Abstract
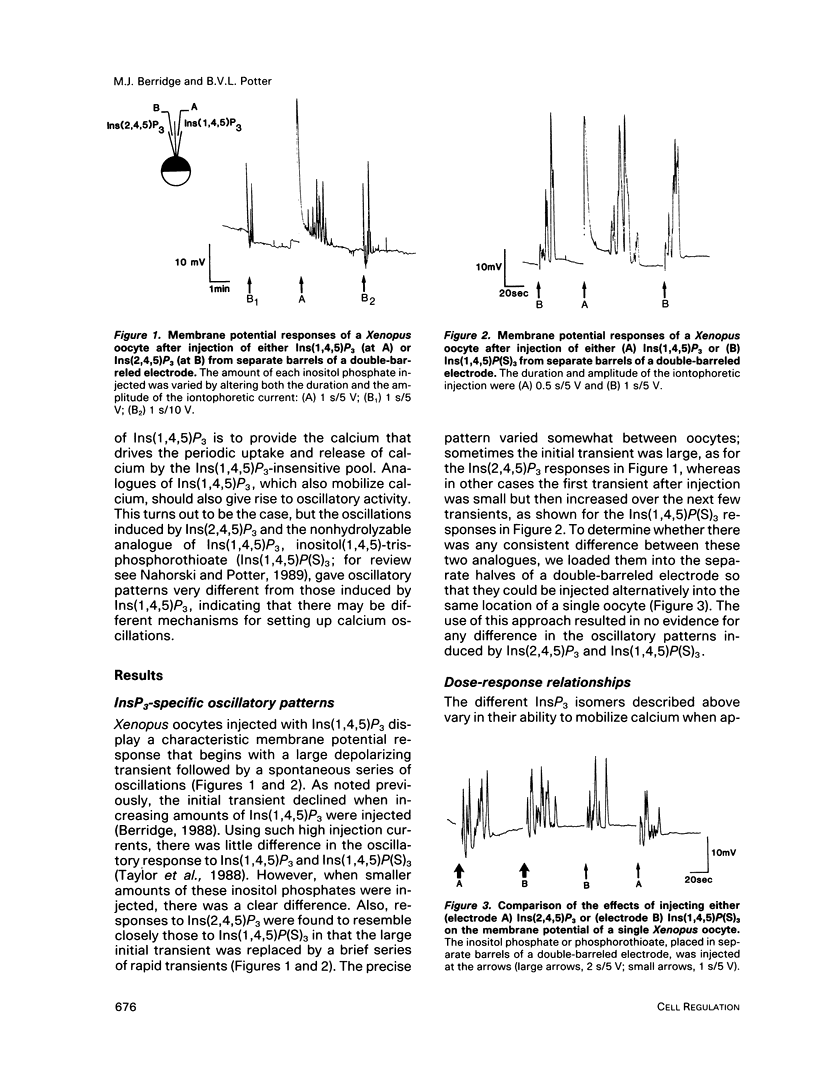
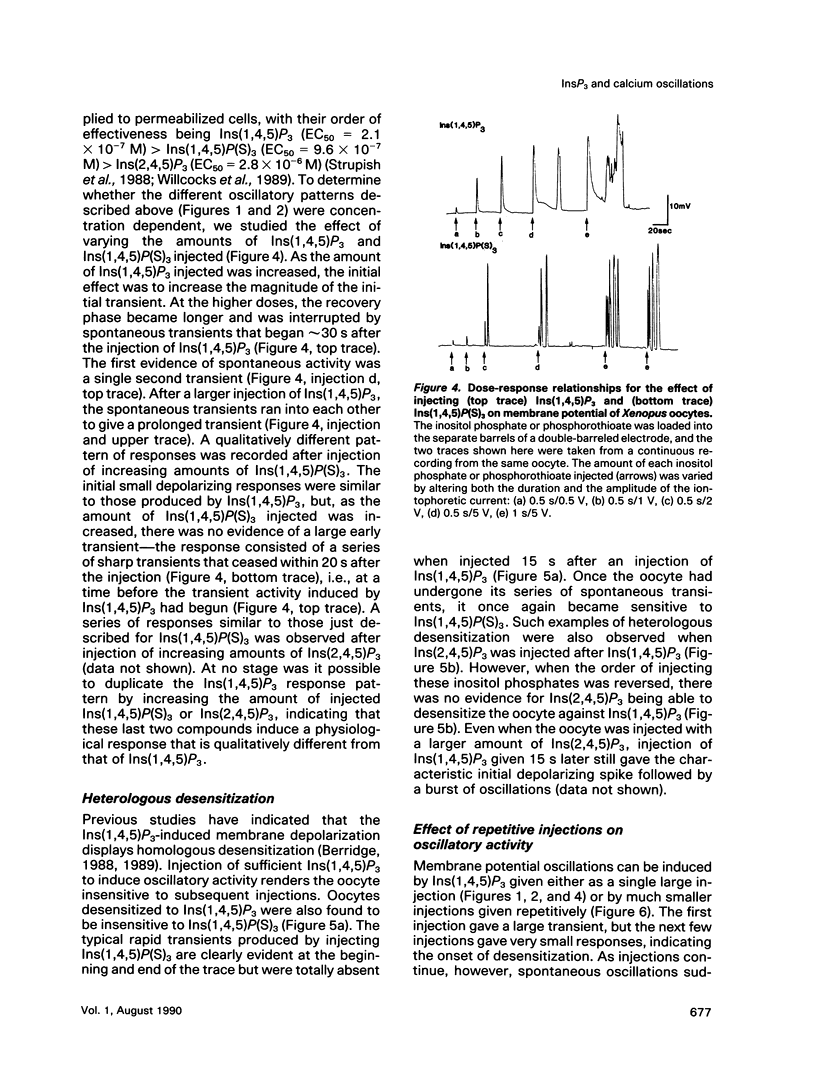
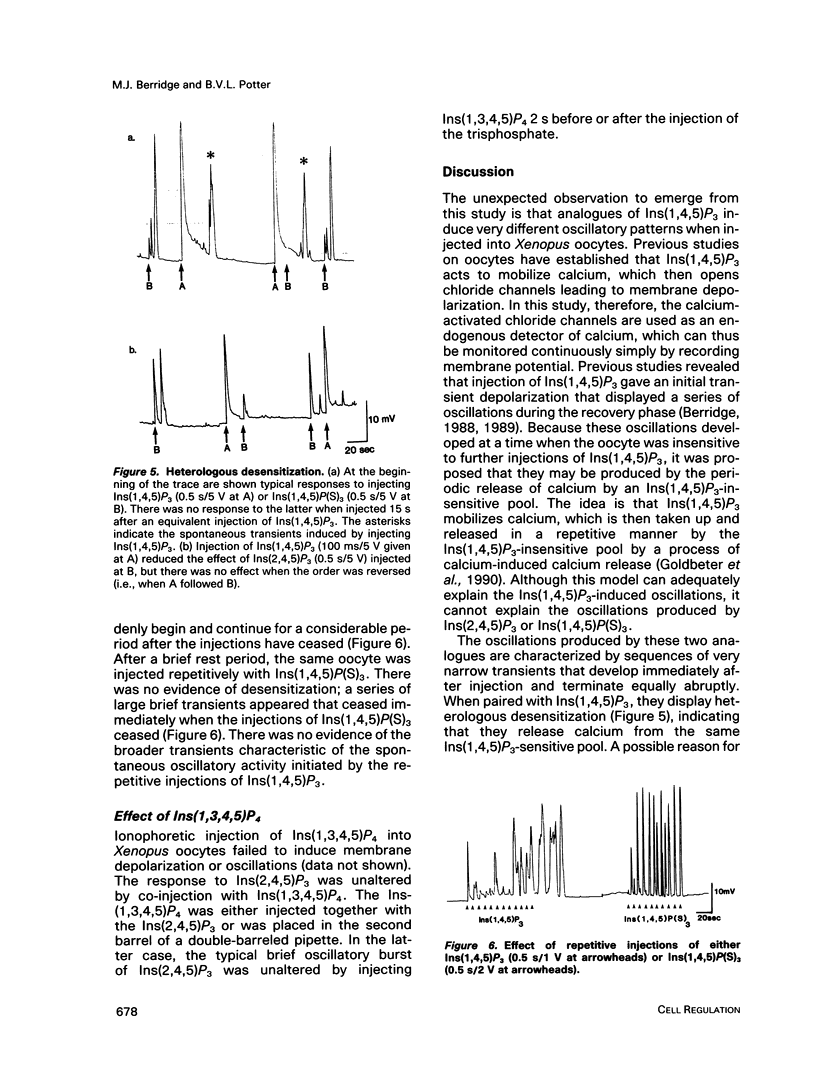
Agonists that utilize the calcium-mobilizing second messenger inositol(1,4,5)trisphosphate Ins(1,4,5)P3 usually generate oscillations in intracellular calcium. Such oscillations, based on the periodic release of calcium from the endoplasmic reticulum, can also be induced by injecting cells with Ins(1,4,5)P3. The mechanism responsible for oscillatory activity was studied in Xenopus oocytes by injecting them with different inositol trisphosphates. The plasma membrane of Xenopus oocytes has calcium-dependent chloride channels that open in response to calcium, leading to membrane depolarization. Oscillations in calcium were thus monitored by recording membrane potential. The naturally occurring Ins(1,4,5)P3 produced a large initial transient followed by a single transient or a burst of oscillations. By contrast, two analogues (Ins(2,4,5)P3 and Ins(1,4,5)P(S)3) produced a different oscillatory pattern made up of a short burst of sharp transients. Ins(1,3,4,5)P4 had no effect when injected by itself, and it also failed to modify the oscillatory responses to either Ins(2,4,5)P3 or Ins(1,4,5)P(S)3. Both analogues failed to induce a response when injected immediately after the initial Ins(1,4,5)P3-induced response, indicating that they act on the same intracellular pool of calcium. The existence of different oscillatory patterns suggests that there may be different mechanisms for setting up calcium oscillations. The Ins(2,4,5)P3 and Ins(1,4,5)P(S)3 analogues may initiate oscillations through a negative feedback mechanism whereby calcium inhibits its own release. The two-pool model is the most likely mechanism to describe the Ins(1,4,5)P3-induced oscillations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol 1,4,5-trisphosphate-induced calcium mobilization is localized in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1989 Dec 22;238(1292):235–243. doi: 10.1098/rspb.1989.0079. