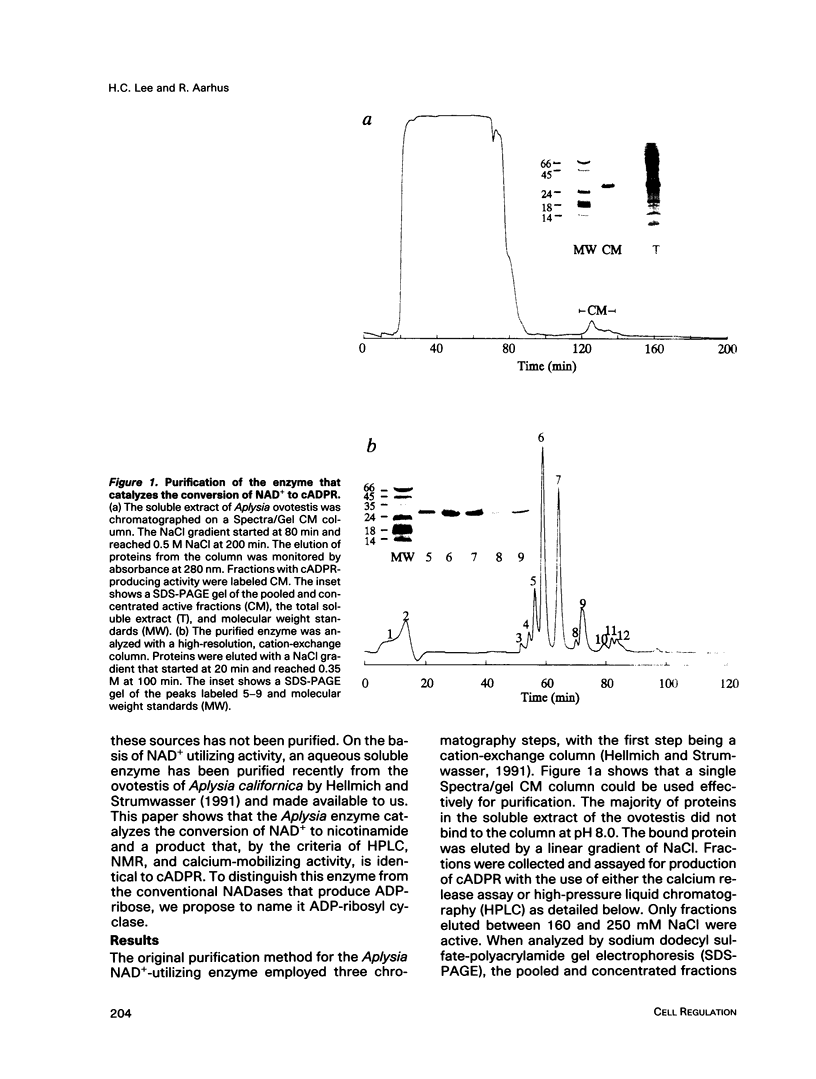
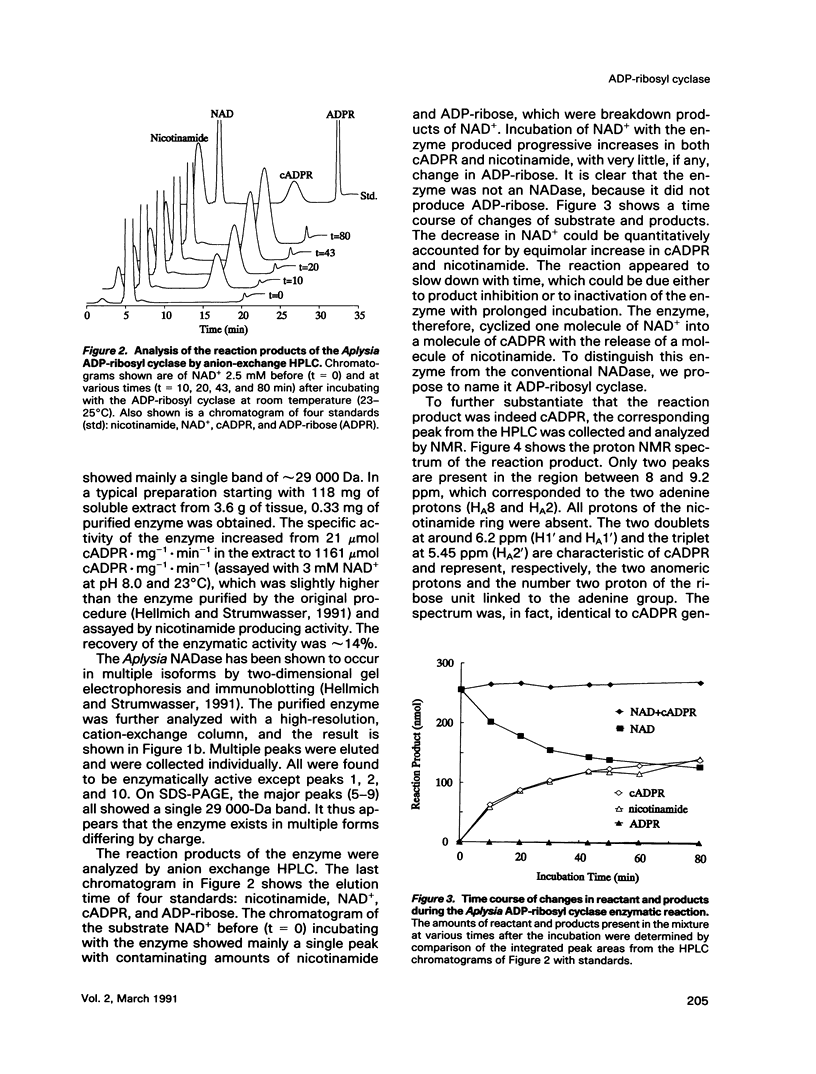
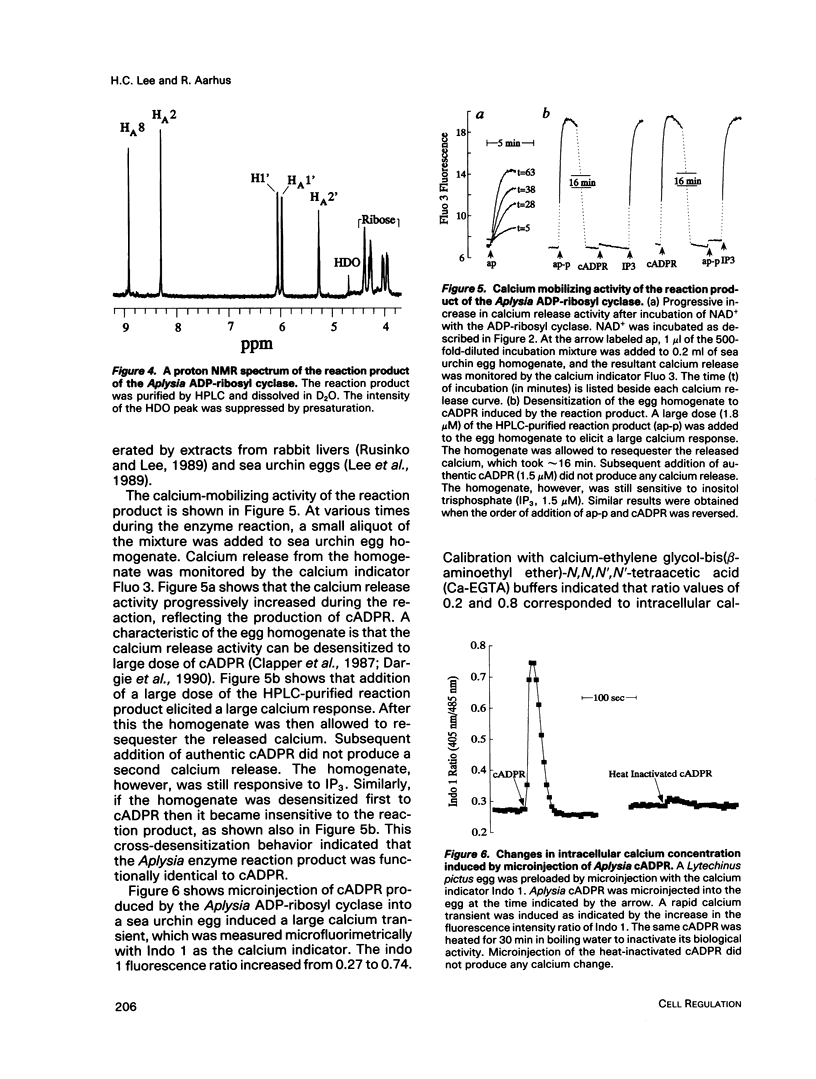
Abstract
Cyclic ADP-ribose (cADPR) is a metabolite of NAD+ that is as active as inositol trisphosphate (IP3) in mobilizing intracellular Ca2+ in sea urchin eggs. The activity of the enzyme responsible for synthesizing cADPR is found not only in sea urchin eggs but also in various mammalian tissue extracts, suggesting that cADPR may be a general messenger for Ca2+ mobilization in cells. An aqueous soluble enzyme, thought to be an NADase, has been purified recently from the ovotestis of Aplysia californica (Hellmich and Strumwasser, 1991). This paper shows that the Aplysia enzyme catalyzes the conversion of NAD+ to cADPR and nicotinamide. The Aplysia enzyme was purified by fractionating the soluble extract of Aplysia ovotestis on a Spectra/gel CM column. The purified enzyme appeared as a single band of approximately 29,000 Da on SDS-PAGE but could be further separated into multiple peaks by high-resolution, cation-exchange chromatography. All of the protein peaks had enzymatic activity, indicating that the enzyme had multiple forms differing by charge. Analysis of the reaction products of the enzyme by anion-exchange high-pressure liquid chromatography (HPLC) indicated no ADP-ribose was produced; instead, each mole of NAD+ was converted to equimolar of cADPR and nicotinamide. The identification of the product as cADPR was further substantiated by proton NMR and also by its Ca(2+)-mobilizing activity. Addition of the product to sea urchin egg homogenates induced Ca2+ release and desensitized the homogenate to authentic cADPR but not to IP3. Microinjection of the product into sea urchin eggs elicited Ca2+ transients as well as