Abstract
Conventional understanding suggests that simultaneous infection with more than one species of Leishmania is unlikely. In Peru, co-infections are clinically relevant because causative species dictates prognosis, treatment response, and follow-up. We describe a case of Leishmania (Viannia) braziliensis and L. (V.) lainsoni co-infection in a Peruvian patient with cutaneous leishmaniasis.
Introduction
Cutaneous leishmaniasis (CL) is caused mainly by Leishmania (Viannia) species in the New World.1,2 Among the subgenus L. (Viannia), different species are widespread in South American regions to which leishmaniasis is endemic, which include areas from the tropical jungle to Andean valleys.1 Moreover, Leishmania (Viannia) species are responsible for different clinical characteristics, such as the capacity of L. (V.) braziliensis,2,3 L. (V.) guyanensis,4 and L. (V.) panamensis,5 although predominantly L. (V.) braziliensis, to metastasize in the human host, leading to mucosal leishmaniasis, or the variable clinical response to antimonial chemotherapy seen in patients infected with L. (Viannia) parasites in different geographic settings.6
It has been previously demonstrated that at 12 months post-treatment, 30% of L. (V.) braziliensis-infected patients showed treatment failure with pentavalent antimony compared with 25% of L. (V.) peruviana-infected patients and 8% of L. (V.) guyanensis-infected patients, thus reiterating a species specificity to treatment response.6 In Peru, where approximately 10,000 new cases of leishmaniasis are reported annually,7 four species of the L. (Viannia) subgenus, namely L. (V.) peruviana, L. (V.) braziliensis, L. (V.) guyanensis, and L. (V.) lainsoni, in addition to Leishmania (Leishmania) amazonensis, have been reported.1,6
Traditionally, it is assumed that leishmaniasis vectors and human hosts are infected with one single species, However, it has been reported in Brazil and Mexico that two different species have naturally co-infected the same reservoir.8–13 Moreover, previous work in Bolivia using molecular methods detected patients with co-infection of different Leishmania species as well as Leishmania-Trypanosoma cruzi co-infections.14 We report a case of two different Leishmania (Viannia) species, namely L. (V.) braziliensis and L. (V.) lainsoni, identified by molecular means as causing distinct lesions in a Peruvian patient with CL.
Case report
A 33-year-old Peruvian woman presented to our center for evaluation of three painless ulcerative skin lesions with a duration of six weeks, which appeared simultaneously. Two months before presentation, the patient had spent two weeks working in the Iberia District of Madre de Dios, Peru. She did not normally reside in an endemic area for CL, but during the 12 months before admission, she had traveled to several jungle regions in Peru, including Ucayali, Loreto, Junin, and San Martin, for periods no longer than 20 days. The patient reported that her skin lesions developed as three papules, one on each of the left arm, right arm, and left leg two weeks after leaving Iberia, three months after leaving Ucayali, and five months after leaving Loreto. All papules quickly progressed to painless, moist, ulcerative lesions. Her past medical history was unremarkable and she was otherwise well without fever, weight loss, cough, or other systemic symptoms.
At examination, the patient appeared well and vital signs were normal. The three lesions in question were moist ulcers with raised, well-circumscribed violaceous borders. There was no evidence of cellulitis, lymphangitic streaking, or regional lymphadenopathy. The ulcer on the right arm was 1.0 × 1.2 cm, the ulcer on the left arm was 1.4 × 1.6 cm, and the ulcer on the left leg was 2.2 × 2.9 cm. There was no evidence of mucosal involvement in the nose, palate, or larynx.
