Abstract
Sickle cell disease (SCD) is a hemoglobinopathy that affects one in 500 African Americans. Although it is well established that patients with SCD have left ventricular (LV) diastolic dysfunction, it is not clear whether they have subtle LV systolic dysfunction despite preserved ejection fraction (EF). We used three-dimensional speckle tracking echocardiography (3D STE) to assess changes in both systolic and diastolic LV function in SCD.
Methods
Transthoracic real-time 3D images were obtained (Philips iE33) in 56 subjects, including 28 stable outpatients with SCD (age 33±7 years) and 28 normal controls (age 35±9 years). 3D-STE was performed using prototype software (4DLV Analysis, TomTec) to obtain LV volume and deformation time-curves, from which indices of systolic and diastolic LV function were calculated.
Results
In SCD patients, 3D-STE derived LV filling parameters were significantly different from normal controls, reflecting an increase in both rapid and atrial filling volumes and prolonged active relaxation, depicted by a decrease in filling volume fractions at fixed times and an increase in rapid filling duration. Global LV systolic function was not only preserved but increased compared to controls, as reflected by significantly increased global longitudinal strain. Importantly, twist angle and torsion as well as radial and circumferential components of 3D strain were similar in both groups.
Conclusions
3D-STE was able to confirm diastolic dysfunction, as expected in some patients with SCD. However, 3D-STE strain analysis did not reveal any changes in LV systolic function. These findings provide novel insight into the pathophysiology of the cardiovascular complications of SCD.
Keywords: three-dimensional echocardiography, left ventricle, diastole, ventricular function
Introduction
Sickle cell disease (SCD) is a hemoglobinopathy that affects one in 500 African Americans. The cardiovascular complications associated with this disease are underappreciated because these patients are not routinely evaluated by cardiologists and because of the lack of experience with older SCD patients, whose survival rates have significantly improved in recent decades [1]. Accordingly, the effects of SCD on the cardiovascular system need to be better understood in order to optimally manage these patients. It is well established that patients with SCD frequently develop left ventricular (LV) diastolic dysfunction [2, 3] and/or pulmonary hypertension [4], both of which are independent predictors of mortality [2, 4]. Although in the majority of these patients overall LV systolic performance is preserved [5, 6], there may be subtle changes in LV contractility that may be caused by microvascular occlusions due to red blood cell sickling, as is known to occur in other organ systems. These changes may not necessarily manifest themselves early in the disease process as a reduced ejection fraction, but rather as abnormalities in individual components of myocardial deformation [7–10].
In the past decade, multiple studies have demonstrated the ability of echocardiography to accurately assess myocardial deformation in a variety of disease states. Specifically, tissue Doppler imaging (DTI) and more recently two-dimensional (2D) speckle tracking echocardiography (STE) were used to detect subtle changes in LV systolic function in the presence of preserved ejection fraction [7–10], and also changes in LV filling [11, 12]. Since real-time three-dimensional (3D) echocardiography (RT3DE) has evolved as a useful modality for the imaging of cardiac chambers [13], its ability to provide accurate estimates of LV volume throughout the cardiac cycle has been well established [14–17], including the ability to quantitatively evaluate LV filling. Moreover, recent developments in RT3DE imaging technology allow automated tracking and dynamic measurements of not only LV volume, but also of myocardial deformation [18].
Accordingly, we hypothesized that 3D speckle tracking echocardiography (3D-STE) could be used to identify both LV systolic and diastolic dysfunction in patients with SCD who had preserved LV ejection fraction (EF), as reflected by changes in both myocardial deformation and LV filling. To test this hypothesis, we compared 3D-STE derived indices of systolic and diastolic LV function in adult patients with SCD and in normal subjects.
Methods
Study population
Sixty-one patients were initially screened for the study, and 5 subjects in whom adequate endocardial visualization was not achieved in at least 2 of the 16 LV segments were excluded. We studied 56 subjects, including 28 stable African American outpatients with SCD (age 33±7 years, 12 males) and 28 normal African American controls (age 35±9 years, 13 males). All SCD patients were free of vaso-occlusive crisis at least one month prior to imaging. The clinical characteristics of the patients with SCD are shown in Table 1, whereas the conventional echocardiographic indices of LV systolic and diastolic function of the entire study group are summarized in Table 2. The protocol was approved by the Institutional Review Board, and each subject provided informed consent.
Table 1.
Clinical characteristic of the 28 patients with sickle cell disease (data refers to events over lifetime).
| mean±SD | |
|---|---|
| Body surface area (m2) | 1.73±0.16 |
| Systolic blood pressure (mmHg) | 120±13 |
| Diastolic blood pressure (mmHg) | 67±10 |
| Heart rate (bpm) | 73±16 |
| Hemoglobin (gr/dL) | 7.8±1.5 |
| Creatinine (mg/dL) | 0.7±0.2 |
| Glomerular filtration rate (mL/min) | 101±23 |
| median [range] | |
| Blood transfusions | 19 [3–82] |
| Sickle cell crises/year | 5 [0–30] |
| # of patients | |
| Hemoglobin SS | 23 |
| Iron overload | 14 |
| History of acute chest syndrome | 18 |
| Iron chelation | 7 |
| Hydroxyurea | 15 |
| Exchange transfusion | 5 |
Table 2.