the cortical exocytosis reaction. Therefore, by the criteria of HPLC, NMR, and calcium-mobilizing activity, the product was identical to cADPR. To distinguish the Aplysia enzyme from the conventional NADases that produce ADP-ribose, we propose to name it ADP-ribosyl cyclase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelson J. T., Bodley J. W., Walseth T. F. A volatile liquid chromatography system for nucleotides. Anal Biochem. 1981 Sep 15;116(2):357–360. doi: 10.1016/0003-2697(81)90371-7. [DOI] [PubMed] [Google Scholar]
- Chow S. C., Jondal M. Polyunsaturated free fatty acids stimulate an increase in cytosolic Ca2+ by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in T cells through a mechanism independent of phosphoinositide turnover. J Biol Chem. 1990 Jan 15;265(2):902–907. [PubMed] [Google Scholar]
- Clapper D. L., Walseth T. F., Dargie P. J., Lee H. C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987 Jul 15;262(20):9561–9568. [PubMed] [Google Scholar]
- Dargie P. J., Agre M. C., Lee H. C. Comparison of Ca2+ mobilizing activities of cyclic ADP-ribose and inositol trisphosphate. Cell Regul. 1990 Feb;1(3):279–290. doi: 10.1091/mbc.1.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J., Gill D. L. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990 Jun 29;248(4963):1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Hellmich M. R., Strumwasser F. Purification and characterization of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991 Mar;2(3):193–202. doi: 10.1091/mbc.2.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C. Specific binding of cyclic ADP-ribose to calcium-storing microsomes from sea urchin eggs. J Biol Chem. 1991 Feb 5;266(4):2276–2281. [PubMed] [Google Scholar]
