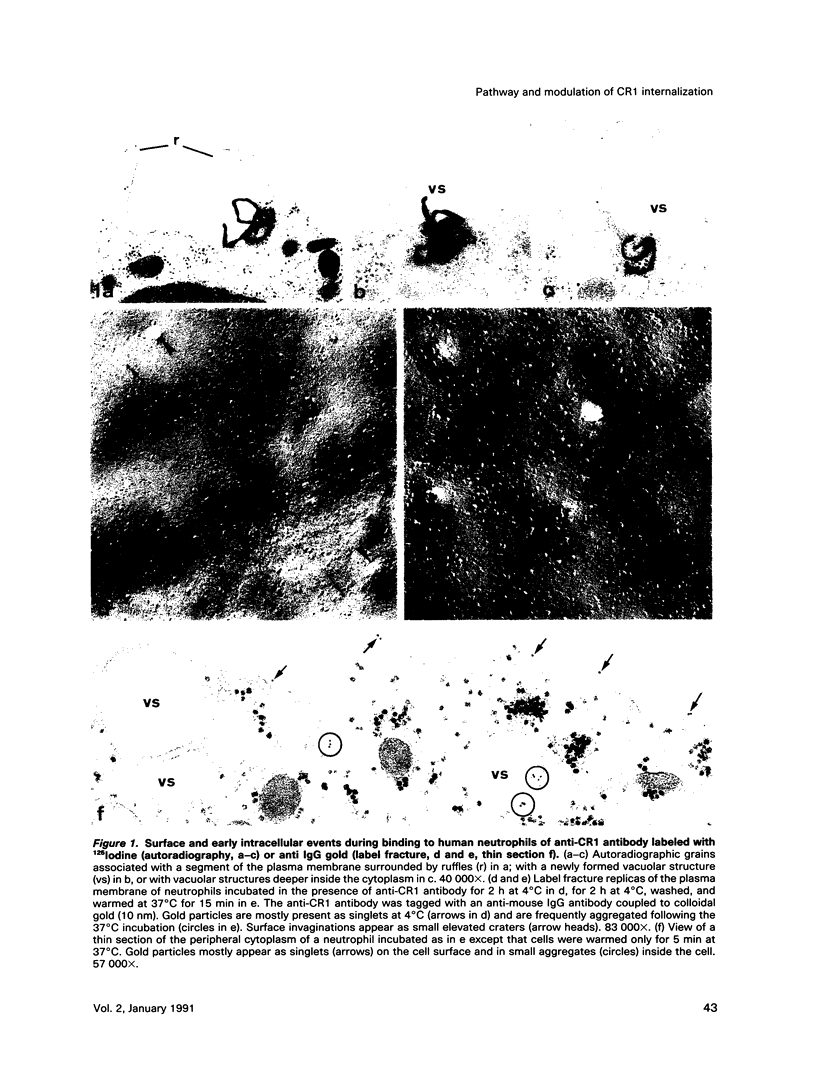
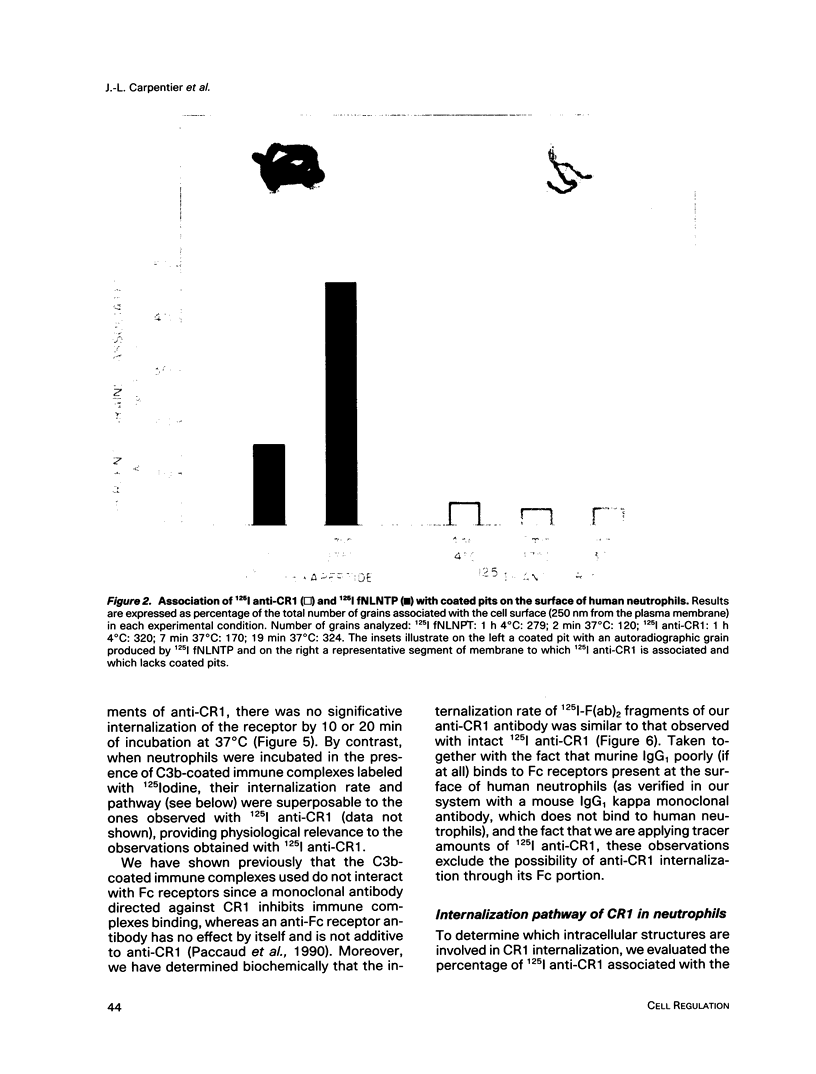
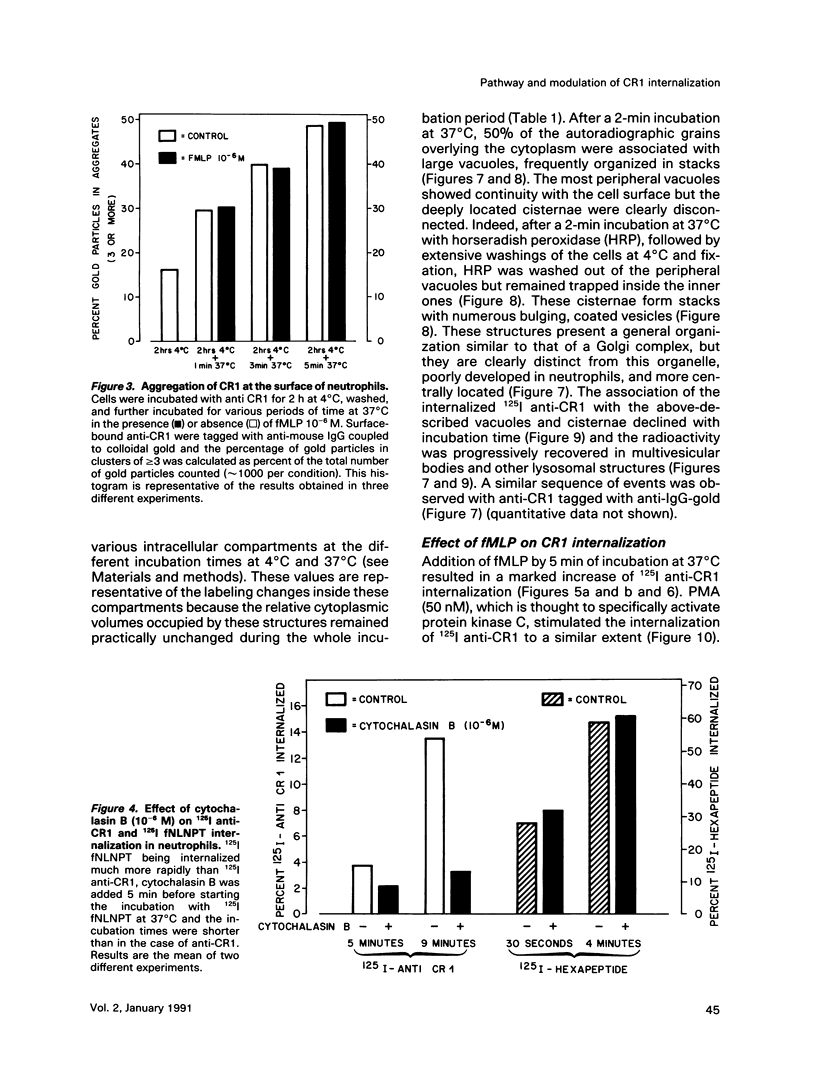
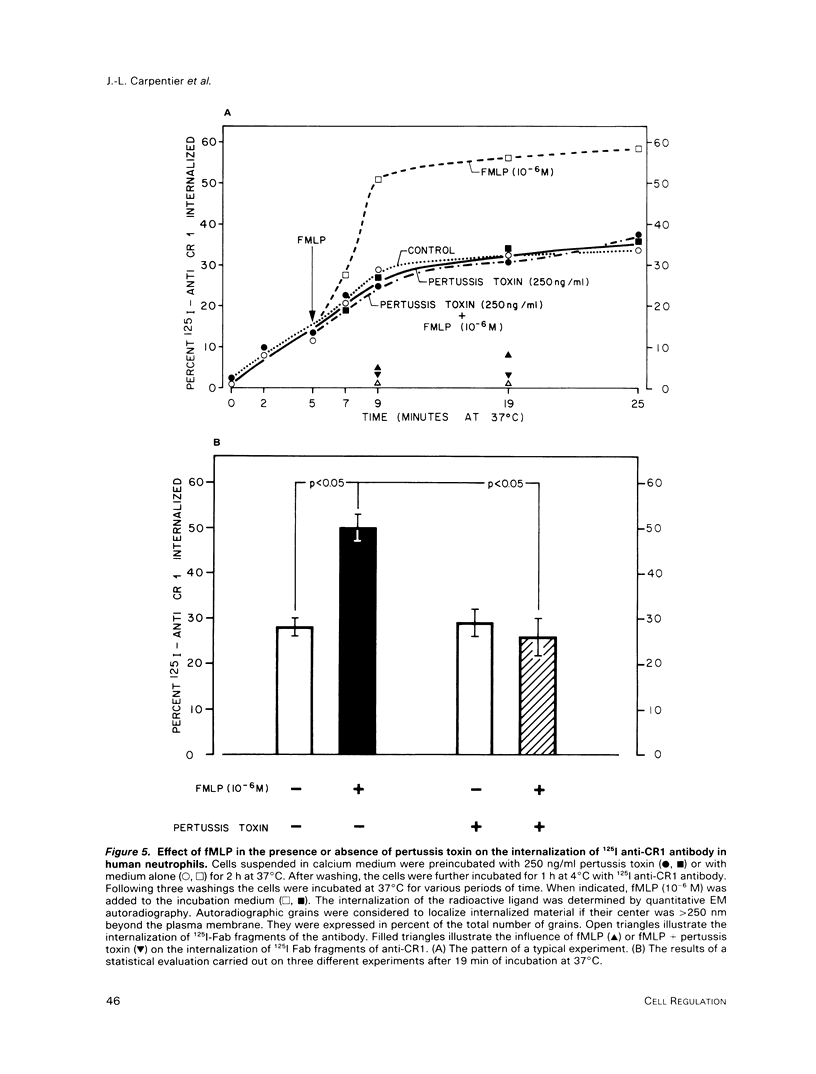
Abstract
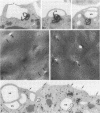
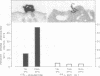
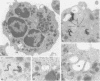
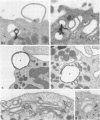
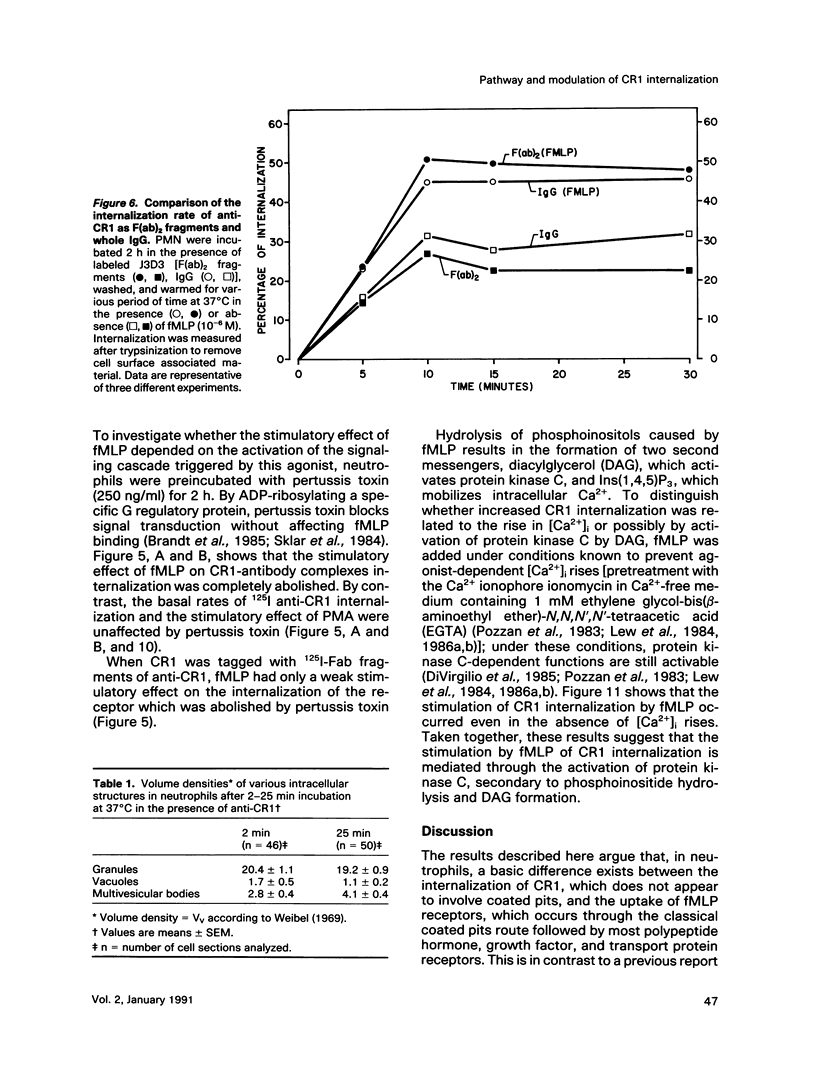
On the surface of phagocytes, C3b receptors (CR1) bind C3b-coated particles and promote their ingestion after activation by appropriate stimuli such as lymphokines or the chemoattractant formyl methionyl leucyl phenylalanine (fMLP) and fibronectin. The aims of the present study were 1) to define at the electron microscopic level the nature of the process responsible for CR1 internalization and 2) to dissect the mechanism by which a physiological activator (fMLP) stimulates this process. CR1 was visualized either by the immunogold technique or by quantitative electron microscopic autoradiography using a monoclonal anti-CR1 antibody. Both techniques revealed that after anti-CR1 binding, CR1 cluster on the neutrophil surface in a