Abstract
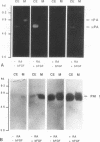
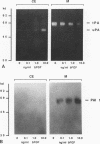
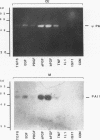
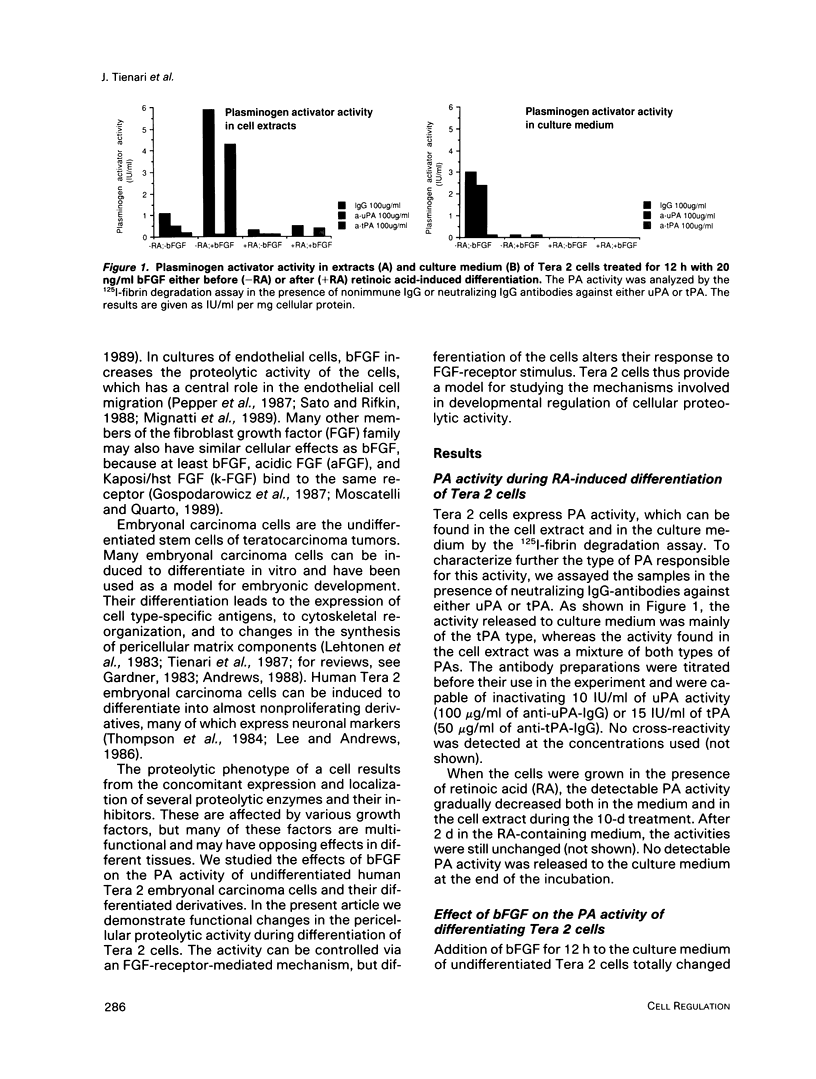
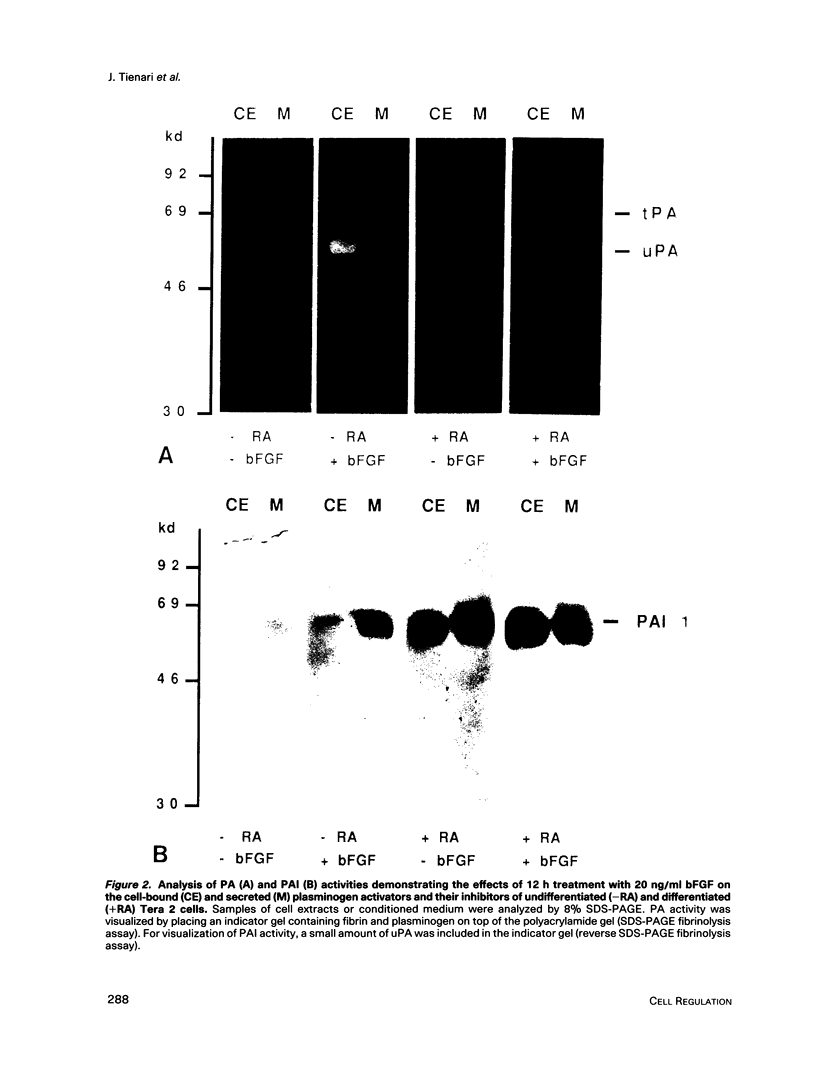
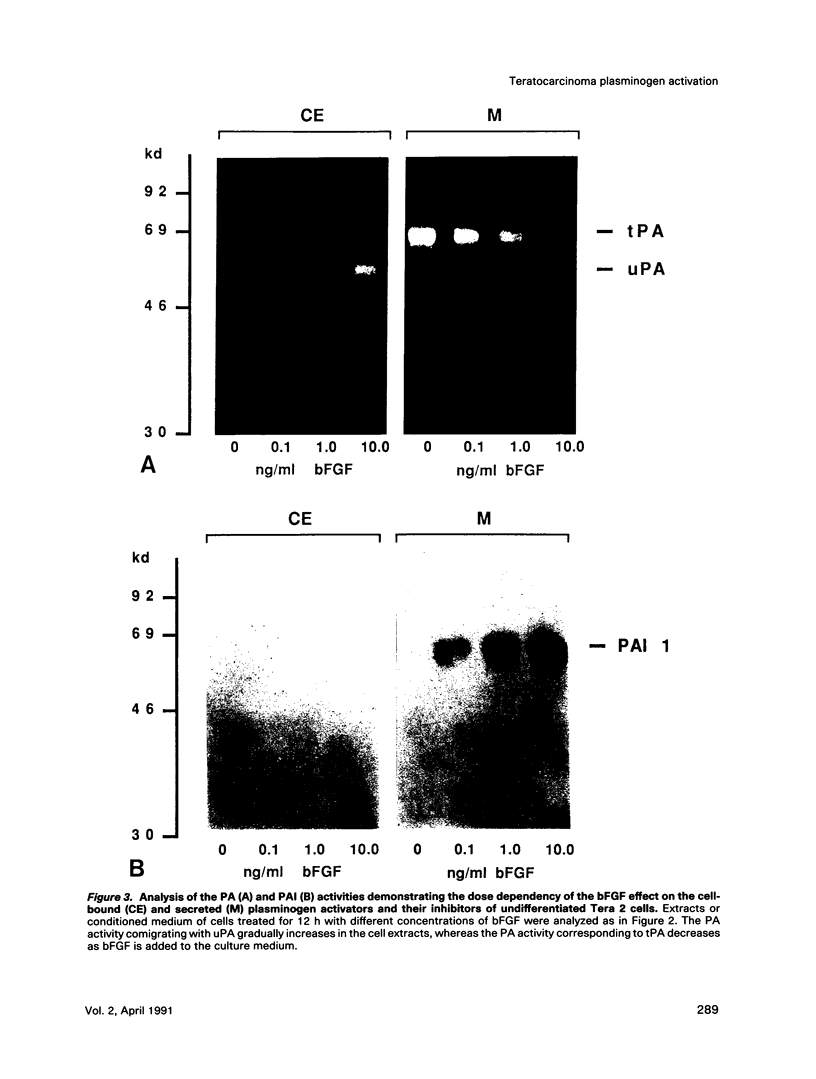
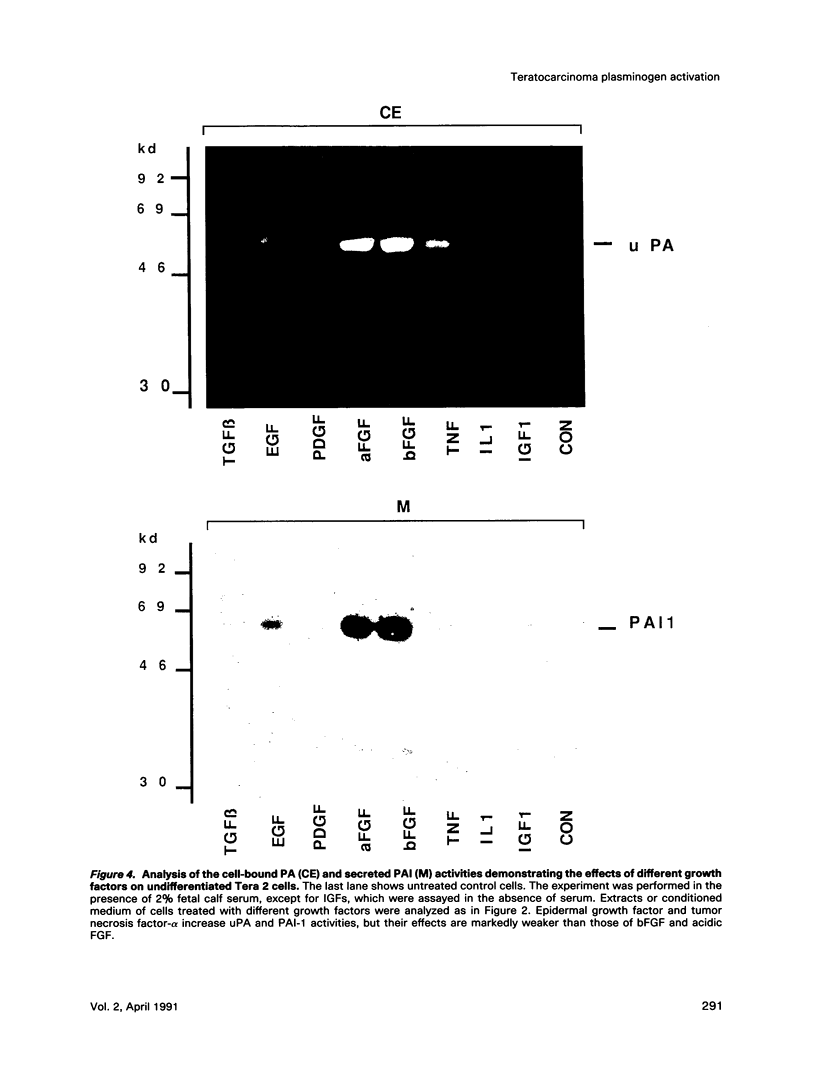
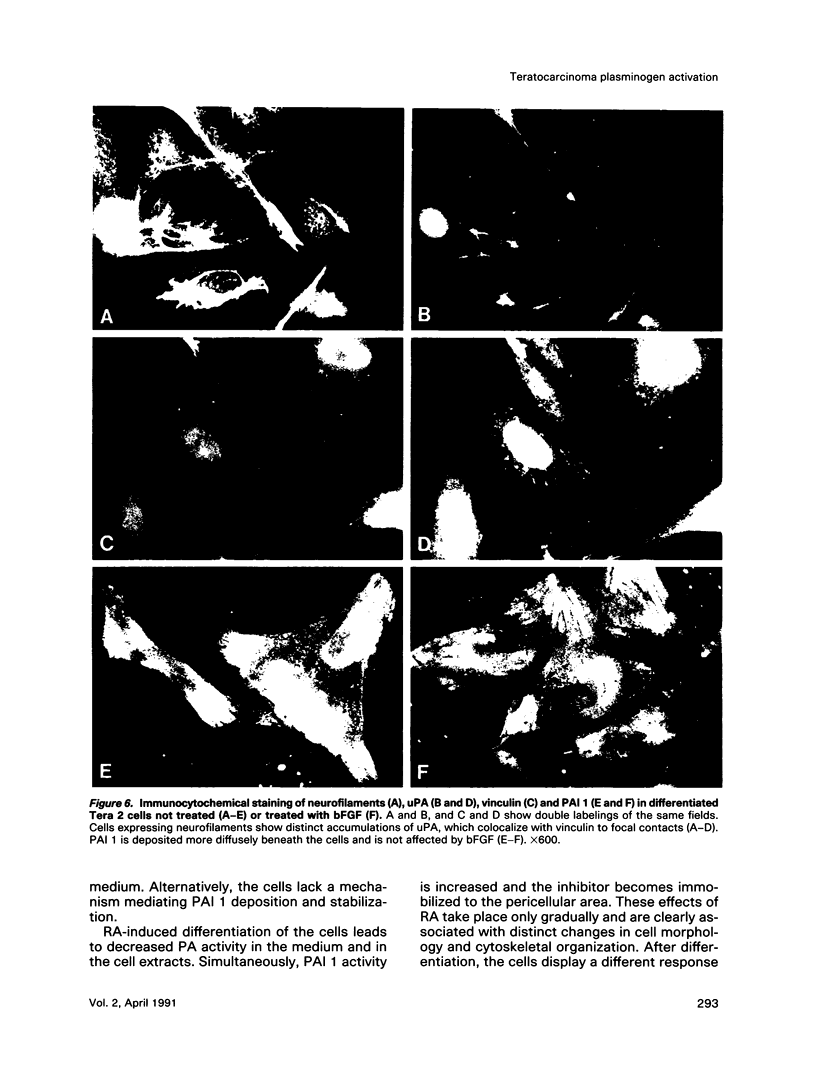
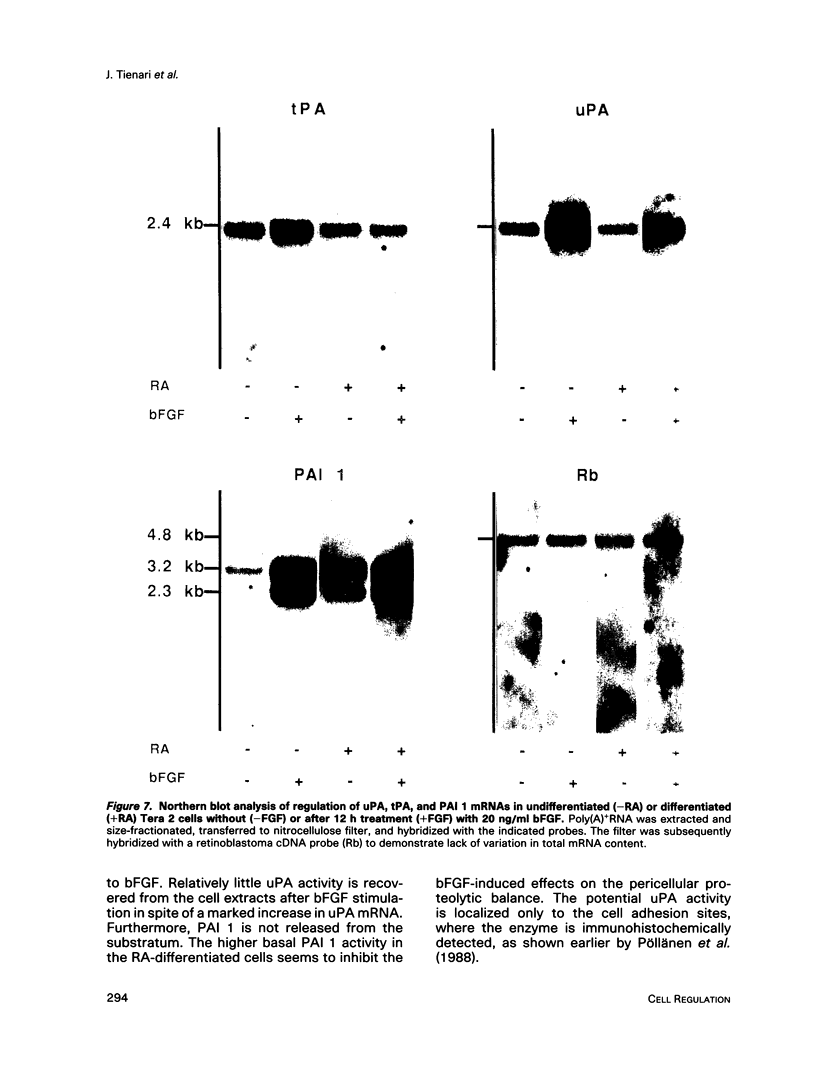
Human Tera 2 embryonal carcinoma cells switch gradually from rapidly growing undifferentiated cells to almost nonproliferating cells during retinoic acid (RA)-induced neuronal differentiation. This process is associated with the increased expression of type 1 plasminogen activator inhibitor (PAI 1) mRNA, and the secreted inhibitor is immobilized to the pericellular area. Furthermore, the differentiation is accompanied by a decrease in the amount of both the secreted tissue-type PA (tPA) and the mainly cell-associated urokinase-type PA (uPA) activity. In RA-differentiated cells, uPA becomes localized at the vinculin-rich cell-substratum adhesion sites. Fibroblast growth factor activity has been associated with various events during embryonal growth and with the regulation of proteolytic enzymes. A short-term treatment of the undifferentiated Tera 2 cells with basic fibroblast growth factor (bFGF) increases uPA mRNA levels and the cell-associated uPA activity, whereas the secretory tPA activity decreases. bFGF induces