Conventional echocardiographic indices of LV systolic and diastolic function shown for the normal controls and the patients with sickle cell disease (SCD).
| EDV(ml) | EF(%) | E (cm/s) | A (cm/s) | Mitral E/A | DT(ms) | e' (cm/s) | E/e' | LAVi(ml/m2) | |
|---|---|---|---|---|---|---|---|---|---|
| Normal (N=28) | 158 ± 36 | 66 ± 9 | 77 ± 21 | 63 ± 19 | 1.7 ± 0.7 | 188 ± 41 | 12.5 ± 2.1 | 6.7 ± 1.5 | 23 ± 7 |
| SCD (N=28) | 235 ± 68 * | 67 ± 10 | 99 ± 33 * | 47 ± 12 * | 1.6 ± 0.6 | 207 ± 53 | 11.2 ± 2.7 * | 9.0 ± 2.8 * | 46 ± 18* |
p<0.05 SCD versus Normal.
Abbreviations: EDV – end-diastolic volume, ESV – end-systolic volume, EF – ejection fraction, DT – mitral flow deceleration time, LAVi – left atrial volume index, PASP – peak pulmonary arterial systolic pressure.
Echocardiographic imaging
Transthoracic imaging was performed by an experienced cardiac sonographer using an iE33 cardiac ultrasound system (Phillips Healthcare, Andover, Massachusetts) with the patient in the left lateral decubitus position. First, standard 2D and Doppler images were acquired using the S5 transducer. Then, RT3DE datasets were acquired using the X5 matrix-array transducer from the apical position. To ensure inclusion of the entire left ventricle within the pyramidal scan volume, datasets were acquired using the wide-angled full-volume mode over four consecutive cardiac cycles during a single breath-hold. After gain settings were optimized for endocardial visualization, three datasets were obtained for each patient.
Image analysis
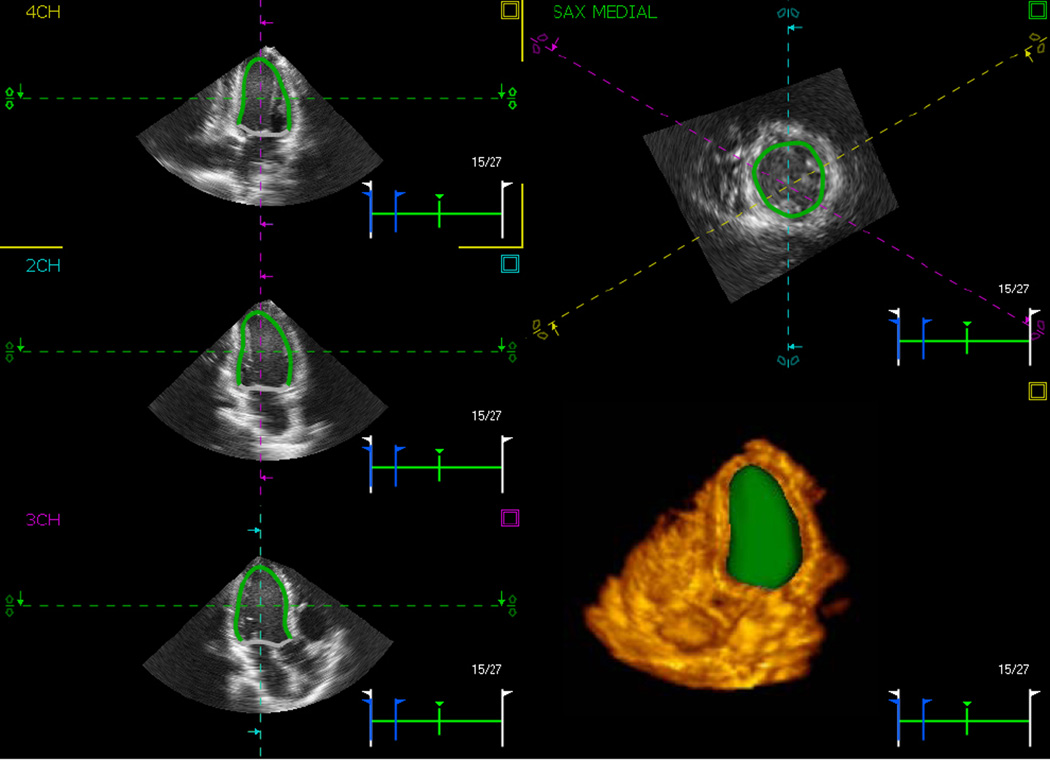
2D and Doppler images were reviewed and analyzed off-line by an experienced investigator using the Xcelera workstation (Philips) to obtain standard measurements of left ventricular and atrial volumes, mitral flow E/A ratio and deceleration time, as well as septal and lateral mitral annular velocities to assess LV diastolic function according to the recent guidelines of the American Society of Echocardiography [19]. LV ejection fraction (EF) was calculated from the end-systolic and end-diastolic volumes (ESV, EDV) using standard formula. The best RT3DE dataset with minimal amount of drop-out was analyzed using pre-commercial prototype software which utilizes 3D-STE (4D LV Analysis, TomTec, Unterschleissheim, Germany). After the LV long axis was manually aligned in three apical views (four-, three-, and two-chamber), the software automatically identified the LV endocardial border, while including the papillary muscles in the LV cavity, and tracked it throughout the cardiac cycle, resulting in a dynamic cast of the LV cavity (figure 1). Endocardial contours were manually adjusted when necessary to optimize boundary position and tracking. Finally, a time-curve of LV volume was obtained. In addition, using the standard 16-segment model, time-curves of segmental 3D strain, as well as its 3 principal components (longitudinal, radial and circumferential) were obtained. All time curves were interpolated using the standard cubic spline technique, resulting in effective temporal resolutions of 150–200 samples per second. These data were saved digitally for further analysis.