time-, temperature-, and antibody-dependent fashion, but do not concentrate in coated pits. CR1 internalization requires receptor cross-linking (does not occur in the presence of Fab fragments of anti-CR1) and intact microfilaments. It results in the association of the internalized material with large flattened vacuoles, organized in stacks. Together with the surface localization of CR1 close to cytoplasmic projections (ruffles), these observations suggest that uptake of CR1 occurs through a macropinocytotic process. Eventually, CR1 concentrate in lysosomal structures. fMLP markedly stimulates this pattern of CR1 internalization without affecting their clustering or their lack of association with coated pits. Stimulation by fMLP is inhibited by pertussis toxin, unaffected by preventing receptor-triggered cytosolic free calcium [Ca2+]i elevations, and mimicked by phorbol myristate acetate. Taken together our data demonstrate 1) that, in neutrophils, CR1 is internalized via a coated pit independent macropinocytotic process, dependent on intact microfilaments and receptor cross-linking; 2) that, in the same cells, fMLP is internalized via the classical coated pits pathway; and 3) that fMLP amplifies CR1 uptake possibly via protein kinase C stimulation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
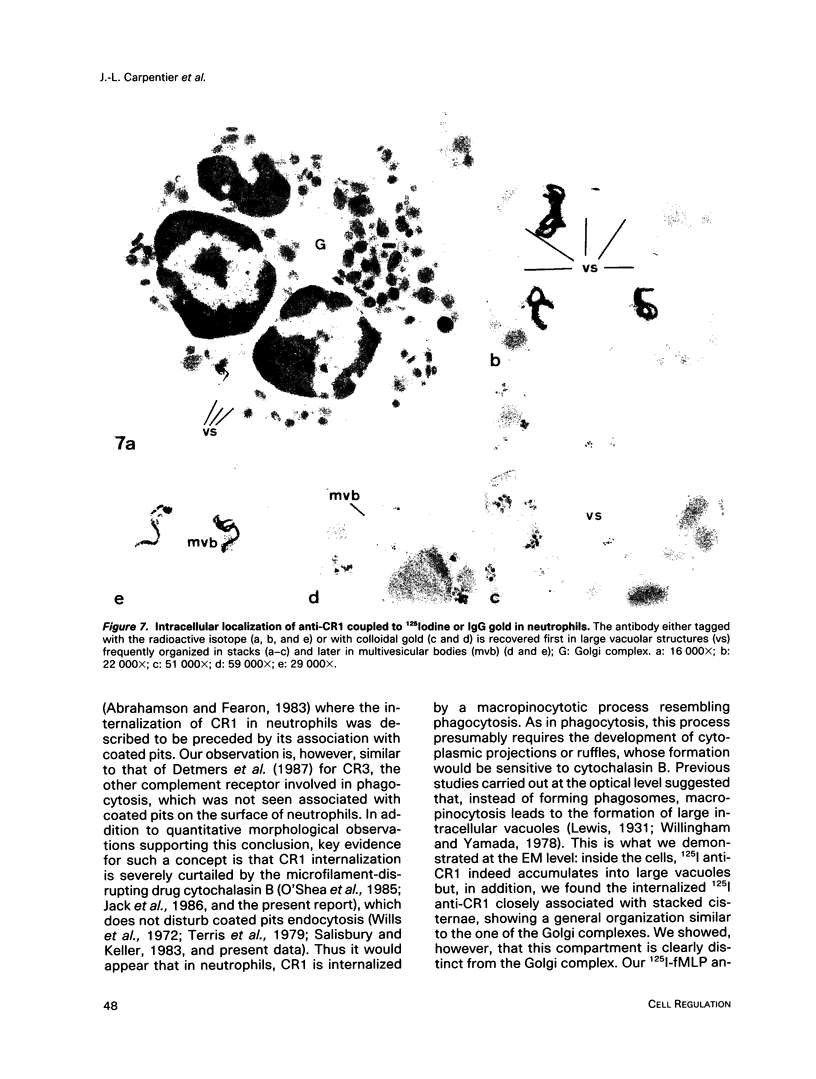
- Abrahamson D. R., Fearon D. T. Endocytosis of the C3b receptor of complement within coated pits in human polymorphonuclear leukocytes and monocytes. Lab Invest. 1983 Feb;48(2):162–168. [PubMed] [Google Scholar]
- Andersson T., Schlegel W., Monod A., Krause K. H., Stendahl O., Lew D. P. Leukotriene B4 stimulation of phagocytes results in the formation of inositol 1,4,5-trisphosphate. A second messenger for Ca2+ mobilization. Biochem J. 1986 Dec 1;240(2):333–340. doi: 10.1042/bj2400333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Differential control of azurophilic and specific granule exocytosis in Sendai-virus-permeabilized rabbit neutrophils. J Physiol. 1987 Feb;383:115–124. doi: 10.1113/jphysiol.1987.sp016399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J. The role of extracellular matrix proteins in the control of phagocytosis. J Leukoc Biol. 1986 May;39(5):579–591. doi: 10.1002/jlb.39.5.579. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Brown D., Iacopetta B., Orci L. Detection of surface-bound ligands by freeze-fracture autoradiography. J Cell Biol. 1985 Sep;101(3):887–890. doi: 10.1083/jcb.101.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Fehlmann M., Van Obberghen E., Gorden P., Orci L. Redistribution of 125I-insulin on the surface of rat hepatocytes as a function of dissociation time. Diabetes. 1985 Oct;34(10):1002–1007. doi: 10.2337/diab.34.10.1002. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Freychet P., Le Cam A., Orci L. Lysosomal association of internalized 125I-insulin in isolated rat hepatocytes. Direct demonstration by quantitative electron microscopic autoradiography. J Clin Invest. 1979 Jun;63(6):1249–1261. doi: 10.1172/JCI109420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Van Obberghen E., Gorden P., Orci L. Surface redistribution of 125I-insulin in cultured human lymphocytes. J Cell Biol. 1981 Oct;91(1):17–25. doi: 10.1083/jcb.91.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., White M. F., Orci L., Kahn R. C. Direct visualization of the phosphorylated epidermal growth factor receptor during its internalization in A-431 cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2751–2762. doi: 10.1083/jcb.105.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changelian P. S., Fearon D. T. Tissue-specific phosphorylation of complement receptors CR1 and CR2. J Exp Med. 1986 Jan 1;163(1):101–115. doi: 10.1084/jem.163.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changelian P. S., Jack R. M., Collins L. A., Fearon D. T. PMA induces the ligand-independent internalization of CR1 on human neutrophils. J Immunol. 1985 Mar;134(3):1851–1858. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Detmers P. A., Wright S. D., Olsen E., Kimball B., Cohn Z. A. Aggregation of complement receptors on human neutrophils in the absence of ligand. J Cell Biol. 1987 Sep;105(3):1137–1145. doi: 10.1083/jcb.105.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
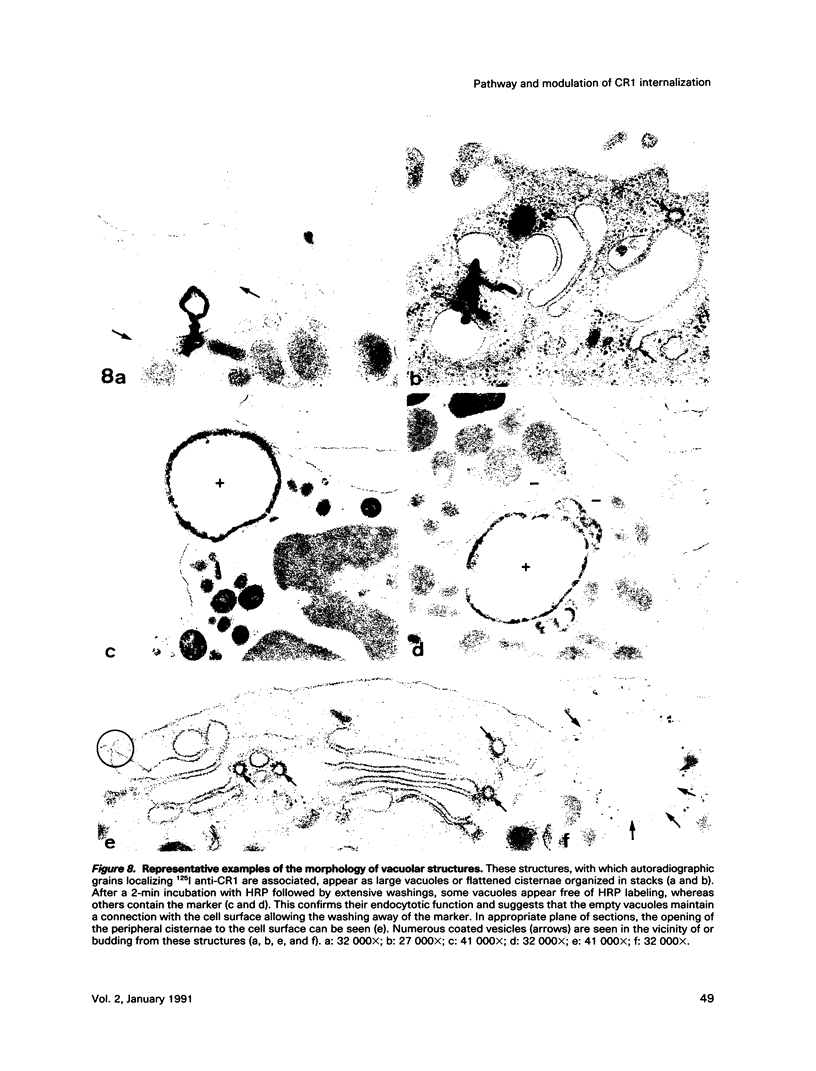