PAI 1 mRNA expression in the undifferentiated cells, but unlike PAI 1 protein after RA-treatment, the inhibitor does not accumulate around the cells but is released in the medium. A similar exposure to bFGF has less effect on the RA-differentiated Tera 2 cells. Under these conditions bFGF treatment leads to an increase in the amounts of PAI 1 and uPA mRNAs, but no changes in the localization of these components can be seen. Differentiation of human embryonal carcinoma cells is thus connected with an altered response to bFGF.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. A., Georg B., Lund L. R., Riccio A., Stacey S. N. Plasminogen activator inhibitors: hormonally regulated serpins. Mol Cell Endocrinol. 1990 Jan 2;68(1):1–19. doi: 10.1016/0303-7207(90)90164-4. [DOI] [PubMed] [Google Scholar]
- Andrews P. W. Human teratocarcinomas. Biochim Biophys Acta. 1988 Aug 3;948(1):17–36. doi: 10.1016/0304-419x(88)90003-0. [DOI] [PubMed] [Google Scholar]
- Blasi F., Vassalli J. D., Danø K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987 Apr;104(4):801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes S., Smith R., Casey G., Dickson C., Peters G. Sequence organization of the human int-2 gene and its expression in teratocarcinoma cells. Oncogene. 1989 Apr;4(4):429–436. [PubMed] [Google Scholar]