Figure 1.
3D speckle tracking analysis. Example of three apical (left) and one short-axis views (right, top) of the left ventricle with the automatically detected endocardial borders. These borders are tracked throughout the cardiac cycle, resulting in a dynamic LV endocardial cast (right, bottom), from which volume time curve is obtained. In addition, three components of 3D myocardial strain are calculated in 16 segments and reported over time as well. See text for details.
3D-STE indices of systolic function
The following parameters of LV deformation were obtained using the TomTec analysis software: (1) global longitudinal strain (GLS), (2) twist angle, defined as the maximum difference between basal and apical rotation, and (3) torsion, defined as the twist angle divided by the end-diastolic LV length. In addition, 3D strain and its radial, longitudinal and circumferential components were averaged over the 16 segments. The peak value of each index was defined as its maximum absolute value with the sign.
3D-STE indices of diastolic function
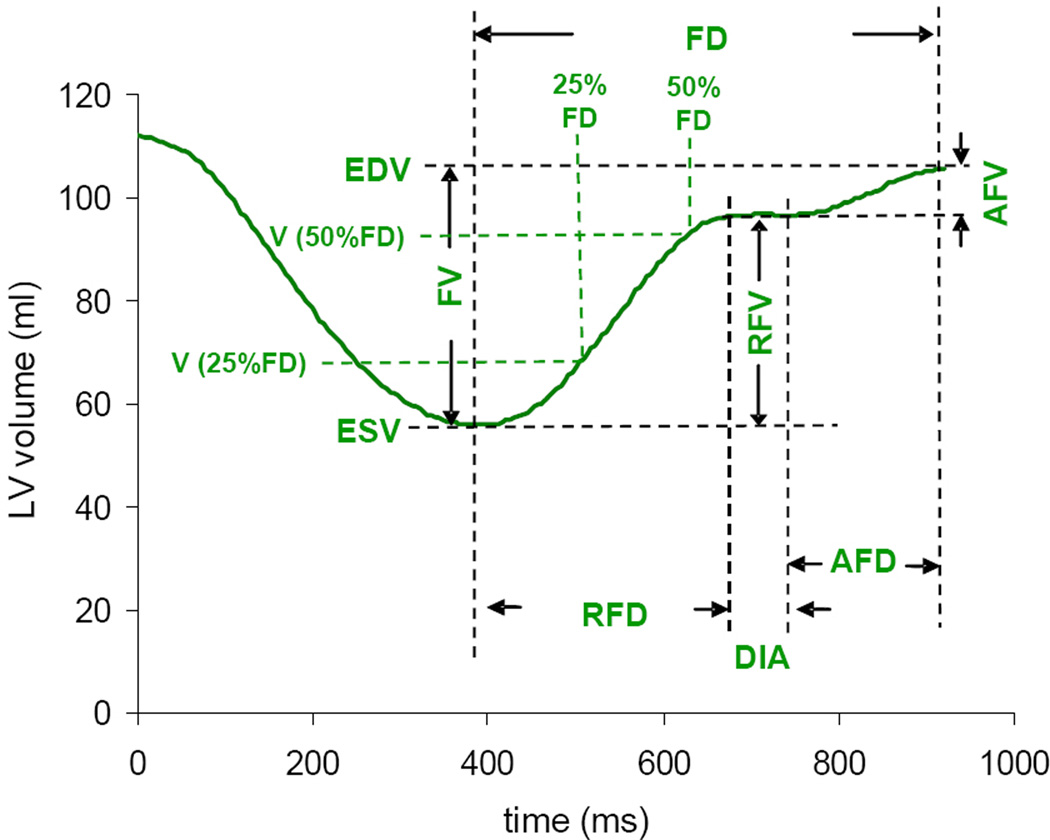
Volume time curves were analyzed using Microsoft Excel software. First, the time derivative of the LV volume was calculated in order to determine the timing of the different phases of the cardiac cycle, including rapid filling, diastasis and atrial filling. Using these data, LV volume curves were analyzed (figure 2) to measure the following parameters of LV filling: rapid and atrial filling volumes (RFV, AFV), and volumes at 25 and 50% of LV filling duration (FD) which were expressed in percent of end-diastolic volume, and referred to as volume index, LVVi at 25 and 50% of FD.