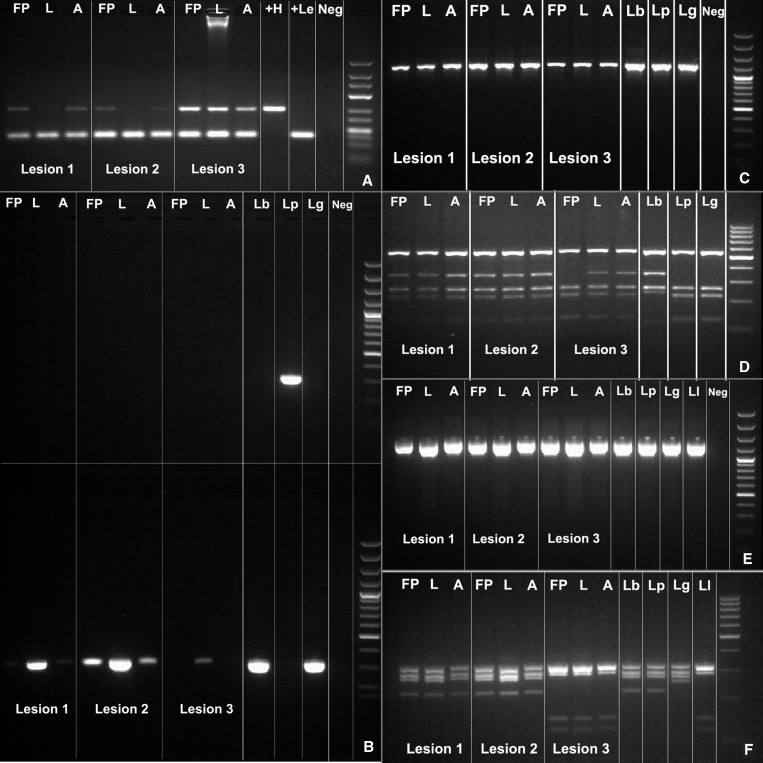
The patient was enrolled in a diagnostic study reported elsewhere,15 and as such, she underwent lesion scraping, aspiration, and filter paper impression of all three ulcers. Material from lesion scrapings was smeared on glass slides for Giemsa-stained microscopy and assayed for detection of Leishmania (Viannia) kinetoplast DNA (kDNA) by polymerase chain reaction (PCR) as described (Figure 1A).15 Tissue fluid from lesion aspirates was inoculated into traditional Leishmania Novy-MacNeal-Nicolle culture medium as described,16 and assayed by kDNA PCR. Filter paper lesion impressions (FPLIs) were assayed by kDNA PCR as described.15,16 Identification of Leishmania (Viannia) causative species was performed by using mannose phosphate isomerase (mpi), cysteine proteinase B, and heat shock protein 70 PCR and PCR–restriction fragment length polymorphism (PCR-RFLP) analysis according to the algorithm described elsewhere.17
Figure 1.
Results of polymerase chain reaction (PCR) and PCR–restriction fragment length polymorphism (RFLP) analysis for three types of clinical specimens (filter paper lesion impressions [FP], lancet scrapings [L], and aspirates [A]) obtained from three skin lesions of a Peruvian patient with cutaneous leishmaniasis. A, kinetoplast DNA PCR; B, mannose phosphate isomerase (mpi) PCR. Upper gel: Leishmania (Viannia) peruviana (Lp) mpi PCR; lower gel: L. (V.) braziliensis [Lb] mpi PCR, indicating amplification of L. (V.) braziliensis mpi target in all 3 lesions (note: only 1 of 3 specimens from lesion 3); C, cysteine proteinase B (cpb) PCR; D, RFLP analysis of cpb PCR products, indicating L. (V.) braziliensis pattern of banding for lesions 1 and 2, and a mixed banding pattern (L. (V.) peruviana or L. (V.) guyanensis [Lg] with filter paper specimen and L. (V.) braziliensis with lancet and aspirate specimens) for lesion 3; E, heat shock protein 70 (hsp70) PCR; F, RFLP analysis of hsp70 PCR products, indicating an L. (V.) braziliensis or L. (V.) peruviana banding pattern for lesions 1 and 2, and an L. (V.) lainsoni banding pattern for lesion 3. Neg = negative. Right lane in each panel is a molecular mass marker.
Specimens from lesions 1 and 2 on the arms amplified product in the mpi PCR when mpi Leishmania (V.) braziliensis primer was used, allocating their identity to either L. (V.) braziliensis or L. (V.) guyanensis (Figure 1B).17 In contrast, specimens from lesion 3 on the leg did not yield any amplification product with mpi PCR; mpi PCRs distinguish L. (V.) peruviana from L. (V.) braziliensis and L. (V.) guyanensis.17 When cysteine proteinase B PCR-RFLP analysis, which distinguishes L. (V.) braziliensis from L. (V.) peruviana and L. (V.) guyanensis, was performed on specimens from lesions 1 and 2, the resulting band pattern identified them as L. (V.) braziliensis (Figure 1C and D). As per our Leishmania species identification strategy,17 specimens from lesion 3 were then subjected to heat shock protein 70 PCR-RFLP analysis, which discriminates other L. (Viannia) species, and the resulting band pattern identified them as L. (V.) lainsoni (Figure 1E and F).