- Cubellis M. V., Andreasen P., Ragno P., Mayer M., Danø K., Blasi F. Accessibility of receptor-bound urokinase to type-1 plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4828–4832. doi: 10.1073/pnas.86.13.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson L. A., Lawrence D. A., Loskutoff D. J. Reverse fibrin autography: a method to detect and partially characterize protease inhibitors after sodium dodecyl sulfate--polyacrylamide gel electrophoresis. Anal Biochem. 1984 Mar;137(2):454–463. doi: 10.1016/0003-2697(84)90113-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. L., Moscatelli D., Jaffe E. A., Rifkin D. B. Plasminogen activator and collagenase production by cultured capillary endothelial cells. J Cell Biol. 1982 Dec;95(3):974–981. doi: 10.1083/jcb.95.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen B. S., Nachman R. L. Matrix plasminogen activator inhibitor. Modulation of the extracellular proteolytic environment. J Biol Chem. 1988 Jul 5;263(19):9476–9481. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 1989 May 15;49(10):2533–2553. [PubMed] [Google Scholar]
- Laiho M., Saksela O., Andreasen P. A., Keski-Oja J. Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-beta. J Cell Biol. 1986 Dec;103(6 Pt 1):2403–2410. doi: 10.1083/jcb.103.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Lee V. M., Andrews P. W. Differentiation of NTERA-2 clonal human embryonal carcinoma cells into neurons involves the induction of all three neurofilament proteins. J Neurosci. 