Figure 2.
Volume time curve based analysis of LV function. Detailed analysis of the diastolic portion of the LV volume time curve provides a variety of indices of LV filling, including: filling volume (FV) and filling duration (FD), rapid filling volume and duration (RFV, RFD), atrial filling volume and duration (AFV, AFD), diastasis (DIA), as well as filling volumes at 25 and 50% of FD.
Statistical analysis
For each parameter, unpaired two-tailed student’s t-tests were used to test differences between patients with SCD and normal controls. P-values <0.05 were considered significant.
Reproducibility analysis
To determine the reproducibility of the 3D-STE derived indices of systolic and diastolic function, image analysis was repeated by an independent observer in 30 study subjects, including randomly selected 15 patients with SCD and 15 controls. Inter-measurement variability was calculated for each index as an absolute difference between each pair of repeated measurements in percent of their mean, and averaged over the 30 subjects.
Results
The mean frame rate of the RT3DE datasets in our study subjects was 18±3 frames per second. Table 2 shows the non-STE derived echocardiographic parameters in the two study groups. All study subjects were in sinus rhythm. Of the 28 patients with SCD, 7 had normal diastolic function, 7 had grade I, 11 had grade II and 3 had grade III diastolic dysfunction using the current classification guidelines recommended by the ASE [19]. All normal controls had normal diastolic function, when evaluated using the same criteria.
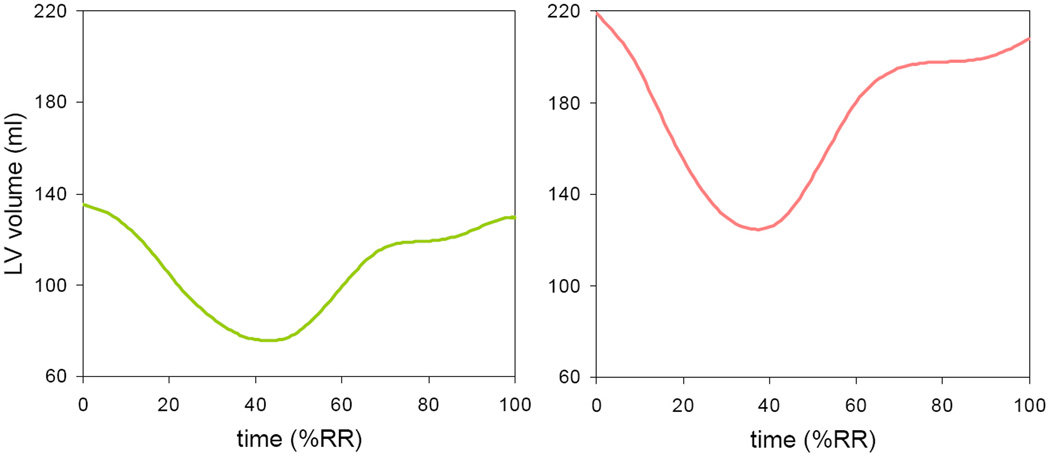
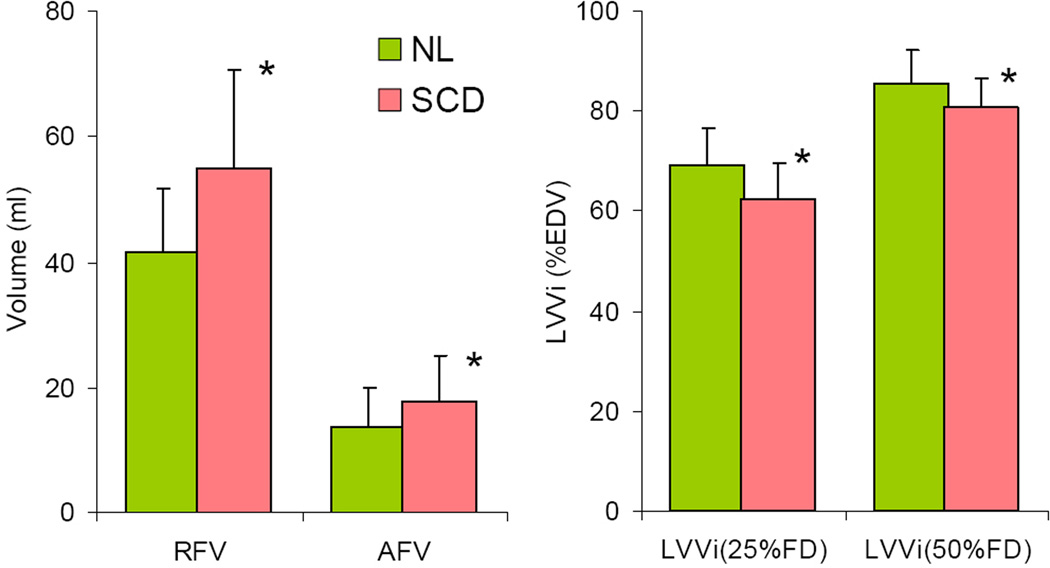
Figure 3 shows examples of LV volume curves obtained in a normal control subject and a patient with SCD. These curves depicted the increased volumes noted in patients with SCD (Table 2). Despite the fact that LV volume time-curves had similar general shape in subjects with SCD and normal controls, indices of LV diastolic function derived from these curves were significantly different in the two study groups (figure 4). Both rapid and atrial filling volumes were increased in patients with SCD compared to controls, in keeping with the increased LV volumes. In addition, LV volume index at 25 and 50% of FD was decreased in patients with SCD compared to controls (Table 3), reflecting a decrease in the rate of rapid filling.