The patient was treated with 20 mg/kg/day of sodium stibogluconate for 20 days. By the end of treatment, the ulcers on the left arm (lesion 2) and left leg (lesion 3) showed complete re-epithelialization, and the ulcer on the right arm (lesion 1) showed more than 90% re-epithelialization and little inflammation. During subsequent follow-up evaluations at 1, 2, 3, 6, 12, and 24 months after therapy, the three ulcers remained completely re-epithelialized with characteristic scars and no evidence of inflammation and or mucosal involvement.
Discussion
We report a case of human Leishmania (V.) braziliensis and L. (V.) lainsoni causing different lesions in the same person. This case is notable for several reasons. First, in countries such as Peru with multiple co-endemic species, the occurrence of co-infections in humans is important to document because different causative species portend different clinical outcomes,2 and demonstrate notable differences in intrinsic sensitivity to different anti-leishmanial drugs.6,18–20 For economic reasons, if species identification is performed, it is typically conducted for just one lesion. Our documentation of two causative species infecting a single host suggests that species identification for each lesion may be justified, particularly in areas where L. (V.) braziliensis, the main causative species of mucosal leishmaniasis, is highly endemic. Had this particular patient only undergone diagnostic sampling of her lower extremity lesion, her infection with L. (V.) braziliensis would have gone undocumented, potentially resulting in a shortened duration of follow-up and lack of counseling regarding potential future risk of mucosal involvement.
In addition to the clinical significance of simultaneous Leishmania spp. infections, these cases also have pathophysiologic importance. It has been generally believed that leishmaniasis is caused by “infection with one of several different species of protozoan parasites of the genus Leishmania,”21 rather than one or more species of Leishmania. However, simultaneous infection with more than one causative species of Leishmania has been documented.8–14 It has long been postulated that infection of the host with one species of Leishmania might protect against re-infection with homologous or heterologous species.22,23 However, in vitro studies of individual macrophage co-infections with different species of Leishmania suggest an absence of mutual exclusion if a second infection with a different strain occurs within a short window,24 and recurrent infections in humans also imply some plasticity of this protective immune phenomenon.25,26 The patient described in this report probably acquired the infection in the last leishmaniasis-endemic region (Iberia), and although we cannot demonstrate definitively where this patient was infected, she was likely bitten by sand flies carrying different species over a short period in the same geographic location, thereby precluding development of any kind of partially protective immune response to infection by a second species. Irrespective of this suggestion, the patient had a favorable response to standard pentavalent antimony treatment, and no further relapsing cutaneous disease or mucosal involvement was identified.
Finally, this report adds significantly to our understanding of the molecular epidemiology of leishmaniasis in Peru. Leishmania (V.) lainsoni is known to exist in the Brazilian Amazon region,27 the Department of La Paz, Bolivia,28 and the sub-Andean or high jungle regions of Peru.1,29 Although the preferred vector of L. (V.) lainsoni, Lutzomyia ubiquitalis, has been found in Madre de Dios,27 this report is the first documented case of probable human infection occurring from this region. In their study of the geographic distribution of leishmaniasis in Peru based on 350 clinical samples from patients in 15 Peruvian departments, Lucas and others isolated L. (V.) lainsoni from seven patients in high jungle regions.1 Of 136 samples from Madre de Dios, all isolates were confirmed to be L. (V.) braziliensis.1 Until this report, L. (V.) lainsoni human infections have only been documented in residents of areas 600–2,000 meters above sea level.27–29 Thus, this case adds to the scientific literature regarding the epidemiology of this protozoan parasite.
In summary, we have reported the first documented case of simultaneous L. (V.) braziliensis and L. (V.) lainsoni infection in a patient from Madre de Dios, Peru, an area not known to be endemic for L. (V.) lainsoni. Documentation of Leishmania co-infections is essential to our understanding of the epidemiology, pathophysiology, and clinical course of cutaneous leishmaniasis, particularly in highly leishmaniasis-endemic countries such as Peru.
ACKNOWLEDGMENTS
We thank Ana Luz Quispe and Carmen Medina (Instituto de Medicina Tropical Alexander von Humboldt) for logistical support.
Footnotes
Financial support: Andrea K. Boggild was supported by a Detweiler Traveling Fellowship from the Royal College of Physicians and Surgeons of Canada. Personnel and facility fees for the Arevalo molecular laboratory (Nicolas Veland, Milena Alba, Vanessa Adaui, and Jorge Arevalo) were supported by the Institutional Collaboration Framework Agreement 3 from the Belgian Directorate-General for Development Cooperation.