1986 Feb;6(2):514–521. doi: 10.1523/JNEUROSCI.06-02-00514.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto V. P., Hovi T., Vartio T., Badley R. A., Virtanen I. Reorganization of cytoskeletal and contractile elements during transition of human monocytes into adherent macrophages. Lab Invest. 1982 Oct;47(4):391–399. [PubMed] [Google Scholar]
- Lehtonen E., Lehto V. P., Badley R. A., Virtanen I. Formation of vinculin plaques precedes other cytoskeletal changes during retinoic acid-induced teratocarcinoma cell differentiation. Exp Cell Res. 1983 Mar;144(1):191–197. doi: 10.1016/0014-4827(83)90453-6. [DOI] [PubMed] [Google Scholar]
- Levin E. G. Quantitation and properties of the active and latent plasminogen activator inhibitors in cultures of human endothelial cells. Blood. 1986 May;67(5):1309–1313. [PubMed] [Google Scholar]
- Mignatti P., Tsuboi R., Robbins E., Rifkin D. B. In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol. 1989 Feb;108(2):671–682. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimuro J., Loskutoff D. J. Purification of a protein from bovine plasma that binds to type 1 plasminogen activator inhibitor and prevents its interaction with extracellular matrix. Evidence that the protein is vitronectin. J Biol Chem. 1989 Jan 15;264(2):936–939. [PubMed] [Google Scholar]