Figure 3.
Examples of LV volume over time throughout the cardiac cycle from 0 to 100% of the RR interval, obtained in a normal control subject (left) and a patient with SCD (right). See text for details.
Figure 4.
Summary of RT3DE indices of LV diastolic function extracted from the volume time curves, averaged for the normal controls and the patients with SCD, including rapid and atrial filling volumes (RFV, AFV), as well as volumes at 25 and 50% of filling duration (FD) normalized by the end-diastolic volume (EDV). *p<0.05. See text for details.
Table 3.
3D-STE derived indices of diastolic (top) and systolic (bottom) LV function measured in a group of 28 patients with sickle cell disease (SCD) and 28 normal controls.
| Normal | SCD | ||
|---|---|---|---|
| RFV (ml) | 42 ± 10 | 56 ± 15* | |
| AFV (ml) | 14 ± 6 | 18 ± 7* | |
| LVVi (25%FD) | 69 ± 7 | 62 ± 7* | |
| LVVi (50%FD) | 85 ± 7 | 81 ± 6* | |
| 3D strain | GLS (%) | −14.2 ± 2.9 | −16.0 ± 2.9* |
| Twist angle (°) | 8.8 ± 4.6 | 8.2 ± 5.0 | |
| Torsion (°/cm) | 1.14 ± 0.65 | 1.06 ± 0.69 | |
| --Longitudinal (%) | −15.9 ± 2.8 | −17.2 ± 2.3* | |
| --Radial (%) | 70 ± 21 | 78 ± 20 | |
| --Circumferential (%) | −19.8 ± 3.0 | −20.7 ± 3.6 | |
Abbreviations: RFV, AFV – rapid and atrial filling volumes; LVVi(25%FD), LVVi(50%FD) – LV volumes at 25 and 50% time of total filling duration (FD), normalized by end-diastolic volumes; GLS – global longitudinal strain;
p<0.05.
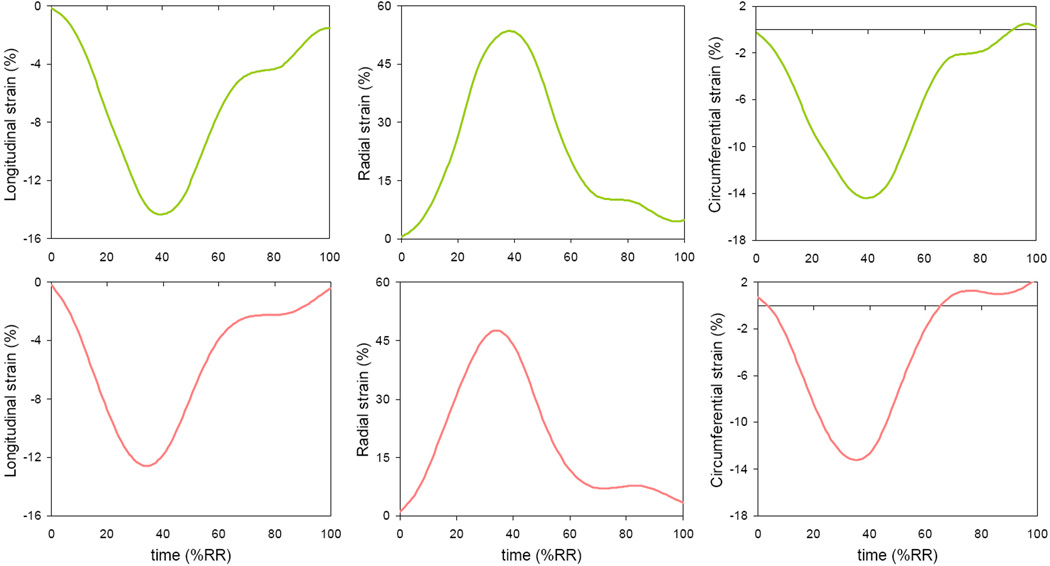
Global LV systolic function was not only preserved in patients with SCD (Table 2), but increased compared to controls, as reflected by a significant increase in GLS (Table 3). Figure 5 shows longitudinal, radial and circumferential components of the 3D-STE derived strain obtained in a normal subject and a patient with SCD. As expected, the shape of the strain curves was similar to that of LV volume curves, except that the radial strain curves were inverted. In other words, radial strain was positive throughout the cardiac cycle, reflecting a systolic increase in myocardial thickness, while the longitudinal and circumferential strains were negative reflecting systolic shortening of the ventricle in these two dimensions. Importantly, there were no obvious differences in the strain curves between the patient with SCD and the normal subject shown in figure 5. Mean values of the 3D-STE derived indices of LV systolic function also showed no significant differences between the two groups for either twist angle or torsion (Table 3). In addition, patients with SCD showed a trend towards an increase in both radial and circumferential strains.