- Lee H. C., Walseth T. F., Bratt G. T., Hayes R. N., Clapper D. L. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989 Jan 25;264(3):1608–1615. [PubMed] [Google Scholar]
- Matozaki T., Göke B., Tsunoda Y., Rodriguez M., Martinez J., Williams J. A. Two functionally distinct cholecystokinin receptors show different modes of action on Ca2+ mobilization and phospholipid hydrolysis in isolated rat pancreatic acini. Studies using a new cholecystokinin analog, JMV-180. J Biol Chem. 1990 Apr 15;265(11):6247–6254. [PubMed] [Google Scholar]
- Rusinko N., Lee H. C. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989 Jul 15;264(20):11725–11731. [PubMed] [Google Scholar]
- Saluja A. K., Powers R. E., Steer M. L. Inositol trisphosphate independent increase of intracellular free calcium and amylase secretion in pancreatic acini. Biochem Biophys Res Commun. 1989 Oct 16;164(1):8–13. doi: 10.1016/0006-291x(89)91675-6. [DOI] [PubMed] [Google Scholar]
- Snyder P. M., Krause K. H., Welsh M. J. Inositol trisphosphate isomers, but not inositol 1,3,4,5-tetrakisphosphate, induce calcium influx in Xenopus laevis oocytes. J Biol Chem. 1988 Aug 15;263(23):11048–11051. [PubMed] [Google Scholar]
- Wilson D. B., Connolly T. M., Bross T. E., Majerus P. W., Sherman W. R., Tyler A. N., Rubin L. J., Brown J. E. Isolation and characterization of the inositol cyclic phosphate products of polyphosphoinositide cleavage by phospholipase C. Physiological effects in permeabilized platelets and Limulus photoreceptor cells. J Biol Chem. 1985 Nov 5;260(25):13496–13501. [PubMed] [Google Scholar]
- Wolf B. A., Turk J., Sherman W. R., McDaniel M. L. Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-trisphosphate in isolated pancreatic islets. J Biol Chem. 1986 Mar 15;261(8):3501–3511. [PubMed] [Google Scholar]