- Moscatelli D., Quarto N. Transformation of NIH 3T3 cells with basic fibroblast growth factor or the hst/K-fgf oncogene causes downregulation of the fibroblast growth factor receptor: reversal of morphological transformation and restoration of receptor number by suramin. J Cell Biol. 1989 Nov;109(5):2519–2527. doi: 10.1083/jcb.109.5.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Vassalli J. D., Montesano R., Orci L. Urokinase-type plasminogen activator is induced in migrating capillary endothelial cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2535–2541. doi: 10.1083/jcb.105.6.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Hedman K., Nielsen L. S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1988 Jan;106(1):87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzino A., Kuszynski C., Ruff E., Tiesman J. Production and utilization of growth factors related to fibroblast growth factor by embryonal carcinoma cells and their differentiated cells. Dev Biol. 1988 Sep;129(1):61–71. doi: 10.1016/0012-1606(88)90161-3. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A., Pöllänen J., Stephens R., Andreasen P., Mayer M., Danø K., Gailit J., Ruoslahti E. Interaction of plasminogen activator inhibitor (PAI-1) with vitronectin. J Biol Chem. 1989 Apr 15;264(11):6339–6343. [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S., Stern P. L., Webb M., Walsh F. S., Engstrom W., Evans E. P., Shi W. K., Hopkins B., Graham C. F. Cloned human teratoma cells differentiate into neuron-like cells and other cell types in retinoic acid. J Cell Sci. 1984 Dec;72:37–64. doi: 10.1242/jcs.72.1.37. [DOI] [PubMed] [Google Scholar]