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Jack R. M., Ezzell R. M., Hartwig J., Fearon D. T. Differential interaction of the C3b/C4b receptor and MHC class I with the cytoskeleton of human neutrophils. J Immunol. 1986 Dec 15;137(12):3996–4003. [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
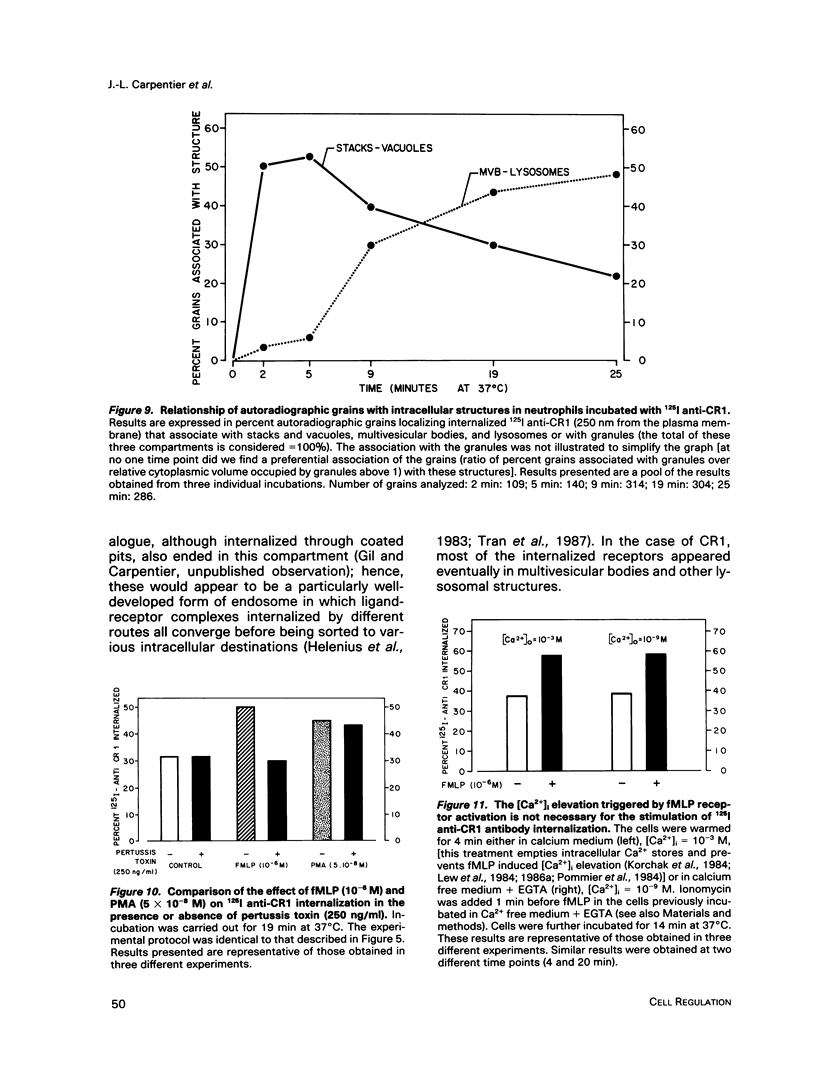
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Gaither T. A., Takahashi T., Frank M. M. Tumor-promoting phorbol esters induce rapid internalization of the C3b receptor via a cytoskeleton-dependent mechanism. J Immunol. 1985 Aug;135(2):1325–1330. [PubMed] [Google Scholar]
- Paccaud J. P., Carpentier J. L., Schifferli J. A. Difference in the clustering of complement receptor type 1 (CR1) on polymorphonuclear leukocytes and erythrocytes: effect on immune adherence. Eur J Immunol. 1990 Feb;20(2):283–289. doi: 10.1002/eji.1830200209. [DOI] [PubMed] [Google Scholar]
- Pauli B., Weinstein R. S., Soble L. W., Alroy J. Freeze-fracture of monolayer cultures. J Cell Biol. 1977 Mar;72(3):763–769. doi: 10.1083/jcb.72.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Kan F. W. Label-fracture: a method for high resolution labeling of cell surfaces. J Cell Biol. 1984 Sep;99(3):1156–1161. doi: 10.1083/jcb.