Figure 5.
Examples of time curves of the longitudinal, radial and circumferential components of 3D strain obtained in the same two subjects as in figure 3: a normal control subject (top) and a patient with SCD (bottom). See text for details.
Table 4 summarizes the results of the reproducibility analysis of both systolic and diastolic 3D-STE derived indices. While diastolic indices were reasonably reproducible, systolic deformation indices generally showed higher levels of variability. Among these systolic indices, the longitudinal components were the most reproducible, while the radial components showed higher levels of both inter- and intra-observer variability, and twist angle and torsion were the least reproducible.
Table 4.
Intra- and inter-observer variability of the 3D-STE derived indices of diastolic (top) and systolic (bottom) function, expressed in percent of the mean of each pair of repeated measurements. Values in the table represent mean±SD of a group of 30 randomly selected study subjects (15 normals and 15 patients with SCD).
| Inter-observer variabilty (%) |
Intra-observer variabilty (%) |
||
|---|---|---|---|
| RFV | 15 ± 17 | 17 ± 21 | |
| AFV | 23 ± 20 | 22 ± 15 | |
| LVVi (25%FD) | 10 ± 8 | 7 ± 6 | |
| LVVi (50%FD) | 5 ± 4 | 4 ± 4 | |
| 3D strain | GLS | 17 ± 12 | 10 ± 8 |
| Twist angle | 57 ± 37 | 58 ± 40 | |
| Torsion | 58 ± 40 | 59 ± 40 | |
| --Longitudinal | 17 ± 10 | 10 ± 8 | |
| --Radial | 30 ± 30 | 30 ± 29 | |
| --Circumferential | 24 ± 11 | 9 ± 9 | |
Abbreviations: RFV, AFV – rapid and atrial filling volumes; LVVi(25%FD), LVVi(50%FD) – LV volumes at 25 and 50% time of total filling duration (FD), normalized by end-diastolic volumes; GLS – global longitudinal strain.
Discussion
This is the first study to use 3D STE for the evaluation of systolic and diastolic LV function in patients with SCD. Several studies have used tissue Doppler imaging and/or 2D-STE to assess myocardial strain in a variety of cardiac disorders [20–23]. Since tissue Doppler methodology is known to be angle-dependent [24], and 2D-STE can only track motion within the imaging plane, while through-plane motion adversely affects tracking when speckles move out of the imaging plane [25, 26], the accuracy of these techniques is limited. In contrast, 3D-STE is likely better suited for the assessment of myocardial deformation, because it is mostly angle-independent and moving speckles remain in the imaging volume and can thus be tracked from frame to frame.
Accordingly, our hypothesis was that this novel methodology could identify not only diastolic dysfunction known to be present in patients with SCD, but also detect subtle changes in systolic myocardial deformation, which may presumably be present in these patients despite preserved EF. If confirmed, such early signs of systolic dysfunction could have potentially be used to predict which patients are likely to develop more overt systolic dysfunction. To test this hypothesis, we studied a group of patients with moderate to severe SCD, as evidenced by the high number of patients experiencing episodes of acute chest syndrome, hepatic iron overload, and the large number of blood transfusions and sickle cell crises per year (Table 1).
We used prototype software that tracks ultrasound speckles frame-by-frame in 3D space to reconstruct a beating endocardial surface, from which LV volume over time is calculated [17], and also to measure the three principal components of myocardial strain throughout the cardiac cycle [27]. We analyzed the diastolic portion of the LV volume time-curves to identify the timing of events during LV filling, including rapid filling and atrial contraction, and to measure LV volumes at different phases of LV filling, as well as the duration of rapid and atrial filling. The systolic function was assessed using myocardial deformation indices derived by 3D-STE, which is capable of separating the different spatial components of strain.
Because in patients with SCD, the left ventricle is frequently enlarged, LV volumes measured at different phases of LV filling were normalized by end-diastolic LV volume, thus eliminating the confounding effects of LV dilatation. In addition, to eliminate the confounding effects of differences in heart rate, rapid and atrial filing duration indices were heart-rate adjusted (i.e. expressed in %RR rather than milliseconds).