- Tienari J., Virtanen I., Soinila S., Lehtonen E. Neuron-like derivatives of cultured F9 embryonal carcinoma cells express characteristics of parietal endoderm cells. Dev Biol. 1987 Oct;123(2):566–573. doi: 10.1016/0012-1606(87)90415-5. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Miettinen M., Lehto V. P., Kariniemi A. L., Paasivuo R. Diagnostic application of monoclonal antibodies to intermediate filaments. Ann N Y Acad Sci. 1985;455:635–648. doi: 10.1111/j.1749-6632.1985.tb50441.x. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weima S. M., van Rooijen M. A., Feijen A., Mummery C. L., van Zoelen E. J., de Laat S. W., van den Eijnden-van Raaij A. J. Transforming growth factor-beta and its receptor are differentially regulated in human embryonal carcinoma cells. Differentiation. 1989 Sep;41(3):245–253. doi: 10.1111/j.1432-0436.1989.tb00753.x. [DOI] [PubMed] [Google Scholar]
- Wun T. C., Palmier M. O., Siegel N. R., Smith C. E. Affinity purification of active plasminogen activator inhibitor-1 (PAI-1) using immobilized anhydrourokinase. Demonstration of the binding, stabilization, and activation of PAI-1 by vitronectin. J Biol Chem. 1989 May 15;264(14):7862–7868. [PubMed] [Google Scholar]