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., O'Shea J., Chused T., Yancey K., Frank M. M., Takahashi T., Brown E. J. Studies on the fibronectin receptors of human peripheral blood leukocytes. Morphologic and functional characterization. J Exp Med. 1984 Jan 1;159(1):137–151. doi: 10.1084/jem.159.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Badwey J. A., Karnovsky M. L., Karnovsky M. J. Cell surface dynamics of neutrophils stimulated with phorbol esters or retinoids. J Cell Biol. 1987 Jul;105(1):417–426. doi: 10.1083/jcb.105.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J. L., Keller G. A. Structural investigations on the role of microfilaments in ligand translocation. Methods Enzymol. 1983;98:368–375. doi: 10.1016/0076-6879(83)98165-x. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G. The neutrophil N-formyl peptide receptor: dynamics of ligand-receptor interactions and their relationship to cellular responses. Contemp Top Immunobiol. 1984;14:29–82. doi: 10.1007/978-1-4757-4862-8_2. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Smith C. D., Verghese M. W. Model for leukocyte regulation by chemoattractant receptors: roles of a guanine nucleotide regulatory protein and polyphosphoinositide metabolism. J Leukoc Biol. 1986 Dec;40(6):785–800. doi: 10.1002/jlb.40.6.785. [DOI] [PubMed] [Google Scholar]
- Terris S., Hofmann C., Steiner D. F. Mode of uptake and degradation of 125I-labelled insulin by isolated hepatocytes and H4 hepatoma cells. Can J Biochem. 1979 Jun;57(6):459–468. doi: 10.1139/o79-059. [DOI] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M., Berger M. Intracellular degradation of the complement C3b/C4b receptor in the absence of ligand. J Biol Chem. 1988 Apr 5;263(10):4914–4920. [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S. A mechanism for the destruction of pinosomes in cultured fibroblasts. Piranhalysis. J Cell Biol. 1978 Aug;78(2):480–487. doi: 10.1083/jcb.78.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. J., Davies P., Allison A. C., Haswell A. D. Cytochalasin B fails to inhibit pinocytosis by macrophages. Nat New Biol. 1972 Nov 8;240(97):58–60. doi: 10.1038/newbio240058a0. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]