Our 3D-STE based LV volume analysis confirmed the known differences in LV dimensions between patients with SCD and normal subjects, in agreement with previous studies [6]. In addition, this analysis revealed differences in LV filling patterns, specifically a decrease in the rate of rapid filling (figure 4), thus confirming the presence of diastolic dysfunction in the SCD group. Of note, the majority of these patients (21 of 28, 75%) had diastolic dysfunction based on current ASE guidelines using 2D and Doppler criteria, and the prevalence of diastolic dysfunction was considerably higher than reported in previous studies using older criteria (approximately 20%) [2, 3]. Assuming that this difference was due to an increase in left atrial volume, which may be unrelated to diastolic dysfunction in patients with SCD, we reclassified our patients using previous criteria, including E/A<1 indicating abnormal relaxation, E/A>1 indicating pseudo-normalized filling patterns if either septal e’ velocity or lateral e’ velocity was abnormally low: <8 cm/sec and <10 cm/sec, respectively [18]. Interestingly, the result was that the incidence of diastolic dysfunction was the same (21/28), despite the fact that septal e’ velocity could potentially be affected by right ventricular dysfunction. However, two patients were reclassified. On the other hand, when requiring both septal and lateral e’ velocities to be abnormal for the diagnosis of diastolic dysfunction, only 9/28 patients had diastolic dysfunction. While the high prevalence of diastolic dysfunction may reflect the advanced stages of SCD in our cohort, our findings also indicate that the choice of criteria is crucial in terms of determining the prevalence of diastolic dysfunction in this population, which affects predicting outcomes in individual patients. This issue will need to be further evaluated in future studies.
As in several prior studies that reported normal systolic function in patients with SCD [5, 6], in our patients, global systolic function was not only preserved as evidenced by the conventional echocardiographic indices (Table 2), but even increased when compared to normal controls, as reflected by a significant increase in global longitudinal strain (Table 3). This can probably be explained by compensatory mechanisms within the cardiovascular system aimed at maintaining adequate oxygenation in the anemic patients [28]. However, our analysis did not confirm the hypothesis of possible subtle changes in systolic LV function, as reflected by similar values in both radial and rotational myocardial deformation (including circumferential strain, twist angle and torsion) in patients with SCD and a normal control group of similar age and gender. This is despite the fact that our patients had advanced SCD.
Of note, the wide inter-measurement variability of the radial and rotational deformation indices (Table 4) may raise concerns regarding the reliability of these measurements. This variability is likely the result of suboptimal tracking, which depends on image quality and may indeed affect the calculated strains. One may expect however, that with the continuing improvement in RT3DE image quality and the fine-tuning of tracking algorithms, the reproducibility of 3D-STE strain measurements would improve as well.
Limitations
Despite the recent improvements in RT3DE image quality and frame rates, the two limitations of the 3D-STE technique are still the relatively low temporal and spatial resolution, compared to the 2D techniques previously used to study myocardial deformation. Both these factors are likely to affect the frame-to-frame correlation of local image features, and thus affect the quality of tracking. However, the issue of low temporal resolution was partially addressed by interpolating the time curves and thus effectively reducing noise and allowing analysis that relies on their time derivatives.
One limitation of our study population is that the SCD group is mixed in terms of presence and degree of diastolic dysfunction, resulting in mean values of some of the diastolic indices that are not significantly different from the normal controls. However, our SCD group was not large enough to be subdivided according to the severity of diastolic dysfunction.
One might view as a limitation of our study the lack of an independent reference technique to compare the 3D-STE derived measurements. However, 3D echocardiography derived regional LV volume time curves have been validated against cardiac magnetic resonance [14]. As far as myocardial strain is concerned, there is no noninvasive “gold standard” technique that can be used in humans to validate strain in three dimensions. Although cardiac magnetic resonance imaging is widely used as a reference technique for LV function, it is not a true 3D imaging technique, but a fundamentally 2D, albeit a multislice, imaging modality. In addition, there are no widely accepted tools to quantify radial, longitudinal and circumferential strain from cardiac magnetic resonance images.
Conclusion
This is the first study that used current RT3DE technology, which allows the combined quantitative evaluation of myocardial deformation and LV filling, to study patients with SCD. Our findings confirmed the presence of diastolic dysfunction in these patients, as demonstrated by prolonged rapid filling. However, this novel analysis of principal LV strains has not confirmed the hypothesis that there may be subtle systolic dysfunction that does not manifest in global parameters of LV performance. These findings not only contribute to the growing understanding of the cardiovascular pathophysiology in SCD, but suggest that the RT3DE assessment of LV diastolic dysfunction may become a useful addition to the current criteria recommended by the ASE guidelines.
Acknowledgments
Disclosures:
EG is a recipient of a research grant from the Fondation pour la Recherche Medicale (FRM), Paris, France. CY received a fellowship from the Department of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok Thailand
Footnotes
None of the other authors have any potential conflicts of interest to disclose.
