doi: 10.1007/BF02592286. [DOI] [PubMed] [Google Scholar]
- 46.Gibson CL, Jones NC, Prior MJ, Bath PM, Murphy SP. G-CSF suppresses edema formation and reduces interleukin-1beta expression after cerebral ischemia in mice. J Neuropathol Exp Neurol. 2005;64(9):763–769. doi: 10.1097/01.jnen.0000179196.10032.dd. [DOI] [PubMed] [Google Scholar]
- 47.Kim BR, Shim JW, Sung DK, Kim SS, Jeon GW, Kim MJ, Chang YS, Park WS, Choi ES. Granulocyte stimulating factor attenuates hypoxic-ischemic brain injury by inhibiting apoptosis in neonatal rats. Yonsei Med J. 2008;49(5):836–842. doi: 10.3349/ymj.2008.49.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lubics A, Reglodi D, Tamas A, Kiss P, Szalai M, Szalontay L, Lengvari I. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res. 2005;157(1):157–165. doi: 10.1016/j.bbr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 49.Fan LW, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, Cai Z. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res. 2005;165(1):80–90. doi: 10.1016/j.bbr.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 50.Stola A, Perlman J. Post-resuscitation strategies to avoid ongoing injury following intrapartum hypoxia-ischemia. Semin Fetal Neonatal Med. 2008;13(6):424–431. doi: 10.1016/j.siny.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Latini G, De Mitri B, Del Vecchio A, Chitano G, De Felice C, Zetterstrom R. Foetal growth of kidneys, liver and spleen in intrauterine growth restriction:"programming" causing"metabolic syndrome" in adult age. Acta Paediatr. 2004;93(12):1635–1639. doi: 10.1080/08035250410023106. [DOI] [PubMed] [Google Scholar]
- 52.Chvojkova Z, Ostadalova I, Ostadal B. Low body weight and cardiac tolerance to ischemia in neonatal rats. Physiol Res. 2005;54(4):357–362. [PubMed] [Google Scholar]
- 53.Platzbecker U, Prange-Krex G, Bornhauser M, Koch R, Soucek S, Aikele P, Haack A, Haag C, Schuler U, Berndt A, et al. Spleen enlargement in healthy donors during G-CSF mobilization of PBPCs. Transfusion. 2001;41(2):184–189. doi: 10.1046/j.1537-2995.2001.41020184.x. [DOI] [PubMed] [Google Scholar]
- 54.Spandou E, Papadopoulou Z, Soubasi V, Karkavelas G, Simeonidou C, Pazaiti A, Guiba-Tziampiri O. Erythropoietin prevents long-term sensorimotor deficits and brain injury following neonatal hypoxia-ischemia in rats. Brain Res. 2005;1045(1–2):22–30. doi: 10.1016/j.brainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Matchett GA, Calinisan JB, Matchett GC, Martin RD, Zhang JH. The effect of granulocyte-colony stimulating factor in global cerebral ischemia in rats. Brain Res. 2007;1136(1):200–207. doi: 10.1016/j.brainres.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khatibi NH, Jadhav V, Saidi M, Chen W, Martin R, Stier G, Tang J, Zhang JH. Granulocyte colony-stimulating factor treatment provides neuroprotection in surgically induced brain injured mice. Acta Neurochir Suppl. 2011;111:265–269. doi: 10.1007/978-3-7091-0693-8_44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimozaki K, Nakajima K, Hirano T, Nagata S. Involvement of STAT3 in the granulocyte colony-stimulating factor-induced differentiation of myeloid cells. J Biol Chem. 1997;272(40):25184–25189. doi: 10.1074/jbc.272.40.25184. [DOI] [PubMed] [Google Scholar]
- 58.Hubel K, Engert A. Clinical applications of granulocyte colony-stimulating factor: an update and summary. Ann Hematol. 2003;82(4):207–213. doi: 10.1007/s00277-003-0628-y. [DOI] [PubMed] [Google Scholar]
- 59.Hubel K, Engert A. Granulocyte transfusion therapy for treatment of infections after cytotoxic chemotherapy. Onkologie. 2003;26(1):73–79. doi: 10.1159/000069868. [DOI] [PubMed] [Google Scholar]