Authors' addresses: Nicolas Veland, University of Texas, MD Anderson Cancer Center, Smithville, TX, E-mail: nicolasveland@yahoo.com. Braulio Mark Valencia and Alejandro Llanos-Cuentas, Institute of Tropical Medicine Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: braulio.valencia@upch.pe and elmer.llanos@upch.pe. Milena Alba, Vanessa Adaui, and Jorge Arevalo, Departmento de Bioquimica, Biologia Molecular y Farmacologia, Facultad de Ciencias, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: milenacollado@yahoo.es, vanessa.adaui@upch.pe, and biomoljazz@gmail.com. Andrea K. Boggild, Tropical Disease Unit, UHN-Toronto General Hospital, Toronto, Ontario, Canada, E-mail: andrea.boggild@utoronto.ca.
References
- 1.Lucas CM, Franke ED, Cachay MI, Tejada A, Cruz ME, Kreutzer RD, Barker DC, McCann SH, Watts DM. Geographic distribution and clinical description of leishmaniasis cases in Peru. Am J Trop Med Hyg. 1998;59:312–317. doi: 10.4269/ajtmh.1998.59.312. [DOI] [PubMed] [Google Scholar]
- 2.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 3.David CV, Craft N. Cutaneous and mucocutaneous leishmaniasis. Derm Ther. 2009;22:491–502. doi: 10.1111/j.1529-8019.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 4.Santrich C, Segura I, Arias AL, Saravia NG. Mucosal disease caused by Leishmania braziliensis guyanensis. Am J Trop Med Hyg. 1990;42:51–55. doi: 10.4269/ajtmh.1990.42.51. [DOI] [PubMed] [Google Scholar]
- 5.Osorio LE, Castillo CM, Ochoa MT. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg. 1998;59:49–52. doi: 10.4269/ajtmh.1998.59.49. [DOI] [PubMed] [Google Scholar]
- 6.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verástegui C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F, Dujardin JC, Llanos-Cuentas A. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 7.Pan American Health Organization, Peru Resumen del Analisis de Situacion y Tendencias de Salud. http://www.paho.org/spanish/dd/ais/cp_604.htm Available at. Accessed April 23, 2012.
- 8.Silveira FT, Lainson R, Shaw JJ, Ribeiro R. Cutaneous leishmaniasis in Amazonia. Report of the 1st human case of mixed infection, determined by 2 different Leishmania species: Leishmania brasiliensis and Leishmania mexicana amazonensis. Rev Inst Med Trop Sao Paulo. 1984;26:272–275. doi: 10.1590/s0036-46651984000500008. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira Neto MP, Marzochi MCA, Grimaldi G, Pacheco RS, Toledo LM, Momen H. Concurrent human infection with Leishmania donovani and Leishmania braziliensis braziliensis. Ann Trop Med Parasitol. 1985;80:587–592. doi: 10.1080/00034983.1986.11812072. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Montes O, Monroy-Ostria A, McCann S, Barker DC. Identification of Mexican Leishmania species by analysis of PCR amplified DNA. Acta Trop. 1998;71:139–153. doi: 10.1016/s0001-706x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 11.Martinez E, Mollinedo S, Torrez M, Munoz M, Banuls A-L, Le Pont F. Co-infection by Leishmania amazonensis and L. infantum/L. chagasi in a case of diffuse cutaneous leishmaniasis in Bolivia. Trans R Soc Trop Med Hyg. 2002;96:529–532. doi: 10.1016/s0035-9203(02)90428-1. [DOI] [PubMed] [Google Scholar]
- 12.Aldiwany LJ, Alawkati NA, Atia M, Rassam MB. Concomitant natural infection with L. donovani and L. major: a case report from Iraq. Soz Praventivmed. 1995;40:234–238. doi: 10.1007/BF01354478. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim ME, Smyth AJ, Ali MH, Barker DC, Kharazmi A. The polymerase chain reaction can reveal the occurrence of naturally mixed infections with Leishmania parasites. Acta Trop. 1994;57:327–332. doi: 10.1016/0001-706x(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 14.Bastrenta B, Mita N, Buitrago R, Vargas F, Flores M, Machane M, Yacsik N, Torrez M, Le Pont F, Brenière F. Human mixed infections of Leishmania spp. and Leishmania-Trypanosoma cruzi in a sub Andean Bolivian area: identification by polymerase chain reaction/hybridization and isoenzyme. Mem Inst Oswaldo Cruz. 2003;98:255–264. doi: 10.1590/s0074-02762003000200015. [DOI] [PubMed] [Google Scholar]