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Melo J. R., Dean N. M., Ali H., Beaven M. A. Formation of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate from inositol 1,3,4,5-tetrakisphosphate and their pathways of degradation in RBL-2H3 cells. J Biol Chem. 1988 Oct 5;263(28):14245–14250. [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höer D., Kwiatkowski A., Seib C., Rosenthal W., Schultz G., Oberdisse E. Degradation of inositol 1,3,4,5-tetrakisphosphates by porcine brain cytosol yields inositol 1,3,4-trisphosphate and inositol 1,4,5-trisphosphate. Biochem Biophys Res Commun. 1988 Jul 29;154(2):668–675. doi: 10.1016/0006-291x(88)90191-x. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium dependent inositol trisphosphate-induced calcium release in the guinea-pig taenia caeci. Biochem Biophys Res Commun. 1987 Jan 15;142(1):47–52. doi: 10.1016/0006-291x(87)90449-9. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M., Pollock W. K., Smith P. M., Wreggett K. A. Inositol phosphates: proliferation, metabolism and function. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):281–298. doi: 10.1098/rstb.1988.0077. [DOI] [PubMed] [Google Scholar]
- Jean T., Klee C. B. Calcium modulation of inositol 1,4,5-trisphosphate-induced calcium release from neuroblastoma x glioma hybrid (NG108-15) microsomes. J Biol Chem. 1986 Dec 15;261(35):16414–16420. [PubMed] [Google Scholar]