References
- 1.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N.Engl.J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 2.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–479. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankins JS, McCarville MB, Hillenbrand CM, et al. Ventricular diastolic dysfunction in sickle cell anemia is common but not associated with myocardial iron deposition. Pediatr.Blood Cancer. 2010;55:495–500. doi: 10.1002/pbc.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N.Engl.J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 5.Batra AS, Acherman RJ, Wong WY, et al. Cardiac abnormalities in children with sickle cell anemia. Am J Hematol. 2002;70:306–312. doi: 10.1002/ajh.10154. [DOI] [PubMed] [Google Scholar]
- 6.Westwood MA, Shah F, Anderson LJ, et al. Myocardial tissue characterization and the role of chronic anemia in sickle cell cardiomyopathy. J Magn Reson Imaging. 2007;26:564–568. doi: 10.1002/jmri.21018. [DOI] [PubMed] [Google Scholar]
- 7.Gorcsan J, III, Strum DP, Mandarino WA, Gulati VK, Pinsky MR. Quantitative assessment of alterations in regional left ventricular contractility with color-coded tissue Doppler echocardiography. Comparison with sonomicrometry and pressure-volume relations. Circulation. 1997;95:2423–2433. doi: 10.1161/01.cir.95.10.2423. [DOI] [PubMed] [Google Scholar]
- 8.Kukulski T, Jamal F, Herbots L, et al. Identification of acutely ischemic myocardium using ultrasonic strain measurements. A clinical study in patients undergoing coronary angioplasty. J Am Coll Cardiol. 2003;41:810–819. doi: 10.1016/s0735-1097(02)02934-0. [DOI] [PubMed] [Google Scholar]
- 9.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313–1327. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 10.Bijnens B, Claus P, Weidemann F, Strotmann J, Sutherland GR. Investigating cardiac function using motion and deformation analysis in the setting of coronary artery disease. Circulation. 2007;116:2453–2464. doi: 10.1161/CIRCULATIONAHA.106.684357. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–1383. doi: 10.1161/CIRCULATIONAHA.106.662882. [DOI] [PubMed] [Google Scholar]
- 12.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol. 2008;101:1504–1509. doi: 10.1016/j.amjcard.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 13.Mor-Avi V, Sugeng L, Lang RM. Real-time 3-dimensional echocardiography: an integral component of the routine echocardiographic examination in adult patients? Circulation. 2009;119:314–329. doi: 10.1161/CIRCULATIONAHA.107.751354. [DOI] [PubMed] [Google Scholar]
- 14.Corsi C, Lang RM, Veronesi F, et al. Volumetric quantification of global and regional left ventricular function from real-time three-dimensional echocardiographic images. Circulation. 2005;112:1161–1170. doi: 10.1161/CIRCULATIONAHA.104.513689. [DOI] [PubMed] [Google Scholar]
- 15.Sugeng L, Mor-Avi V, Weinert L, et al. Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation. 2006;114:654–661. doi: 10.1161/CIRCULATIONAHA.106.626143. [DOI] [PubMed] [Google Scholar]
- 16.Mor-Avi V, Jenkins C, Kuhl HP, et al. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC.Cardiovasc Imaging. 2008;1:413–423. doi: 10.1016/j.jcmg.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Nesser HJ, Mor-Avi V, Gorissen W, et al. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: comparison with MRI. Eur Heart J. 2009;30:1565–1573. doi: 10.1093/eurheartj/ehp187. [DOI] [PubMed] [Google Scholar]
- 18.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Abraham TP, Belohlavek M, Thomson HL, et al. Time to onset of regional relaxation: feasibility, variability and utility of a novel index of regional myocardial function by strain rate imaging. J Am Coll Cardiol. 2002;39:1531–1537. doi: 10.1016/s0735-1097(02)01768-0. [DOI] [PubMed] [Google Scholar]
- 21.Voigt JU, Exner B, Schmiedehausen K, et al. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003;107:2120–2126. doi: 10.1161/01.CIR.0000065249.69988.AA. [DOI] [PubMed] [Google Scholar]
- 22.Park TH, Nagueh SF, Khoury DS, et al. Impact of myocardial structure and function postinfarction on diastolic strain measurements: implications for assessment of myocardial viability. Am J Physiol Heart Circ.Physiol. 2006;290:H724–H731. doi: 10.1152/ajpheart.00714.2005. [DOI] [PubMed] [Google Scholar]
- 23.Park SM, Miyazaki C, Prasad A, et al. Feasibility of prediction of myocardial viability with Doppler tissue imaging following percutaneous coronary intervention for ST elevation anterior myocardial infarction. J Am Soc Echocardiogr. 2009;22:183–189. doi: 10.1016/j.echo.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Burgess MI, Jenkins C, Chan J, Marwick TH. Measurement of left ventricular dyssynchrony in patients with ischaemic cardiomyopathy: a comparison of real-time three-dimensional and tissue Doppler echocardiography. Heart. 2007;93:1191–1196. doi: 10.1136/hrt.2006.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meunier J. Tissue motion assessment from 3D echographic speckle tracking. Phys.Med Biol. 1998;43:1241–1254. doi: 10.1088/0031-9155/43/5/014. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Xie H, Erkamp R, et al. 3-D correlation-based speckle tracking. Ultrason.Imaging. 2005;27:21–36. doi: 10.1177/016173460502700102. [DOI] [PubMed] [Google Scholar]
- 27.Maffessanti F, Nesser HJ, Weinert L, et al. Quantitative evaluation of regional left ventricular function using three-dimensional speckle tracking echocardiography in patients with and without heart disease. Am J Cardiol. 2009;104:1755–1762. doi: 10.1016/j.amjcard.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 28.Levine HJ, Wolk MJ, Keefe JF, Bing OH, Snow JA, Messer JV. Myocardial mechanics and energetics in experimental iron-deficiency anemia. Am J Physiol. 1977;232:H470–H477. doi: 10.1152/ajpheart.1977.232.5.H470. [DOI] [PubMed] [Google Scholar]