- 15.Boggild AK, Pilar Ramos A, Veland N, Valencia BM, Calderon F, Arevalo J, Low DE, Llanos-Cuentas A. Diagnostic performance of filter paper lesion impression PCR in both secondarily infected ulcers and non-ulcerative lesions caused by cutaneous leishmaniasis. J Clin Microbiol. 2011;49:1097–1100. doi: 10.1128/JCM.02457-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boggild AK, Valencia BM, Espinosa D, Veland N, Pilar Ramos A, Arevalo J, Llanos-Cuentas A, Low DE. Detection and species identification of Leishmania kDNA from filter-paper lesion impressions for patients with American cutaneous leishmaniasis. Clin Infect Dis. 2010;50:e1–e6. doi: 10.1086/648730. [DOI] [PubMed] [Google Scholar]
- 17.Veland N, Boggild AK, Valencia C, Valencia BM, Llanos-Cuentas A, Van der Auwera G, Dujardin JC, Arevalo J. Leishmania (Viannia) species identification in clinical samples from cutaneous leishmaniasis in Peru: assessment of a molecular step-wise approach. J Clin Microbiol. 2012;50:495–498. doi: 10.1128/JCM.05061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yardley V, Ortuño N, Llanos-Cuentas A, Chappuis F, Doncker SD, Ramirez L, Croft S, Arevalo J, Adaui V, Bermudez H, Decuypere S, Dujardin JC. American tegumentary leishmaniasis: is antimonial treatment outcome related to parasite drug susceptibility? J Infect Dis. 2006;194:1168–1175. doi: 10.1086/507710. [DOI] [PubMed] [Google Scholar]
- 20.Yardley V, Croft SL, De Doncker S, Dujardin JC, Koirala S, Rijal S, Miranda C, Llanos-Cuentas A, Chappuis F. The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am J Trop Med Hyg. 2005;73:272–275. [PubMed] [Google Scholar]
- 21.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 22.Osorio Y, Gonzalez SJ, Gama VL, Travi BL. Reinfection in American cutaneous leishmaniasis: evaluation of clinical outcomes in the hamster model. Mem Inst Oswaldo Cruz. 1998;93:353–356. doi: 10.1590/s0074-02761998000300015. [DOI] [PubMed] [Google Scholar]
- 23.Pérez H, Arredondo B, Machado R. Leishmania mexicana and Leishmania tropica: cross immunity in C57BL/6 mice. Exp Parasitol. 1979;48:9–14. doi: 10.1016/0014-4894(79)90049-3. [DOI] [PubMed] [Google Scholar]
- 24.Abdullah SM, Flath B, Presber W. Mixed infection of human U-937 cells by two different species of Leishmania. Am J Trop Med Hyg. 1998;59:182–188. doi: 10.4269/ajtmh.1998.59.182. [DOI] [PubMed] [Google Scholar]
- 25.Aebischer T. Recurrent cutaneous leishmaniasis: a role for persistent parasites? Parasitol Today. 1994;10:25–28. doi: 10.1016/0169-4758(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 26.Killick-Kendrick R, Bryceson AD, Peters W, Evans DA, Leaney AJ, Rioux JA. Zoonotic cutaneous leishmaniasis in Saudi Arabia: lesions healing naturally in man followed by second infection with the same zymodeme of Leishmania major. Trans R Soc Trop Med Hyg. 1985;79:363–365. doi: 10.1016/0035-9203(85)90381-5. [DOI] [PubMed] [Google Scholar]
- 27.Correa JR, Brazil RP, Soares MJ. Leishmania (Viannia) lainsoni (Kinetoplastida: Trypanosomatidae), a divergent Leishmania of the Viannia subgenus: a mini review. Mem Inst Oswaldo Cruz. 2005;100:587–592. doi: 10.1590/s0074-02762005000600014. [DOI] [PubMed] [Google Scholar]
- 28.Martinez E, Le Pont F, Mollinedo S, Cupolillo E. A first case of cutaneous leishmaniasis due to Leishmania (Viannia) lainsoni in Bolivia. Trans R Soc Trop Med Hyg. 2001;95:375–377. doi: 10.1016/s0035-9203(01)90185-3. [DOI] [PubMed] [Google Scholar]
- 29.Lucas CM, Franke ED, Cachay MI, Tejada A, Carrizales D, Kreutzer RD. Leishmania (Viannia) lainsoni: first isolation in Peru. Am J Trop Med Hyg. 1994;51:533–537. [PubMed] [Google Scholar]