- Joseph S. K., Rice H. L., Williamson J. R. The effect of external calcium and pH on inositol trisphosphate-mediated calcium release from cerebellum microsomal fractions. Biochem J. 1989 Feb 15;258(1):261–265. doi: 10.1042/bj2580261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski S. R., Potter B. V. Molecular recognition of inositol polyphosphates by intracellular receptors and metabolic enzymes. Trends Pharmacol Sci. 1989 Apr;10(4):139–144. doi: 10.1016/0165-6147(89)90165-x. [DOI] [PubMed] [Google Scholar]
- Oberdisse E., Nolan R. D., Lapetina E. G. Thrombin and phorbol ester stimulate inositol 1,3,4,5-tetrakisphosphate 3-phosphomonoesterase in human platelets. J Biol Chem. 1990 Jan 15;265(2):726–730. [PubMed] [Google Scholar]
- Ohya Y., Terada K., Yamaguchi K., Inoue R., Okabe K., Kitamura K., Hirata M., Kuriyama H. Effects of inositol phosphates on the membrane activity of smooth muscle cells of the rabbit portal vein. Pflugers Arch. 1988 Sep;412(4):382–389. doi: 10.1007/BF01907556. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. Injection of inositol 1,3,4,5-tetrakisphosphate into Xenopus oocytes generates a chloride current dependent upon intracellular calcium. Proc R Soc Lond B Biol Sci. 1987 Oct 22;232(1266):59–70. doi: 10.1098/rspb.1987.0061. [DOI] [PubMed] [Google Scholar]
- Payne R., Walz B., Levy S., Fein A. The localization of calcium release by inositol trisphosphate in Limulus photoreceptors and its control by negative feedback. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):359–379. doi: 10.1098/rstb.1988.0082. [DOI] [PubMed] [Google Scholar]
- Prentki M., Glennon M. C., Thomas A. P., Morris R. L., Matschinsky F. M., Corkey B. E. Cell-specific patterns of oscillating free Ca2+ in carbamylcholine-stimulated insulinoma cells. J Biol Chem. 1988 Aug 15;263(23):11044–11047. [PubMed] [Google Scholar]
- Snyder P. M., Krause K. H., Welsh M. J. Inositol trisphosphate isomers, but not inositol 1,3,4,5-tetrakisphosphate, induce calcium influx in Xenopus laevis oocytes. J Biol Chem. 1988 Aug 15;263(23):11048–11051. [PubMed] [Google Scholar]
- Strupish J., Cooke A. M., Potter B. V., Gigg R., Nahorski S. R. Stereospecific mobilization of intracellular Ca2+ by inositol 1,4,5-triphosphate. Comparison with inositol 1,4,5-trisphosphorothioate and inositol 1,3,4-trisphosphate. Biochem J. 1988 Aug 1;253(3):901–905. doi: 10.1042/bj2530901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K., Igusa Y., Miyazaki S. Evidence for an inhibitory effect of protein kinase C on G-protein-mediated repetitive calcium transients in hamster eggs. EMBO J. 1989 Dec 1;8(12):3711–3718. doi: 10.1002/j.1460-2075.1989.tb08546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Berridge M. J., Brown K. D., Cooke A. M., Potter B. V. DL-myo-inositol 1,4,5-trisphosphorothioate mobilizes intracellular calcium in Swiss 3T3 cells and Xenopus oocytes. Biochem Biophys Res Commun. 1988 Jan 29;150(2):626–632. doi: 10.1016/0006-291x(88)90438-x. [DOI] [PubMed] [Google Scholar]
- Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989 May 25;339(6222):317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Willcocks A. L., Strupish J., Irvine R. F., Nahorski S. R. Inositol 1:2-cyclic,4,5-trisphosphate is only a weak agonist at inositol 1,4,5-trisphosphate receptors. Biochem J. 1989 Jan 1;257(1):297–300. doi: 10.1042/bj2570297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule D. I., Gallacher D. V. Oscillations of cytosolic calcium in single pancreatic acinar cells stimulated by acetylcholine. FEBS Lett. 1988 Nov 7;239(2):358–362. doi: 10.1016/0014-5793(88)80951-7. [DOI] [PubMed] [Google Scholar]


